Soft tissue sarcomas are exceptionally located in the elbow region. The aim of this work was to study the soft tissue sarcomas of the elbow region, their epidemiological and histopathological characteristics, anatomical features, the treatment performed, and the results obtained, in a unit of musculoskeletal tumours.
MethodsRetrospective review of ten patients with a mean follow-up of 65.0 ± 11.9 (range 21–132) months with soft tissue sarcomas located in the elbow region operated in our centre between 2008 and 2016.
ResultsMean age was 60.8 ± 6.7 years. Undifferentiated pleomorphic sarcoma was the most frequent histological diagnosis. Limb preservation surgery was performed in 90% of patients. Three patients were previously operated without following surgical oncology guidelines in another hospital, and this was statistically related to the need for more than one surgery to control the disease. R1 margin was obtained in 5 patients and R0 in another 5. Adjuvant radiotherapy was used in 7 cases. In 4 patients, subsequent surgery was performed for local or systemic control of the disease. Local recurrence occurred in 3 cases and in 5 there was distant disease.
ConclusionThe elbow region presents difficulty in achieving wide margins due to the proximity of neurovascular structures, adjuvant and/or neoadjuvant therapies could play an important role in performing limb preservation surgery. It would be advisable to refer these tumours to specialized units with multidisciplinary teams.
Los sarcomas de partes blandas se localizan excepcionalmente en la región del codo. El objetivo de este trabajo fue estudiar los sarcomas de partes blandas de la región del codo, sus características epidemiológicas e histopatológicas, particularidades anatómicas, tratamiento realizado y resultados obtenidos, en una unidad de tumores musculoesqueléticos.
Materiales y métodosRevisión retrospectiva de 10 pacientes con un seguimiento de 65,0 ± 11,9 (rango 21-132) meses con sarcoma de partes blandas situado en la región del codo intervenidos en nuestro centro entre los años 2008 y 2016.
ResultadosLa edad media fue de 60,8 ± 6,7 años. La histología más frecuente fue sarcoma pleomórfico indiferenciado. El 90% de los pacientes fueron sometidos a cirugía de preservación de extremidad. Tres pacientes fueron previamente intervenidos sin criterios oncológicos en otro centro, y esto se relacionó de forma estadísticamente significativa con requerir más de una cirugía para el control de la enfermedad. En 5 pacientes se obtuvo un margen R1 y en 5 R0. La radioterapia adyuvante se utilizó en 7 casos. En 4 pacientes se realizó cirugía posterior para control local o sistémico de la enfermedad. En 3 casos se produjo recidiva local y en 5 se presentó enfermedad a distancia.
ConclusionesLa región del codo presenta dificultad para lograr márgenes amplios por la proximidad de estructuras neurovasculares, por lo que las terapias adyuvantes y/o neoadyuvantes podrían tener un papel importante para poder realizar una cirugía de preservación de la extremidad. Sería recomendable remitir estos tumores a unidades especializadas con equipos multidisciplinares.
In Europe, the approximate incidence of soft tissue sarcoma in adults is 4–5/100,000 per year.1 Of the total number of soft tissue sarcomas, it is estimated that only 14% affect the upper limbs.2 Involvement of the elbow region is rare and represents only 1% of total sarcomas.3
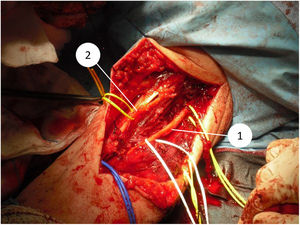
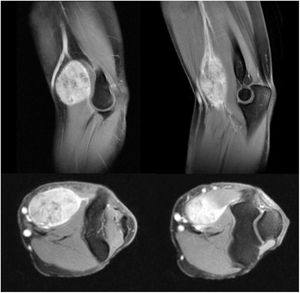
In 2015 Halai et al. published the most extensive series of surgically treated bone tumours of the elbow region.4 To date, we have found no published papers specifically addressing soft tissue sarcomas in the elbow region. Study of the elbow is particularly important due to the anatomical complexity of major neurovascular structures in close proximity to each other (median nerve and brachial artery with its division into ulnar artery and radial artery in the anterior part, ulnar nerve at the medial border and radial nerve at the lateral border) and the small amount of soft tissue, especially in the posterior part. This may make resection with a wide oncological margin difficult, as these neurovascular structures and the options for coverage and reconstruction may be affected.5,6 In addition, it has been described that at elbow level there are usually higher rates of residual disease and therefore a higher incidence of local recurrence.7 Extracompartmental sarcomas in the upper limb, which include tumours of the antecubital fossa, are associated with a higher rate of local recurrence and distant metastasis, as well as a lower survival rate.6
The aim of this study was to study soft tissue sarcomas of the elbow region, their epidemiological and histopathological characteristics, anatomical features, treatment and results obtained, in a musculoskeletal tumour unit.
Material and methodsRetrospective, analytical, observational study of malignant soft tissue tumours of the elbow region operated in our centre between 2008 and 2016. The 533 patients presented to the multidisciplinary committee on musculoskeletal tumours in this period were reviewed and patients were selected according to the following inclusion criteria: 1) soft tissue sarcomas located between the medial and lateral epicondyle as the proximal limit and the distal part of the radius head and olecranon as the distal limit4; 2) who had undergone primary or secondary surgery in our centre between 2008 and 2016. Exclusion criteria: 1) sarcomas in other locations; 2) histological types with special behaviour: dermatofibrosarcoma protuberans, desmoid tumour or rhabdomyosarcoma; 3) tumours whose definitive anatomopathological diagnosis was benign; 4) tumours of bone origin; 5) metastases of primary tumours in other locations. The sample size was determined by these inclusion criteria. Ten patients were included in the study with a mean follow-up of 65.0 ± 11.9 months (range 21–132 months). All the patients were assessed prior to intervention by the multidisciplinary committee on musculoskeletal tumours at our hospital. All the patients signed their informed consent for the various diagnostic and therapeutic procedures.
The data analysed were obtained from the clinical history: 1) sex and age; 2) clinical characteristics of the tumour: laterality, site within the elbow region, history of previous surgery without oncological criteria and staging according to the American Joint Committee on Cancer Staging System (AJCC); 3) anatomopathological result of the surgical specimen; 4) treatment performed (surgical and/or adjuvant or neoadjuvant to the intervention) and 5) follow-up time, local or systemic recurrence, death (and when it occurred).
Patients whose primary consultation was in our unit were studied by core needle biopsy (six patients). The biopsy tract was discussed beforehand in the multidisciplinary committee on musculoskeletal tumours to ensure its removal in subsequent surgery. After the operation, the surgical specimen was analysed. The samples were requested for the patients who had previously undergone surgery at another hospital for evaluation by our centre’s pathological anatomy team.
Histologically, the tumours were registered following the WHO classification for musculoskeletal soft tissue tumours (fourth edition of 2013).8 The system of the Fédération Nationale des Centres de Lutte Contre le Cancer (FNCLCC) was also used, which divides them into G1, G2 or G3 tumours, according to the degree of differentiation, number of mitoses per field and percentage of necrosis.
The main surgical treatment was defined as the first surgical intervention performed in our centre. It was divided into amputation or limb preservation surgery. The need for cooperation from other specialties was also registered: plastic surgery, vascular surgery, or both. Surgical interventions performed in addition or secondary to the main surgery (margin widening, local recurrence treatment or metastasis management), were considered subsequent surgery.
The level of involvement of the surgical specimen margins was determined according to the classification of the Union for International Cancer Control (UICC) which defines a disease-free margin ≥ 1 mm as R0, a margin < 1 mm as R1 and the presence of macroscopic contamination of the edges as R2.9 The classification used, unlike the R-classification, is more demanding since a free margin greater than or equal to 1 mm is required to be defined as R0.9 The use of chemotherapy and/or radiotherapy was also recorded as adjuvant treatment.
The patients were followed up by the orthopaedic surgery team -tumour unit- (local control of disease) and medical oncology (control of systemic disease). Follow-up included magnetic resonance imaging (MRI) with contrast of the operated elbow to assess local recurrence, and thoracoabdominal-pelvic computed tomography (CT) to search for distant disease.8 The controls found local recurrence in one case and systemic recurrence in three cases, after assessment by the multidisciplinary committee, PET-CT was considered to complete staging and prognostic assessment in the follow-up of four patients (cases 2, 6, 7 and 10). Bone scan was not used in any case. After the immediate postoperative follow-up, follow-up was every three months for the first two years, every six months to five years and then an annual control.
Data on local recurrence, systemic recurrence and patient death were recorded. Local recurrence-free survival was defined as the time between surgery and the recurrence of neoplastic tissue in a previously treated location. Metastasis-free survival was defined as the time between diagnosis and the onset of distant disease. In cases of death, overall survival was considered to be the time between diagnosis and the death of the patient.
Statistical analysisFrom the results obtained for the quantitative variables, the arithmetic mean and the standard error of the mean (SEM) were calculated. With the data obtained with the nominal variables, frequency tables were made to calculate the number and percentage of patients presenting each of the options for each variable, and cross tabs and Pearson's test 2 (p < .05) to analyse possible associations between variables. The survival analysis was performed using the Kaplan-Meier method.
ResultsThe epidemiological data from the series are included in Table 1.
Cases from our series of soft tissue sarcoma in the elbow region (2008–2016).
| Caso | Age (years) | Sex | Site | Side | PSWOC | AJCC staging | Initial surgery | Histology | HG | Margins | Adj Tx | Subsequent surgery | Current status (2019) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 78 | F | A-C | L. | NO | IIIA | LPS | Undifferentiated pleomorphic sarcoma | G3 | R1 | Rt | ------ | Disease free |
| 2 | 46 | M | A-C | L. | Yes | LPS | Leiomyosarcoma | G3 | R0 | Rt | Pulmonary segmentectomy for metastasis | Disease free | |
| 3 | 49 | M | A-C | L. | NO | IIIB | LPS | Myxoid liposarcoma | G2 | R1 | Rt | ------ | Death(Metastatic disease) |
| 4 | 76 | M | A-C | L. | NO | IIIA | LPS | Pleomorphic liposarcoma | G3 | R0 | Rt | ----- | Death(Metastatic disease) |
| 5 | 84 | M | A-C | R. | NO | IIIB | Amputation | Leiomyosarcoma | G3 | R0 | NO | ----- | Death(Metastatic disease) |
| 6 | 71 | M | A-C | R. | NO | IIIA | LPS | Undifferentiated pleomorphic sarcoma | G3 | R1 | NO | Further resection + flap for local recurrence/pathological fracture | Disease free |
| 7 | 63 | M | Post | L. | SÍ | LPS | Undifferentiated pleomorphic sarcoma | G3 | R1 | Ct + Rt | Margin widening/above-the-knee amputation due to local recurrence | Disease free | |
| 8 | 83 | F | Post | L. | NO | IIIA | LPS + flap | Undifferentiated pleomorphic sarcoma | G3 | R0 | NO | ----- | Death (not disease related) |
| 9 | 29 | M | A-C | L. | Yes | LPS | Myxoid liposarcoma | G2 | R1 | Rt | Margin widening + flap | Disease free | |
| 10 | 29 | F | A-C | R. | NO | II | LPS + bypass | Epithelioid Sarcoma | G3 | R0 | Rt | ----- | Local recurrence and metastatic disease (Ct and It) |
| 60.8 ± 6.7 | 70% M | 80% A-C | 60% L. | 30% PSWOC | 90% LPS | 80% G3 | 50% R1 | 70% RT | |||||
| 30% F | 20% Post | 40% R. | 50% R0 |
A-C, antero-cubital; (AJCC), American Joint Committee on Cancer Staging System; LPS, limb preservation surgery; PSWOC, prior surgery without oncological criteria; R, right; GH, histological grade; M, Male; It, immunotherapy; L, left; F, female; Post, posterior; CT, chemotherapy; Rt, radiotherapy; Adj Tx, adjuvant treatment.
Three patients were operated in their hospital of origin without oncological criteria (30%), since the suspected preoperative diagnosis was a benign tumour and they were referred to our centre when the pathological anatomy of the surgical specimen was compatible with malignancy (Table 1). The American Joint Committee on Cancer Staging System (AJCC) was used (Table 1). All the patients completed treatment and follow-up in our centre, the seven who were attended from the beginning of the process and the three who were referred.
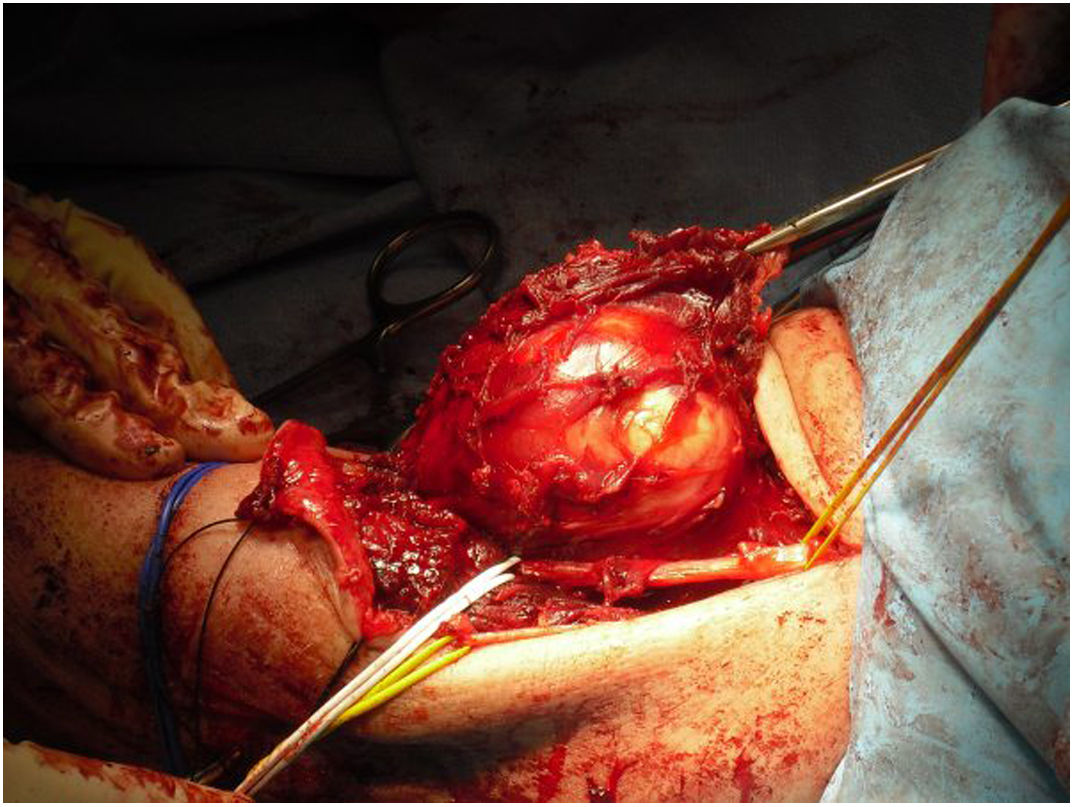
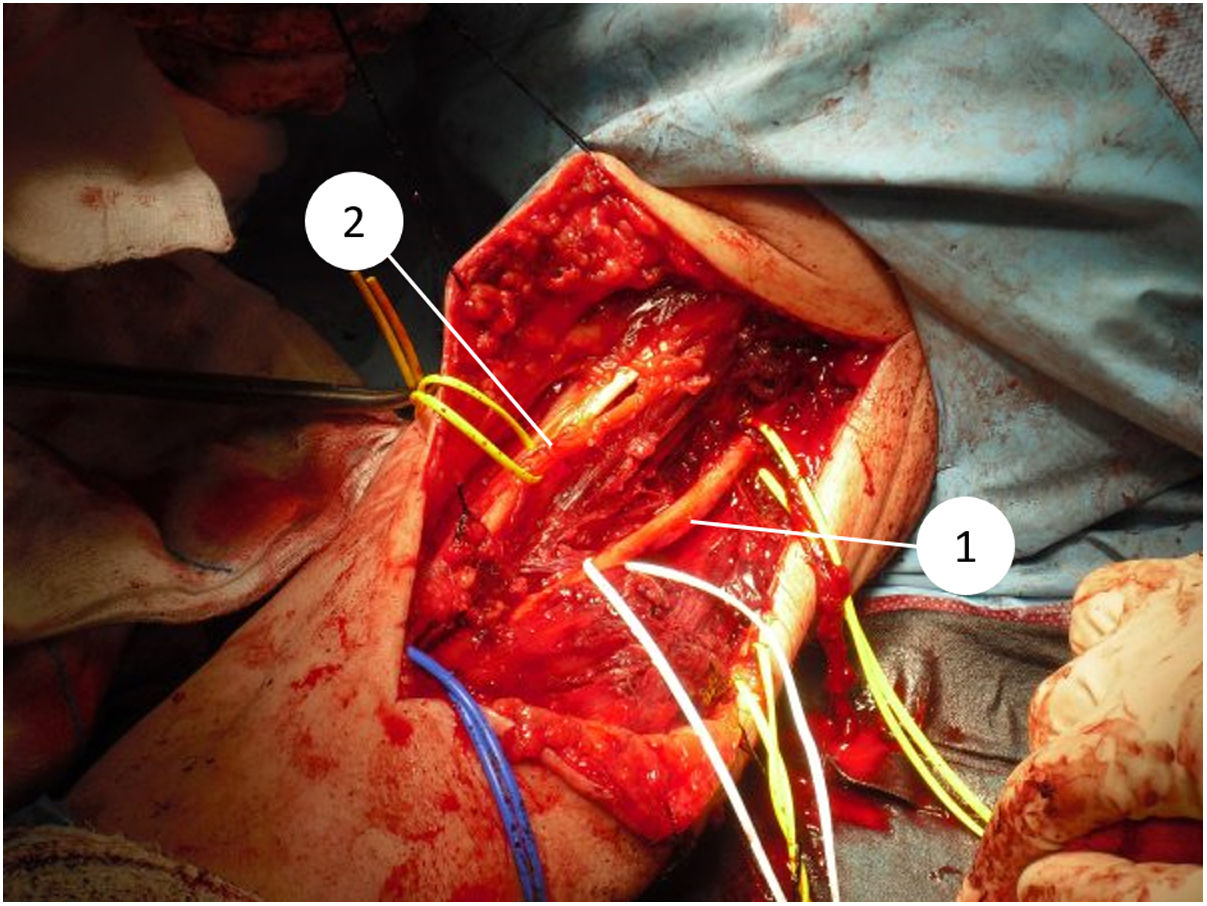
All the patients in our series underwent surgical treatment, with limb preservation in nine of the 10 patients (Fig. 1) (Table 1). Due to advanced age and associated comorbidities, which would have hampered subsequent reconstruction techniques, it was decided to perform a supracondylar amputation in one patient as a first intervention. In four cases, the collaboration of plastic surgery was required at some point in the process, to perform coverage techniques. Two of these patients had been previously operated in another centre without oncological criteria. An association was observed between the tumour site at a posterior level and the need for collaboration in the intervention by the plastic surgery team, although this did not reach statistical significance (P = .053). In one of the cases the vascular surgery team was required to perform a limb bypass. Of the nine patients who underwent limb preservation surgery, two died from metastases, one died from a cause other than the elbow lesion, one recurred locally and has metastasis, and five are free of disease (56%). The patient who was initially amputated died from metastasis.
In 4 (67%) of the 6 patients who underwent core needle biopsy prior to the intervention in our centre, the diagnosis coincided with that obtained from the anatomopathological study of the surgical specimen, although in all six cases malignancy was suspected. The anatomopathological and histological results are shown in Table 1. Within the group of patients with margins classified as R0, in two no additional treatment was performed (40%), and the other three patients underwent adjuvant radiotherapy (60%). With respect to the patients with R1 margins, in two cases further surgery was performed to widen the margins (40%), combined with radiotherapy in one and chemotherapy plus radiotherapy in the other. Two other patients were managed only with adjuvant radiotherapy without additional surgery (40%) and in one of them a wait-and-see approach was taken at the request of the patient (20%). In total, seven patients underwent adjuvant radiotherapy (also combined with chemotherapy in one patient) (Table 1). The decision on adjuvant therapy in each case was made by a multidisciplinary committee taking into account the resection margins, the type and histological grade of the tumour, the general condition of the patient, the tumour site and its relationship to adjacent structures, and the patient's wishes once the options had been explained. A possible association was observed between the location of the tumour at the antecubital level and the need for adjuvant treatment with radiotherapy, without reaching statistical significance (P = .054). None of the patients in our series underwent neoadjuvant treatment.
In two of the operated patients, distal sensory disturbances occurred that resolved spontaneously and without sequelae. No other surgical complications occurred in the patients of the series.
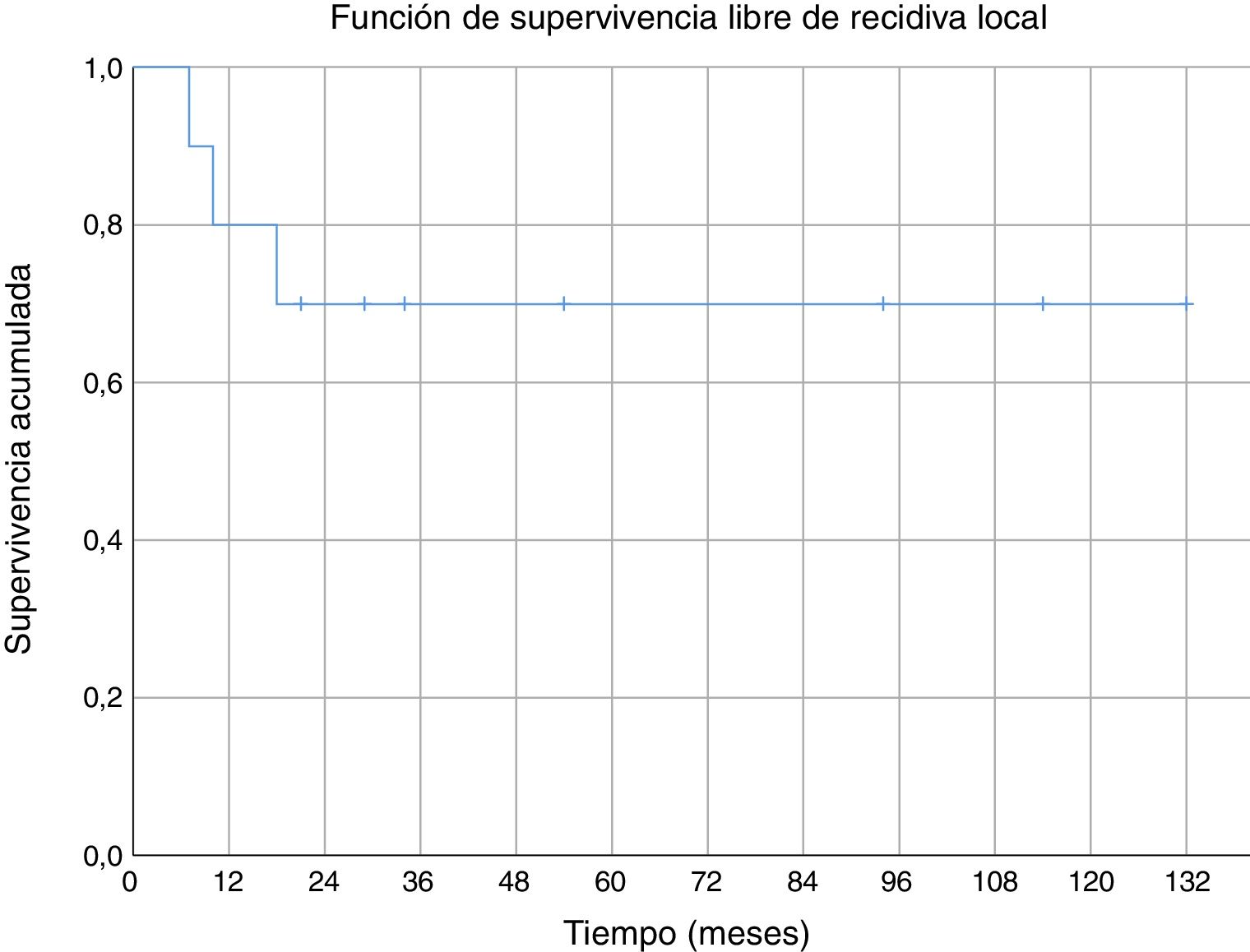
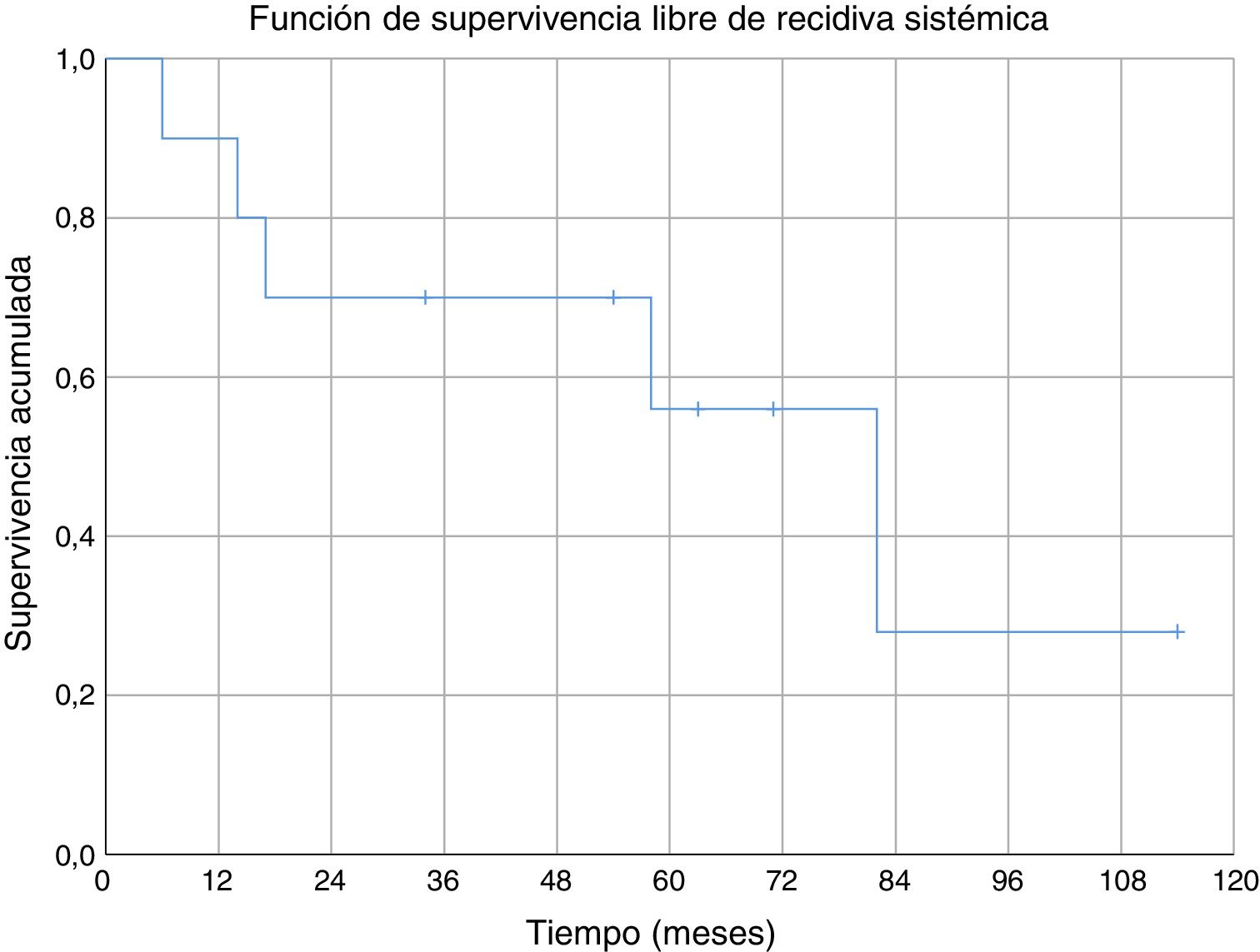
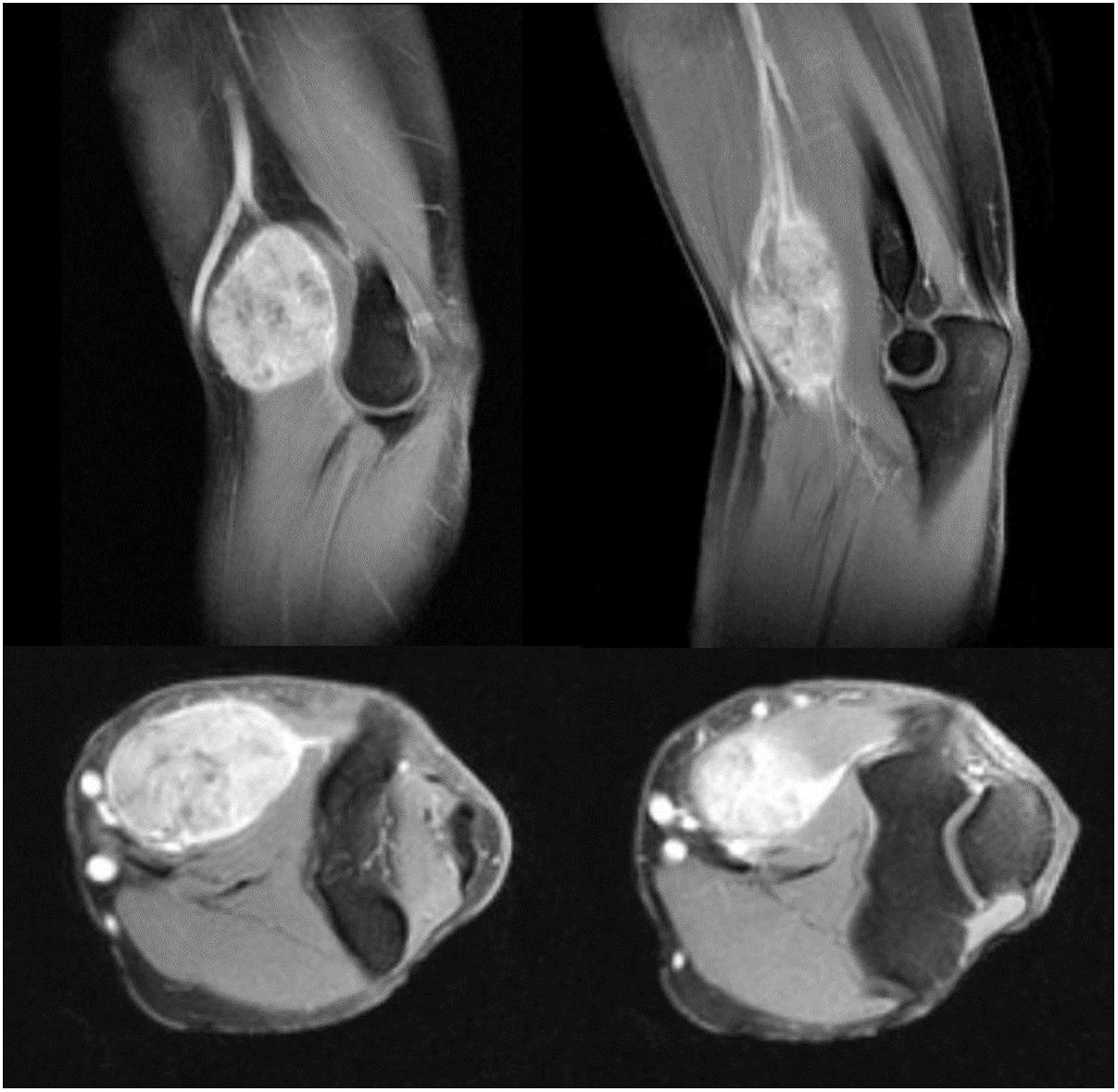
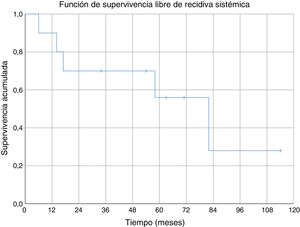
Three of the patients studied presented local recurrence of the disease (30%), in two cases R1 margins had been obtained in the initial surgery and R0 margins in the third case. No statistically significant relationship was observed between the margins obtained and local recurrence. In one of the cases the recurrence was detected seven months after the initial operation, in another case at 10 months and in the third patient local recurrence occurred at 18 months (mean 11.7 ± 3.3 months) (Fig. 2). The first of these patients underwent a new tumour resection combined with a flap for coverage, the second case underwent an above-the-knee amputation, and in the third case no further intervention was performed because, in addition to local recurrence, metastatic disease was present. During the follow-up, five cases of distant disease were detected, four of which presented tumours with G3 histological grading and the remaining case was G2. The time of onset of metastasis was 6, 14, 17, 58 and 82 months after diagnosis, respectively (mean 35.4 ± 14.7 months) (Fig. 3). The five patients presented pulmonary metastases: one was treated by pulmonary segmentectomy and after four years was free of disease; the other four patients, after assessment by the musculoskeletal tumours committee, were considered not to be resectable,10 three patients were treated palliatively (two with metastases in various sites [lung, bone and liver] and one was pluripathological and in poor general condition), and the other patient was treated with chemotherapy and immunotherapy.
Of the total number of patients studied, four required one or more surgical interventions after the initial surgery for local or systemic control of the disease. Two patients underwent margin widening (both combined with flap), one patient underwent pulmonary segmentectomy and one patient underwent resection for local recurrence (Table 1). Of these four patients, three were referred from another centre already having undergone surgery, and there was a statistically significant relationship (P = .04) between previous surgery in another centre and the need for subsequent surgery.
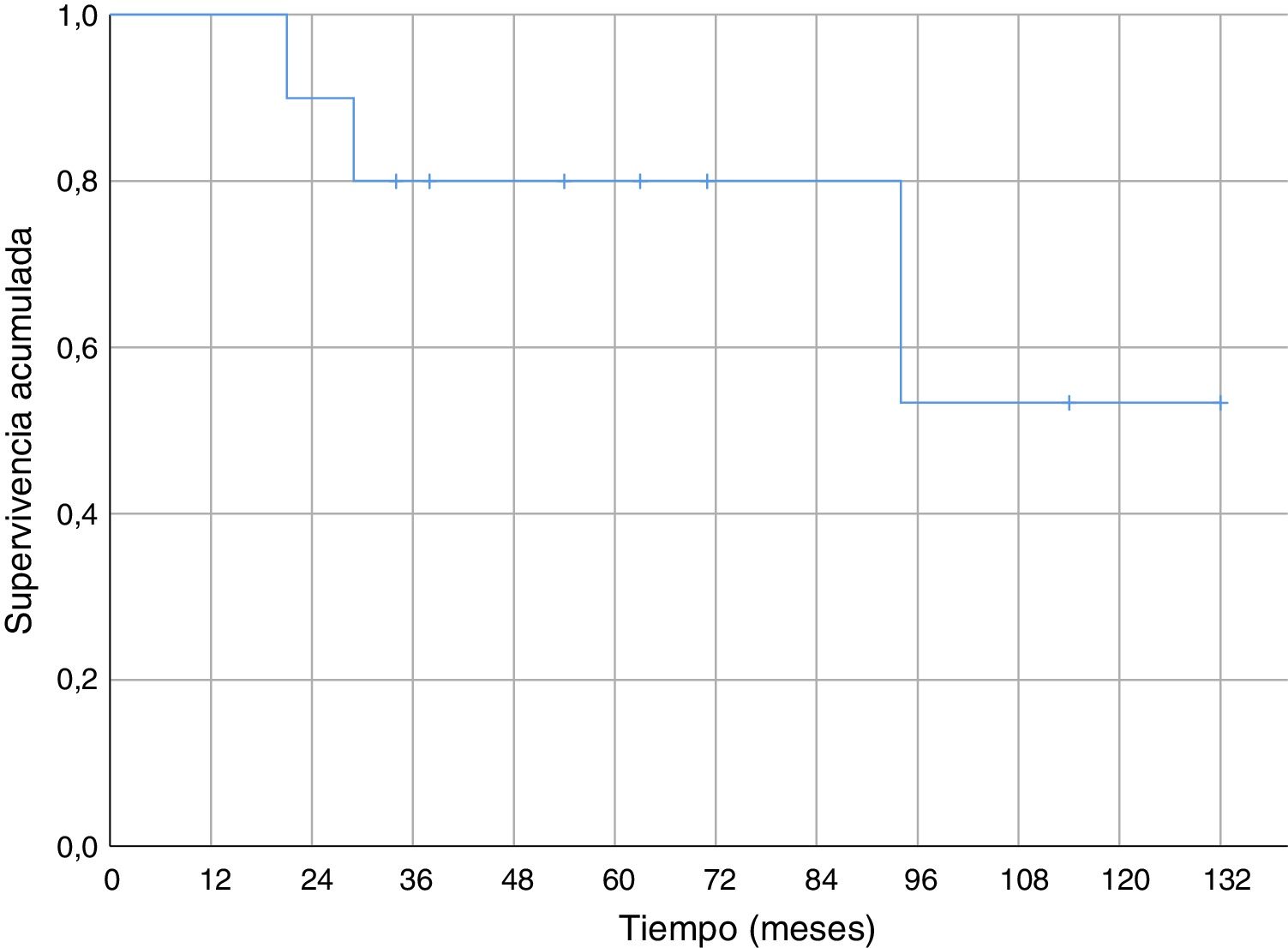
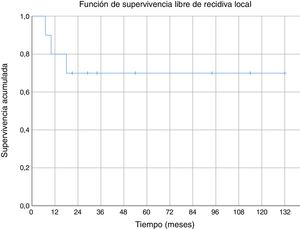
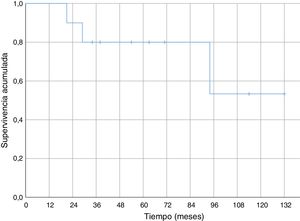
Regarding overall survival throughout the follow-up, four patients died (40%), three of them due to metastatic disease (30% death due to oncological disease) and one of them due to pneumonia without evidence of distant disease. In the cases of metastasis, death occurred 21, 29 and 94 months after diagnosis. Death secondary to pneumonia without disseminated disease occurred 34 months after diagnosis. Survival at one year was 100% and 80% at five years (Fig. 4).
DiscussionThe elbow area is a rare site for soft tissue sarcomas. When considering bone and soft tissue sarcomas together, approximately 1% of them are estimated to originate in the elbow region.3 Creighton et al. reported 10 patients with elbow level tumours in their series of 61 upper extremity soft tissue sarcomas.11 Although our hospital is a referral centre for the treatment of sarcomas in the Community of Valencia, over eight years of review we have only had 10 cases of soft tissue sarcomas located in the elbow region, of the 533 cases presenting to the multidisciplinary committee on musculoskeletal tumours (1.8%). The low frequency of the anatomical site described by other authors3,11 is comparable to our series.
The general recommendations for diagnosis and treatment suggest referral to a specialist centre for musculoskeletal tumours, for all patients with a soft tissue mass deep in relation to the fascia without clear aetiology, and also for those with tumours superficial in relation to the fascial plane but of more than 5 cm in diameter.1 Due to the low frequency of these tumours, it is not uncommon for patients to be referred after having previously undergone surgery without oncological criteria. Different series place patients in whom malignancy is found unexpectedly after the removal of a tumour considered benign before the operation at between 18% and 30%,12–14 which coincides with that observed in our series (30%). Due to their more superficial site and smaller diameter at the time of diagnosis, suspicion of malignant pathology in tumours of the upper limb is usually low and they have been found to have up to twice the risk of being excised without oncological criteria than tumours that occur in the lower limbs.7 In our series, three of the 10 patients (30%) had previously been operated at another centre without oncological criteria. The impact on the long-term prognosis of patients re-operated for a soft tissue sarcoma erroneously diagnosed as benign at the outset remains a matter of debate.15,16 Bianchi et al. state that reintervention in these patients may be particularly difficult in complex anatomical areas such as the axilla or popliteal fossa.16 In our opinion, the anterior region of the elbow is anatomically similar to the areas mentioned and a previous resection, without oncological criteria, could also condition subsequent surgery of greater difficulty. In our case, a statistically significant relationship has been observed between a history of previous intervention in another centre without oncological criteria and the need for at least one operation in addition to the main intervention, for local and systemic control of the disease.
When considering treatment of malignant tumours located in the upper limb, the main objective is oncological control of the disease. Secondly, and provided that control of the disease is not compromised, the highest possible level of functionality will be sought.17 Limb preservation surgery is currently the treatment of choice; this technique is used in 85%–95% of cases diagnosed with the condition.12,18,19 In our series, this percentage is maintained; limb preservation surgery was the choice (nine cases), and only one case, due to the significant associated comorbidities, underwent direct amputation. A disease-free status was obtained in 56% of patients with limb preservation surgery. However, despite the fact that the initial treatment of choice was limb preservation surgery in 90% of cases, two patients required an amputation in a second operation, and therefore limb preservation surgery at the end of follow-up was achieved in 70% of patients.
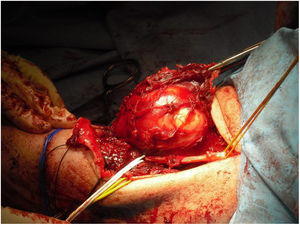
Since 1985, when the National Institute of Health (NIH) recommended limb preservation surgery in its consensus for the treatment of high-grade sarcoma,14 the concept of surgical margins has become increasingly important. However, it has not yet been defined in the literature how wide these margins should be to be considered optimal.1,8,20 Due to the proximity of neurovascular structures at elbow level, it is very difficult to achieve wide margins surgically (Figs. 5 and 6). In our opinion, the complexity of the anatomical area under study may explain the high percentage of patients with R1 margins in our series (50%). Although there is a higher percentage of patients with R1 margins in the cases initially operated without oncological criteria (67%), we were not able to demonstrate statistically significant differences if we compare them with the cases operated after assessment by the multidisciplinary committee (43%); probably because the series is not long enough and also because there is a bias in achieving R1 margins in the patients operated without oncological criteria. On the one hand, in these cases it is more difficult to determine the area to be resected in order to obtain adequate safety margins because in imaging tests we have images of post-surgical tissue and not of a delimited tumour. Secondly, these patients were not referred for assessment by the committee because after the initial study of the disease no malignancy was suspected, which is why referral to our centre was not considered appropriate. Thus, it is possible that these three patients presented less locally aggressive or less evolved tumours and therefore with less involvement or less close to neurovascular structures, than those who were initially referred to the musculoskeletal tumours unit, where the malignancy was indeed suspected by the team that carried out the first assessment. This could also explain why no statistically significant differences in local recurrence were observed between patients with unplanned surgery and those in whom surgery with oncological criteria was performed. Two patients with R1 margins had local recurrence (2/5) and one patient with an R0 margin had local recurrence (1/5), therefore, although we think that the absence of free margins is directly related to local recurrence, we were not able to establish this relationship in a statistically significant way in this series.
Given the difficulty of achieving wide margins, the use of complementary therapies after surgical treatment is of great importance. The efficacy of adjuvant radiotherapy in local control of the disease has been well established,21 but its importance in reducing rates of distant metastasis and overall survival has not been fully confirmed.22,23 Nonetheless, there is a growing body of work that refers to a possible association between postoperative radiotherapy and a positive impact on overall survival rates.23–26 According to the literature, and after each specific case had been studied by the multidisciplinary committee, taking into account the resection margins, the type and histological grade of the tumour, the general condition of the patient, the tumour site and its relationship with adjacent structures and the patient's wishes once the options had been explained, in two cases with R1 margins it was decided to widen the margins and combine radiotherapy in one and chemotherapy plus radiotherapy in the other; two patients were treated with adjuvant radiotherapy alone without additional surgery and in one only a wait-and-see approach was taken at the request of the patient. The finding of a possible relationship, although not statistically significant, between a tumour site in the antecubital area and the indication for adjuvant radiotherapy in our opinion makes sense from a clinical point of view, since precisely in the anterior aspect of the elbow the neurovascular structures may make it difficult to achieve wide disease-free margins and, therefore, this may have influenced the indication for postoperative radiotherapy. This decision follows the trends of current clinical guidelines.1
On the other hand, the posterior aspect of the elbow can be a site where it is easier to achieve wide margins because it is far from neurovascular structures, but more complex when it comes to achieving adequate coverage. For this reason, we believe a trend can be observed in our series between a posterior tumour site and the need for collaboration with the plastic surgery team, although it should be borne in mind that only two cases of a posterior site are presented and no statistical significance was achieved for this association. Therefore, we must be cautious with this statement, which needs to be confirmed or rejected by larger studies.
Over recent years, more evidence has emerged suggesting the use of neoadjuvant techniques in patients with soft tissue sarcoma.1,8,27,28 The justification for their use is a potential increase in achieving negative margins, while performing surgery to preserve major neurovascular structures. This is a great advantage in the treatment of elbow sarcoma, due to the proximity of neurovascular structures that are fundamental for limb function.27 Currently, the clinical guidelines of the SEOM (Spanish Society of Medical Oncology) indicate that chemotherapy and neoadjuvant radiotherapy are not considered standard treatments (they are considered optional) for soft tissue sarcomas and there is no consensus on whether or not they should be used; however, they recommend considering chemotherapy and neoadjuvant radiotherapy in patients with high-grade sarcomas, tumours greater than 5 cm in diameter or in patients in whom a resection with wide margins is difficult to ensure or would be very aggressive.1 In our series, after consideration of each case by the multidisciplinary committee, none of the patients underwent chemotherapy or radiotherapy before the intervention (the date of the papers referenced must be taken into account1,8,27,28 and that the series includes tumours operated between 2008 and 2016), although perhaps in the future, and as the literature provides more specific answers regarding their usefulness, these treatments could be considered according to the characteristics of the tumour, in complex anatomical areas such as the anterior aspect of the elbow and their use and indications could be extended.
We found no specific data for any series of soft tissue sarcomas at the level of the elbow, but the cumulative survival at 5 years (80%) in our series was similar to the survival of other series of soft tissue sarcomas (where they mix several sites).6,29–31 Cumulative survival free of systemic recurrence (56%) and local recurrence (70%) at five years are also comparable to those described in other series of soft tissue sarcomas in the upper limbs.6,31 In our series all local recurrences occurred in the first 18 months; however, metastases appeared after a short time of follow-up (six months the earliest), but also in the medium-long term (58 and 82 months).
This paper presents the limitations of a retrospective study. It is a relatively short series (although relevant, given the low incidence of soft tissue sarcomas in this site). Because the cases were collected over a long period of time, the clinical protocols for action have changed, as have the members of the multidisciplinary team, which may have conditioned the outcome of the different cases. The follow-up time is variable and due to the characteristics of the different soft tissue sarcomas, there is a heterogeneous series of cases, with only one case of some histological types.
In conclusion, the elbow is a rare site for the presentation of soft tissue sarcomas, but it is of great importance due to its anatomical characteristics. It is difficult to achieve wide margins in its antero-cubital aspect, due to the proximity of neurovascular structures, and therefore adjuvant and neoadjuvant therapies could play an important role in limb preservation surgery. Limb preservation surgery is currently the surgical treatment of choice, and it is important to perform surgery with oncological criteria from the outset. Therefore, it would be advisable to refer elbow tumours to specialist units with multidisciplinary teams. With planned and adequate treatment, the results of cumulative survival, local recurrence-free survival and systemic recurrence of soft tissue sarcomas at the elbow in our series are similar to those observed in soft tissue sarcomas in other sites.
Level of evidenceLevel of evidence IV.
FundingNone.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Correa-González N, De La Calva C, Miranda I, Amaya JV, Angulo M, Baixauli-García F. Sarcomas de partes blandas en la región del codo e influencia de sus particularidades anatómicas en su tratamiento. Experiencia en una Unidad de Tumores musculoesqueléticos. Rev Esp Cir Ortop Traumatol. 2020. https://doi.org/10.1016/j.recot.2020.04.010