Despite progress in the development and validation of analytical methods. In the meantime, the challenges in interpretive forensic toxicology remain persistent, since it is no easy to establish a causal link between the concentration of a compound in biological samples and its impact on a real case. A proper interpretation of results is required to ascertain their relevance and to ensure that the investigator or jurist can make the most of the utility of the trace within the framework of the investigation or within the courtroom.
Because of their complexity and frequent presence at toxicology laboratories, in this article, we have focused our attention on the interpretation of results in drug-facilitated offences, in cases where hair samples are involved and in cases related with postmortem investigation. Following this review, the advances in analytical results interpretation in the selected cases, are being mainly conducted towards metabolic studies.
In cases of drug-facilitated crimes, it allows to extend the detection window and identify low concentration consumption markers for fast-clearing compounds. Regarding hair metabolomics analysis, recent studies can assist us to determine the deterioration caused by cosmetic treatments or to elucidate the difference between consumption and extern contamination, which are a well-known factor affecting the interpretation of hair analysis. Concerning postmortem results, progress is intended to ensure better reference data, to the study of drug-to-drug interactions as well as to the application of metabolomics and to the acknowledgment of the genetic variances related with drugs metabolism, location, and mechanism of action.
A pesar de los avances significativos en el desarrollo de nuevas técnicas instrumentales y en la validación de los métodos analíticos, los retos en la toxicología forense interpretativa permanecen ya que no es tarea fácil establecer el vínculo entre la concentración de un compuesto en las muestras biológicas y su relevancia en un caso forense. Para determinar la relevancia y garantizar que el investigador y el jurista puedan aprovechar la prueba en el marco de la investigación o en la sala de juicio, se requiere una adecuada interpretación de los resultados.
Debido a la complejidad y frecuencia con que se presentan en los laboratorios, este artículo se ha focalizado en la interpretación de resultados en los delitos facilitados por drogas, en los casos en que la muestra de pelo se encuentra involucrada y en los relacionados con la investigación postmortem. Tras la revisión realizada, los avances en la interpretación de los resultados analíticos en los casos seleccionados se están enfocando fundamentalmente hacia los estudios metabólicos.
En los casos de delitos facilitados por drogas estas investigaciones se dirigen a ampliar la ventana de detección y detectar marcadores del consumo de bajas cantidades de compuestos de rápida eliminación. Respecto a los análisis de drogas en pelo, los nuevos estudios de metabolitos pueden ayudar a determinar el deterioro provocado por los tratamientos cosméticos o a aclarar la disyuntiva entre consumo y la contaminación externa del cabello, conocidos factores que afectan a la interpretación de las drogas en pelo. En lo referente a los resultados postmortem, los avances en la interpretación se están orientando a la mejora de las bases de datos de referencia, estudios de interacciones entre compuestos, aplicación de la metabolómica y al conocimiento de las variaciones genéticas relacionadas con el metabolismo de los compuestos, de sus vías y lugares de acción.
Forensic toxicology encompasses postmortem investigation, the effects of alcohol and drugs on human behaviour, workplace drug testing, and doping control, which is in turn divided into human and animal control. With the additions of illicit drug analysis, drug-facilitated crimes, and hair analysis, modern forensic toxicology is comprised of a total of 8 subdivisions, unveiling the advancements in this forensic discipline.1
The basic responsibility of the forensic toxicologist is to assist the judicial system in evaluating whether a particular substance could have an impact on the outcome of a legal issue. To do this, they must first determine the presence and unequivocal identity of the chemical substance (whether this be a prescribed substance, an illicit drug, or a general toxicant) in an individual, and establish a relationship between exposure to that substance and the manifestation of a harmful effect or even death.2 Once the compound has been identified and the concentration determined, the forensic toxicologist must interpret the role played by that substance in the case.3
Over the years, many challenges in relation to interpretive toxicology have arisen, which are particularly complex in cases of drug-facilitated crimes, in cases with the involvement of hair samples, and in postmortem investigations, which are the most characteristic of the historical perception of forensic toxicology.
In the cases of drug-facilitated crimes, especially sexual crimes (drug-facilitated sexual assaults, DFSAs), considerable progress has been made with the support of national and international medical/legal4 and toxicological5 recommendations,6,7 in addition to hospital protocols and review articles that classify the criminal act.8–10 However, given the particularities of these cases, interpretation of the analytical results remains complex, especially when compounds such as gammahydroxybutyric acid (GHB) or scopolamine (burundanga) are involved.
Interpretation of analytical findings in hair is still complicated, since the concentration of drugs in hair can be affected, among other factors, by topical, chemical, or physical hair treatments, by growth rates and cycles, and by external contamination. These are frequently referred to as drug limitations in hair analysis.11
Regarding the interpretation of postmortem results, it must be determined whether the concentration of a compound in a sample accurately represents the concentration at the time of death12 and a correlation needs to be established with the medical history, the setting and the seized substances.13,14 After death, a series of processes take place that hinder interpretation of the substances detected in blood and other biological samples. Although much has been published, focusing on redistribution and on related artefacts for particular compounds, studies have not addressed changes in time-dependent postmortem concentrations, nor do they offer advice on best practices that should be adopted to interpret particular cases.15
In recent years, metabolic studies have become a fundamental tool in different fields of forensic toxicology through the proposal of biomarkers that improve the interpretation of results,16 and in some cases, with complementary genetic studies. This article puts forward the advances that have been made in outcome interpretation of the 3 types of issues mentioned, which are highly frequent in the cases of forensic toxicology laboratories.
Opinions and interpretationsAlthough technology aids rapid detection of evidence at the scene, together with its recognition, significance, and analysis, outcome interpretation requires human intervention to determine its relevance and guarantee that the investigator and the jurist can take advantage of the usefulness of evidence within the framework of the investigation and/or in the courtroom.17
The importance of standardisation of analytical methods was highlighted in the National Academy of Sciences (NAS) report and the assurance of the quality of the results has been emphasised by different American organisations, such as the ForensicToxicology Council (FTC) formed by the American Academy of Forensic Science (AAFS), the Society of Forensic Toxicologists (SOFT), and the American Board of Forensic Toxicology (ABFT) and European organisations such as the European Network Forensic Sciences Institutes (ENFSI), among others.1 As a result, the confirmation of laboratory capacity has witnessed the development of accreditation programmes, in keeping with that established in standards and particularly in ISO/IEC 17025:2017. These standards are general requirements for testing and calibration laboratory competence.
ISO/IEC 17025:2017 states that when opinions and interpretations are included in a test report (7.8.7 Information on opinions and interpretations), “the laboratory must ensure that only personnel authorised to express opinions and interpretations release the respective statement. Said opinions and interpretations must be based exclusively on the results obtained from the tested item and must be identified as such.”18
In the Sydney Declaration, which aims to cover the essence of forensic science through the foundational bases beyond organisations, protocols, and technicalities, point 7 refers to the fact that the findings acquire meaning in the context, but it also highlights that the scientist must act with ethics and impartiality, transparency and independence, to ensure that the information provided for the possible resolution of the investigated case is useful and reliable, with avoidance of its adaptation to the recipient of the information.19
Recently, the number of publications related to contextual information and its possible effect on decision-making has increased.20 Although errors due to contextual bias usually appear in those forensic disciplines dedicated to identifications that are made using methods based on human knowledge and experience, similar situations can occur in forensic toxicology, where the methods used can also provide ambiguous data, i.e. data very close to the established limits.
In these cases, if the analyst is knowledgeable about the context, they could be influenced to make an incorrect decision by giving the environment a more solid basis for their decision than the results endorse.21 Therefore, although for the interpretation of results, the toxicology laboratory always requests as much contextual information as possible about the case background and circumstances, its combination with the results should not be made before those results have been obtained.
It is recommended that the laboratory have explicit policies or procedures for minimising contextual bias.20
Interpretation of the results in drug-facilitated crimesGeneralitiesDFSA crimes are the most frequent among drug-facilitated crimes, although it is also known that the true incidence is difficult to quantify,22–25 since victims sometimes do not report the incident or take a long time to do so, with the result that not all of them receive the medical treatment and the corresponding toxicological study.26
Advances in the interpretation of DFSA results have occurred simultaneously with the understanding of the idiosyncrasies of these crimes, fostering a global interest in combating this phenomenon,27 with the involvement of national5,28,29 and international30–32 authorities and organisations. In the toxicology laboratory, the interpretative difficulties in DFSA cases are essentially related to the toxicokinetic characteristics of the substances involved and the analytical techniques used.
One of the key points is to guarantee the shortest possible time between the event and the sampling, in addition to obtaining information about medical treatment. By having more precise information and a better sampling protocol, the interpretation of the toxicological analysis is improved, and so too its usefulness in clarifying the facts.33 Although it is possible to make retrospective estimates in the interpretation of results through calculations based on the toxicokinetic characteristics of the compounds, sample collection, and circumstantial data, it is not possible to determine the administered dose, due to uncontrolled parameters, such as rates of metabolisation or simultaneous consumption, especially of alcohol and drugs. Toxicologists must interpret analytical data with caution and report to judicial authorities from all possible angles, especially when asked about the effects of the drug at the time of the alleged events.34
It is important to remember that many of the drugs implicated in DFSAs, including alcohol, can produce similar clinical symptoms, for example, incapacitation. Therefore, it cannot be concluded that the reported incapacitation is due to a specific substance without evidence that said substance (or a specific marker/metabolite of it) is present in at least one sample from the victim. Moreover, given that most drugs are metabolised and eliminated at different rates, it should never be assumed that a negative toxicology result demonstrates that a drug was not present at the time of the alleged crime,30 and this information should be recorded in the results report.
With consideration of the characteristics that generally hinder the interpretation of these cases, the 2 compounds of GHB and scopolamine (burundanga) deserve special attention with regard to advances in the interpretation of their results in DFSA cases.
Interpretation of gammahydroxybutyric acid results in drug-facilitated crimesGHB is a powerful depressant of the central nervous system that, due to its incapacitating effects, is related to proactive DFSA (GHB-FSA), since it has the perfect characteristics to go unnoticed in these criminal acts: easy availability, amnesic effects—and there is little probability of being detected due to a short half-life or lack of detection with routine analytical procedures.
A vital issue in these forensic investigations is the careful evaluation of the biological matrix (matrices) with which possible unconscious ingestion of GHB can be demonstrated. Taking into account its rapid metabolism and elimination, concentrations in blood and urine quickly decrease to endogenous values.35 Samples must therefore be taken as quickly as possible, since the detection window of exogenous GHB is narrow and hinders testing the use of GHB in cases of DFSA.36 Forensic evidence is often lacking and analytical reports often conclude that GHB was not detected.
From an analytical point of view, the endogenous nature of GHB forces toxicologists to establish a cut-off concentration above which the results are considered positive. These cut-off points must be higher than the highest endogenous concentration and are different for different biological samples.37 To report a positive GHB result, a value greater than 30 mg/l in blood samples38 and between 6 and 10 mg/l in urine samples39 is recommended.
Although the “gold sample” for GHB-FSA cases is urine, the interpretation of the results close to the cut-off point can be complemented with the analysis of GHB in hair samples, which can confirm exposure in the recent or distant past.40 Along these lines, different authors defend that, even after weeks or months of GHB-FSA, the hair segment corresponding to the moment of the event presents an increase in the concentration of GHB with respect to the endogenous concentration determined in other segments.41
However, ensuring that a positive case is positive and a negative case is negative in the hair sample with a single exposure to GHB remains a controversial issue.42 This is mainly due to factors such as the high variability of endogenous GHB in the hair, which does not allow us to propose a single reliable cut-off point that can be applied to discriminate it from exogenous GHB, and the effect on the GHB concentration by washing and/or hair treatments. and by contamination through perspiration.43 In fact, specific recommendations have been published aimed at avoiding erroneous interpretations when using a hair sample in cases of GHB-FSA.35,44,45
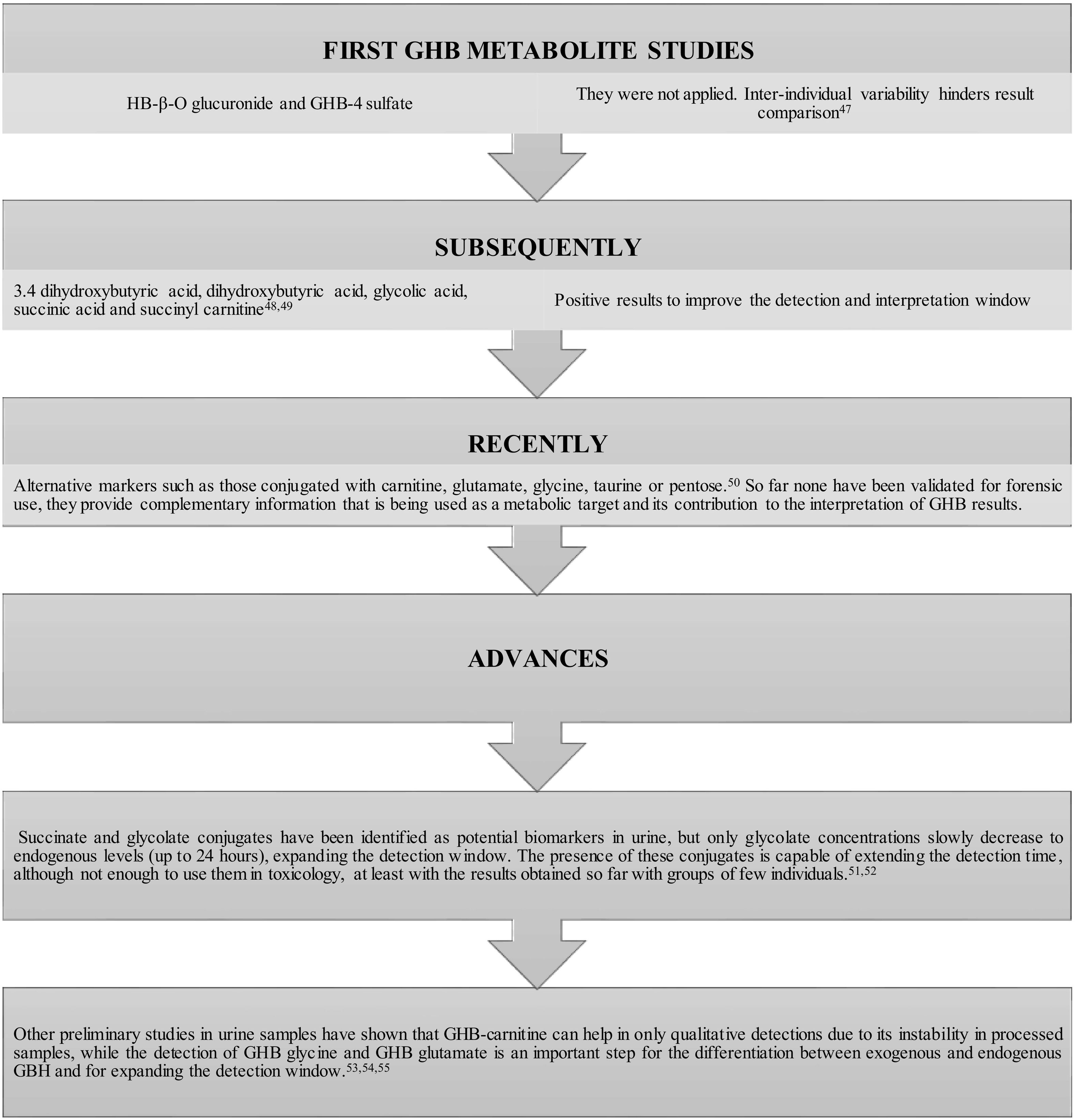
All these difficulties in case analysis and interpretation have necessitated the development of alternative analytical methods with less manipulation of the samples and more sensitive techniques, as well as the use of metabolomics to search for possible biomarkers of GHB consumption that essentially expand the detection window in urine samples.36,46 In the case of the hair sample, to date, neither the GHB glucuronide conjugate nor other metabolites have provided alternatives to the determination of GHB.44
Fig. 1 shows the evolution and advances in studies of GHB metabolites and their possible use as biomarkers to expand the window of analytical detection.
Evolution and advances in GHB metabolite studies to improve and where appropriate, correct the difficulties in interpreting the results in DFSA cases.47–55
In another sense and in the same way that lipid esters of ethanol are formed, phospholipids, esters of fatty acids, and triglycerides represent a new class of GHB metabolites (P-GHB) that are formed through phospholipase D and they can be used as biomarkers. However, as in previous cases, more studies are needed, in this case with real blood samples.56
Interpretation of scopolamine (burundanga) results in drug-facilitated crimesDue to its narcotic properties and rapid elimination, scopolamine (burundanga) is also a compound of choice in these crimes. However, and contrary to alarming urban legends, its widespread use is not proven, as was already evident in 2014.57
Although some specific cases have revealed the involvement of scopolamine in DFSA, the evidence reveals that its actual participation is anecdotal.58 In 2016, its presence was demonstrated in the urine sample along with the anticholinergic syndrome that the victim presented.59 In that same year, there was a widespread rumour about the use of scopolamine, but in a series of similar cases characterised by sample collection within 48 h of the event, the results were always negative.60 Recently, data from the National Institute of Toxicology and Forensic Sciences corroborated the very low presence in the DFSA with positive results for burundanga in 1 of 292 suspected cases.61
Similarly to GHB, but to a much lower degree, advances have focused both on its determination in hair samples62,63 and on experimental studies of its 3 main metabolites: hydroxy-methoxy-scopolamine, scopine, and tropic acid, together with scopolamine within the initial 24 h and scopine and tropic acid in isolated cases 48 h after administration.60
Despite the low incidence, at least in Spain,64 circumstances vary and this can affect the criminal dynamics of burundanga, such as, for example, media attention. Enhanced popular knowledge of the existence of this type of substance can make it more sought after increase its danger, given the ease with which it can be acquired.61
Interpretation of hair sample resultsThe interpretation of hair analysis is generally aimed at elucidating one of the following key points: establishing whether the individual consumed or was exposed to drugs; identifying the drugs that were used; distinguishing between single, occasional, or repeated consumption, and identifying the consumption period.65
Technological advances have made possible the detection of the presence of drugs in hair samples at low concentrations. When the concentrations found are extremely low, interpretation must be cautious and the analytical cut-off points established in the laboratory are recommended to be stated in the report. If the concentrations of the identified drugs are below these cut-off points, the report must clearly reflect the limitations of the analysis, including that use cannot be unequivocally proven without other evidence. A positive or negative interpretation of results close to analytical breakpoints can vary drastically from one laboratory to another which may complicate legal consequences.
In addition to the analytical cut-off points, there are internationally established limits, below which the analytical result of the drug (even if it is identified) must be reported as “negative” or with similar wording. Although these international limits are continually reviewed, their purpose is to minimise risks when an interpretation cannot distinguish active consumption from passive exposure.
Despite the indisputable forensic value of the hair sample, it should not be the primary evidence to prove drug use, as it can potentially be misinterpreted,66 both with false-positive results, especially in those situations in which the individual is exposed to drugs, but does not actively consume them,67 and with false-negative results, in those cases in which the expected concentrations are low and the drug must not only be detected, but also eliminate the possibility of false reporting.62 In relation to ethyl alcohol markers, the interpretation of hair tests is complex due to similar problems, which sometimes call into question whether the alcohol was actually consumed or not.68
Erroneous results can also be due to segments which do not correspond to the exposure time due to the growth phase of the hair; inadequate sampling (not cutting close enough to the scalp); inaccurate alignment of hair strands; incorporation of drugs from sweat or sebum; variations in the rate of hair growth within the sample strand,or due to contact between hairs in the distal direction.69
In the latest consensus of the Society of Hair Testing (2023)70 regarding the interpretation of drug results in hair, it is indicated that the concentration of compounds in hair can be affected by topical, chemical, or physical hair treatments (including, but not limited to, bleaching, dyeing, perming, straightening, and UV exposure). These external contamination and growth rates and cycles must be considered to establish the time frame represented by the sample.
Currently, interpretive evaluation of results in hair samples remains devoid of a full understanding of various variables, including hair colour, cosmetic treatments, or growth inequality, and the possible effects of any “decontamination” procedures used. In recognition of the fact they are key aspects impacting the interpretation of results, this paper will address them in particular.
The specific nature of the sampleColour and cosmetic treatmentsOne of the different factors that affect the incorporation and retention of drugs in the hair is colour.71 Pigmentation is due to the transfer of melanin granules from the melanocytes of the bulb to the cortical keratinocytes and its presence fundamentally favours the incorporation of basic drugs by interacting the protonated amino groups with the negatively charged carboxyl groups of melanin.72 In the case of ethyl glucuronide (EtG) and ethyl palmitate (markers of alcohol consumption), incorporation is not affected by natural hair colour.73
Depending on the nature of the compounds and the type of dye, cosmetic treatments (dyes and bleaches) accentuate the variability in the incorporation of drugs and alcohol metabolites into the hair,74 also causing a degradation of the drugs that is difficult to estimate when treatments are carried out after consumption.75
Various studies have shown that bleaching and dyeing can increase the uptake of cocaine73 and amphetamines, while for MDMA or THC derivatives no differences are found with untreated hair.76 For the particular case of EtG, the effect is so important that the discoloration is even considered an adulteration of the sample, since the high contents of hydrogen peroxide cause important and unpredictable reductions in the EtG contents.49,73,77
It should be noted that some hair “cleansing” procedures suggested on the Internet to negativise drug use have proven to be very effective in reducing analyte concentrations. The hair treated in this way is indistinguishable during visual examination since it does not appear damaged or lose colour when subjected to washing/digestion, making it difficult to appreciate the samples that went through the process, and possibly leading to false-negative results.42
Advances in detecting cosmetic hair adulterations are focused on detecting altered endogenous biomolecules that could be used as biomarkers in the case of oxidative cosmetic hair treatments. Eisenbeiss et al. were able to identify 69 metabolites significantly altered after hair bleaching. Most decreased after bleaching, but fully degraded metabolites showed the most promise as suitable biomarkers. The proportions in which the concentrations of the metabolites increased and decreased improved the discrimination of treated and untreated hair samples. These results offer the possibility of including single biomarkers or selections of biomarkers in routine detection methods to improve the interpretation of hair analysis results.78
Further studies exist, aimed at determining oxidation products. There are few of them but one, cysteic acid, formed through the oxidation of cysteine, is an important component of the keratin structure and is an already known marker of hair damage induced by bleaching.48
Whenever it is confirmed that the hair sample has been treated, the toxicology laboratory must be informed, as it involves considerations in the interpretation of the results73 and the laboratory must reflect this in its report.70
Hair growthHair growth is another bias factor to consider when interpreting results. For years, the consensus that hair grows 1 cm per month has been understood as an oversimplification, since in 1 month hair can grow as little as 0.6 cm or as much as 3.36 cm. Furthermore, hair cut close to the scalp does not show immediate growth, as it is estimated that it takes between 7 and 10 days to reach the surface.79 Other studies have estimated that a more accurate growth range would be 1.1±0.2.80
Taking into account that when the sample is taken after the cut, 0.8±0.1 cm remain on the scalp and that about 2 weeks must be added for the new follicle to reach the scalp, the first 1 cm segment corresponds to a hair formed 1.3±0.2–2.2±0.4 months earlier, which undoubtedly has an impact on results interpretation.81
Related to hair growth is its fragmentation to determine the presence of the compounds over time, generally in monthly periods of 1 cm, according to the growth consensus. Segmental hair analysis is very informative, but is meaningless if the sample collection and handling of the hair sampling is inappropriate. Hair must therefore be carefully aligned, since if not, each segment will contain different portions of hair strands and will represent longer or overlapping periods of time.82
Depending on the type of compound, different authors propose modifications to the segment size. Thus, for THC, segments of 3.0–5.5 cm are proposed.76 In the case of ethyl alcohol metabolites, the minimum proposed size is 3 cm,83 while in the cases of DFSA, smaller segments than recommended (between 10 and 30 mm) are proposed to improve the detection of the compound in a narrower window.84 These recommendations on the most appropriate length of the segment depending on the type of case must be linked to the analytical cut-off values established by the laboratory.
If segmentation is decisive in establishing consumption, it is even more so in defining abstinence. With the cessation of consumption, there is no radical change from positive to negative,85 but there is a transition zone due to changes in hair growth; hair phase; continuity of the incorporation of the drug into the hair after stopping high consumption; inter-individual variations; variability in sample collection, and other routes of incorporation (sebum or sweat) in addition to blood.86
In cases of abstinence, the variability of the elimination constants of the different compounds in the body must be added to the growth variability. Different research studies report variations of the most common drugs, opiates,86 amphetamines,87 or cocaine and benzoyl ecgonine88 which suggest very different times for negative results to be obtained after the last drug use. These times are related to the analytical cut-off values used in each case and must be taken into account when expressing abstinence in the results report.
Regarding alcohol markers, the SoHT consensus in 201973 indicates that EtG in hair samples is the analysis of choice to identify cases of withdrawal, and that a concentration greater than 5 pg/mg in samples of 3–6 cm indicates repeated alcohol consumption. It is not recommended to use fatty acid ethyl ester concentration alone to determine abstinence, although to indicate repeated alcohol consumption, values equal to or greater than 120 pg/mg for 0–3 cm or 150 pg/mg for 0-6 cm73,89 can be used.
External contaminationA critical issue regarding hair analysis, which is still controversial after 30 years of research and data accumulation by numerous scientists, is the possibility of obtaining a positive hair analysis due to external contamination without deliberate consumption of a compound.90
Judicial systems are often interested in establishing whether an individual has actually consumed (ingested, inhaled, or smoked) a drug, so the differentiation between exposure and consumption becomes a frequent interpretation presented to the toxicologist91—which sometimes entails compromising situations. The mere presence of drugs in the hair sample should not be used firmly to discriminate passive exposure from long-term consumption90 but a correct interpretation of the results must be complemented with the identification of specific metabolites, the implementation of a decontamination procedure and analysis of hair washes.92
The application of metabolite ratios can potentially help differentiate consumption from exposure. However, this has also been shown to be inaccurate for some common drugs. Detection of drug metabolites alone cannot prove personal use, as inadvertent absorption from environmental exposure can inevitably lead to their metabolism with the presence of low concentrations of their metabolites in hair and other biological samples. For example, the transfer of the methadone metabolite (EDDP) to a child from the sweat of their caregiver has been demonstrated.66
For greater certainty regarding the origin of drug presence, the use of metabolites has been proposed, the proportion of which can help differentiate consumption from exposure.
In the case of cannabis, smoke containing THC is known to result in drug-positive hair in a non-user, particularly if only THC is being monitored. When the THC metabolites, 11-nor-9-carboxydelta-9-tetrahydrocannabinol (THC COOH) and 11-hydroxy-delta9-tetrahydrocannabinol (THC-OH) are also detected, the probability that the presence comes from active consumption increases.66 However, given the low incorporation rates of these metabolites into the hair, this detection is an analytical challenge that depends on the analytical method and cut-off points established in the laboratory.93 There is also evidence that the solvent washing process to eliminate contamination of the surface of the hair can lead to the drug penetrating the interior compartment of the hair fibres, leading to a possible conclusion that the subject is a user.
Similarly, in the case of cocaine, measurement of benzoylecgonine and one or more of the hydroxy metabolites of cocaine is suggested, but as in the case of THC, due to the low abundance of the hydroxy metabolites of cocaine they are not always detected in active consumers.66 In 2015, the FBI published a protocol that summarises an extensive washing of the sample to eliminate external contamination followed by the analysis of cocaine, ethylcocaine, norcocaine, and hydroxycocaine, establishing the following criteria: cocaine must be identified above 500 pg/mg, after subtracting 5 times the concentration found in the washings and in addition, 2 of the hydroxylated metabolites must be present with concentrations greater than 5 pg/mg. Depending on compliance with these criteria, the results must be issued as “negative”, “contaminated,” or “consistent with exposure to cocaine”.91
Other studies indicate that strong criteria must be applied to clearly differentiate consumption from contamination, since different combinations of metabolites and degradation products can produce false positives. Thus, for concentrations of cocaine in hair greater than 0.1 ng/mg, it must be reported as positive if positive results are found for p or m-OH-benzoylecgonine (BE) or p or m-OH-norcocaine (NC), and/or if the peak area ratios for p, m, o-OH-cocaine (COC) exceed a limit marked as 2 times the maximum ratio estimated for each OH-COC isomer (0.1%, 0.2%, and 0.4%, respectively) and/or if (NC+EC)/COC is greater than 2%.94
Regarding opiates, recent research recommends including the analysis of hydromorphone and of the first washing solution in the procedure, in the case of morphine poisoning.95
These limitations do not generally call into question the value of hair for drug analysis, but both laboratories and recipients of the reports must consider them in their interpretations.66
Interpretation of postmortem resultsFor postmortem toxicological interpretation, it is essential to know whether the concentration of a compound in a sample accurately represents the concentration at the time of death.
In 2011, Moffat et al., had already listed 10 factors to consider for the interpretation of postmortem toxicological data: the inherent pharmacological activity and toxicity of a substance; source and administration route; postmortem changes; exposure time; drug interactions; single or multiple doses; age and natural disease; injuries; pharmacogenomics, and tolerance. Among them, postmortem changes have been the most important challenge for the forensic toxicologist.1
It is therefore prudent to assume that any postmortem concentration in the blood sample should be carefully considered, particularly if the concentration is of any importance in the case, as it is unlikely to reflect the perimortem concentration. Given the variability that has been observed in virtually all publications, it is not possible to calculate a probable perimortem concentration, as this will depend on a variety of factors operating in a particular case (Fig. 2).
Ideally, if the suspicious concentration could be significant in some way, i.e., to possibly contributing to the cause of the death, then other information will be required to inform any subsequent ruling. This may also include analysis of other samples in addition to the blood sample, such as stomach contents or a section of liver.15
If in all matters of forensic toxicology, the importance of the stability of the compounds in the interpretation of the results must be considered, in postmortem cases, it is a relevant factor, since they generally suffer long delays in the processing and analysis of the samples. The data collected in the recent review by Nosbest et al. suggest that quantitative results related to long-term stored samples should be interpreted with caution and that no single temperature, pH, container, or preservative recommendation can be made that is ideal for all drug classes. When long delays in analysis are anticipated, laboratories should consider moving samples to frozen conditions and should take extreme caution with new compounds.96
A key issue in the interpretation of postmortem results is finding reliable data to compare the postmortem concentrations found. Determining whether the concentration found constitutes a lethal concentration is extremely difficult for some compounds due to the overlap between non-toxic, therapeutic, and lethal concentrations. Added to this is both the possible habituation to the effects of a drug, such as the case of some amphetamines and opiates, where high levels may be tolerated, and the fact that they may have ingested a series of medications/drugs/alcohol that may have synergistic effects.97
Obtaining reliable reference data constitutes one of the most important challenges in the interpretation of postmortem cases, since comparing concentrations found in postmortem blood with therapeutic concentrations in serum or plasma from the clinical environment is inappropriate and will lead to incorrect results.With this idea clear and in order to improve diagnostic accuracy in cases of suspected death due to poisoning, different compilations of postmortem reference concentrations of drugs are often used, although they should be used with caution taking into account postmortem circumstances and factors.98
Although at present there are no reference values for all compounds in all biological samples, the published data have evolved from simple data tables to the current ones, which include details of the pharmacokinetics, physicochemical properties and stability of the compounds,99 origin of the sample, and single or multiple drug consumption.100,101
However, the interpretation of the results for compounds affected by postmortem redistribution is unresolved. Some reference data collections have been performed on compound concentrations in femoral blood obtained from autopsies where the cause of death was not poisoning and where the subject was not incapacitated. These data do not reflect any “therapeutic” concentrations, but represent concentrations that could be considered as normally found and not associated with fatal outcome. With the use of these tables, errors can be reduced and the problem associated with postmortem redistribution minimised.102
A promising methodology for obtaining reliable postmortem redistribution data and concentration databases from multiple samples is the procedure established by Kraemer and Steuer et al. through Virtobot technology, taking small guided biopsies of different organs and biological fluids combined with computed tomography and/or magnetic resonance imaging. This technique provides a systematic, time-dependent investigation of postmortem distribution, requiring only minimal amounts of sample and causing minimal damage to the body. Use of this methodology in more cases and in multiple regions could provide improved database sets to significantly assist in the interpretation of postmortem toxicology.55
Given the frequency of multiple drug consumption, the interpretation of postmortem results must take into account interactions between medication and drugs. Interpretations entail knowledge of pharmacokinetics and pharmacodynamics, since interactions can occur at metabolic steps, sometimes affecting the activity of enzyme system, in transport or in receptors. Interaction databases have been published103 and currently there are databases with information on CYP450 inhibitors and inducers and software for pharmacogenetic interpretation. In other databases, genetic differences; the impact on enzymes; side effects of compounds, and changes in lifestyle are considered.
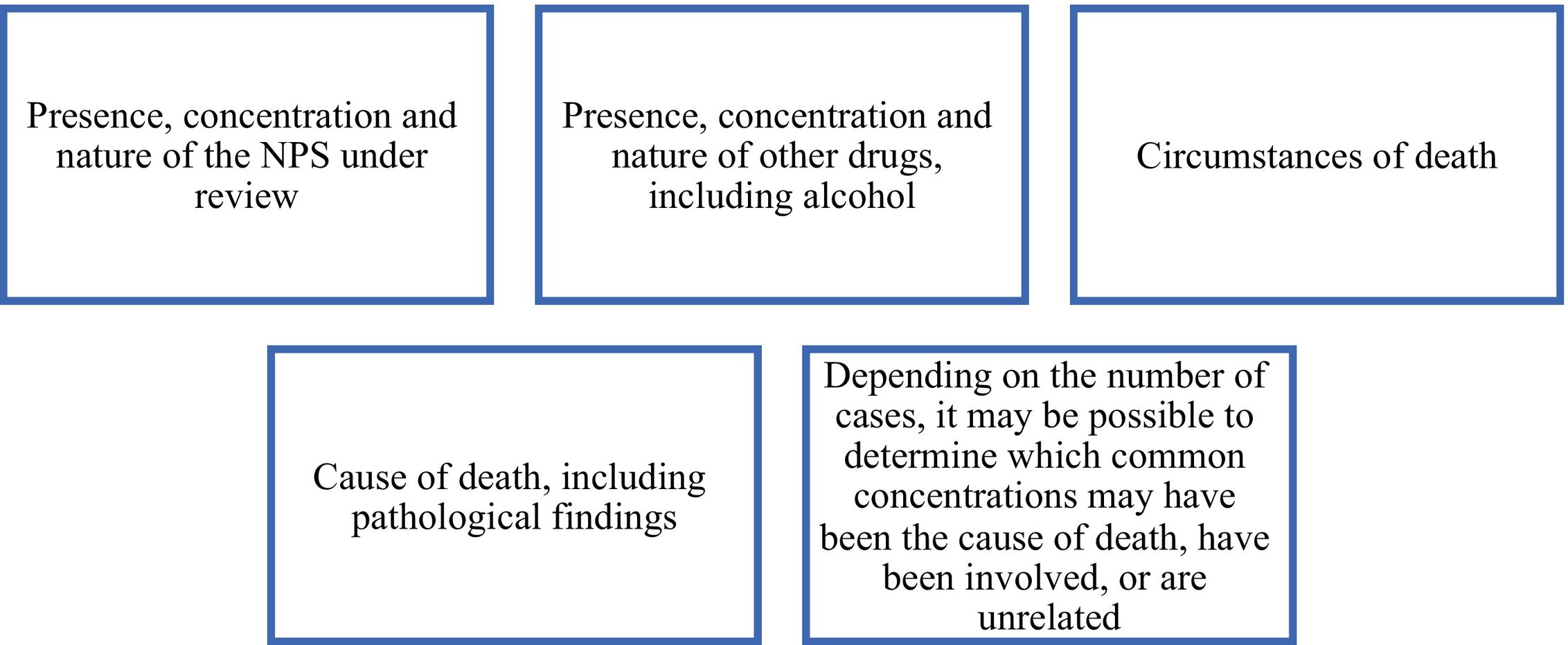
Another scarcely appreciated way to approach the interpretation of postmortem results is by applying what is called the toxicological significance score. This model was initially developed to propose a more systematic, reproducible, and transparent approach to evaluate the toxicological importance of new psychoactive drugs in deaths occurring in serious adverse situations, for the purposes of risk assessment.104 For this evaluation, the factors represented in Fig. 3 are considered.
With this approach, where robust data sets are available, an LR (likelihood ratio) approach could be used to evaluate the evidentiary strength of observations. The LR avoids the definition of a “lethal concentration” and provides an alternative means of deciding on the potential involvement of a compound in the cause of death.105
Inter-individual variationsInter-individual variations also affect postmortem concentrations of compounds and must be taken into account for interpretation. Noteworthy among them are the enzymatic polymorphisms that intervene in the metabolism of the compounds and the genetic variations between individuals.
Recent advances in genetics and metabolomics offer broader knowledge that is already being used to interpret the toxicological significance of a variety of compounds at a more “personalised” level.55
PharmacogenomicsInter-individual variability, adverse reactions to drugs, and tolerance phenomena are closely linked to genetic differences that affect the metabolism of enzymes, transporters, and receptors.106 In forensic toxicology, it is essential to understand the fundamental role of pharmacogenetics since pharmacokinetics, especially the bioavailability of a compound, and pharmacodynamics are conditioned by a genetic substrate. The possible influence of pharmacogenetics on drug metabolism must be considered when interpreting the postmortem concentration of a substance in body fluids or organs.107
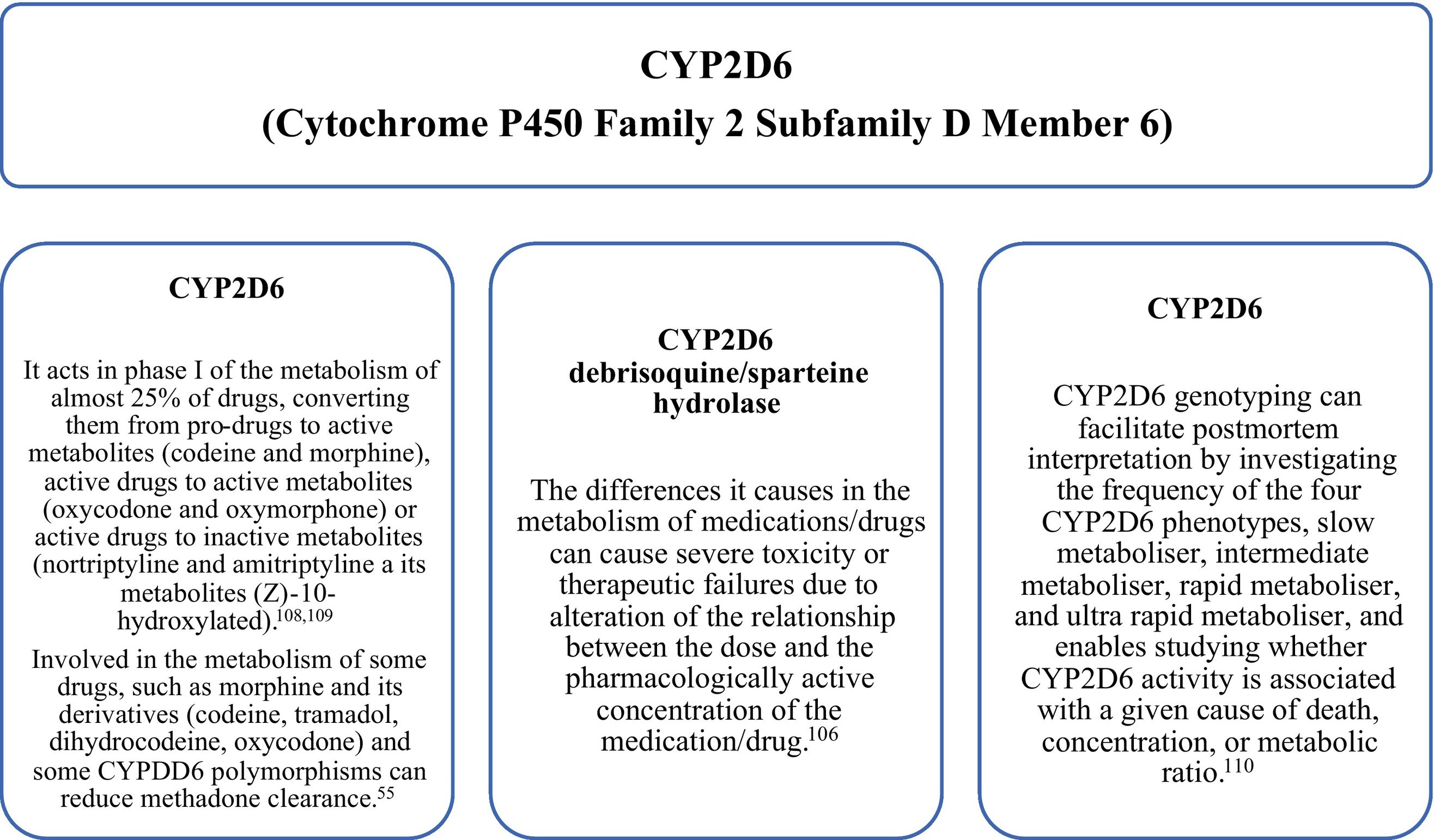
Some gene variants may determine a higher risk of toxic effects, including death, in some individuals who are more sensitive to the substance. Although there are at least 50 P450 genes, the most studied in postmortem toxicology are CYP1A2, CYP2A6, CYP2B6, CYP2C19, CYP2D6 (Fig. 4), CYP2E1, and CYP3A4.
CYP3A4 is involved in the metabolism of benzodiazepines and buprenorphine. Therefore, determining the presence of cytochrome-inducing or suppressing mutations can provide answers in cases of death related to a suspected drug overdose.55
Geneticists of the stature of Budowle or Sajantila indicate that genetic analysis and its effects on metabolism should be applied in sudden deaths, unexplained suicides, or deaths associated with therapies.
In a recent review, they show that pharmacogenetics, through the study of gene polymorphisms, is a very useful tool in toxicology. Genetic variants of enzymes that metabolise or transport drugs change the bioavailability and therapeutic/toxic concentration of the compounds. In these cases, the chronic intake of medications/drugs should also be considered, since there is always an impact due to acquired tolerance. Otherwise, death may occur unexpectedly with very low concentrations compared to other cases in which tolerance has developed.110
The potential of pharmacogenomics promotes the trend towards a “personalised” justice—a general term used to describe each and every application in ante-, peri-, and postmortem investigations. This represents a major challenge in forensic toxicology in which future studies will determine if this tool serves as a complement to determine the cause and manner of death.55,111
MetabolomicsMetabolite research reflects one of the most important fields of postmortem forensic toxicology. Death results in changes in some metabolites in body tissues due to lack of circulating oxygen, altered enzymatic reactions, cellular degradation, and cessation of the anabolic production of metabolites and macromolecules. The new generation of biomarkers (metabolomics) that can provide information about the pathophysiological processes in the deceased can be found in various biological samples such as blood, serum, urine, vitreous humour, or cerebrospinal fluid. The appropriate selection of samples or tissues for analysis can be vital for obtaining the desired metabolite.
Metabolic changes can also provide chemical markers to determine the time since death (postmortem interval), which is difficult to establish with current observation-based methodologies.112–114 Generally, the techniques used in these estimates require information about fluctuations in temperature, rigour mortis, body mass, body coverings, and other environmental conditions that make correct estimation difficult. Today, metabolomics provides answers based on biomarkers that are considered practical and objective indicators.115 Amino acids such as tyrosine, threonine, and lysine, whose ranges of changes among subjects of similar ages and postmortem intervals, are similar and are suggested as biomarker candidates. 116
If we refer specifically to postmortem GHB analysis, the interpretation of the analytical cut-off values is not simple, due to the range of endogenous GHB concentrations and the possibility of postmortem GHB formation, considered as the sum of production since death to the sampling and to the conservation period. The investigation of GHB metabolites in postmortem blood samples, such as 2,4-dihydroxybutyric acid, 3,4-dihydroxybutyric acid, or glycolic acid, may be biomarkers of GHB consumption, considering that concentrations in femoral blood >4 mg/l for 2,4-dihydroxybutyric acid, >5 mg/l for 3,4-dihydroxybutyric acid, and >12 mg/l for glycolic acid are indicators of lethal concentrations. The values of these metabolites in the urine sample are not as decisive as in the blood sample.39
A recent study by Steuer et al. conducted an investigation aimed at finding possible correlations between time-dependent postmortem concentration changes of morphine and methadone and endogenous molecules in a real case. Statistically significant individual correlations were determined between morphine/methadone and endogenous compounds/characteristics such as creatinine; glutaric acid; hypoxanthine; fructose; pentadecanoic acid; palmitoleic acid; alanine, and linoleic acid, along with other unidentified characteristics. Due to the limited number of cases, the reproducibility and robustness of the correlation was not assessed.117
ConsiderationsIn recent years, analytical and technological advances, along with the implementation of quality systems in forensic toxicology laboratories, have enabled the detection of low concentrations of compounds in traditional, alternative, and new matrices to be ensured, using automated workflows and validated methods within the framework of quality assurance.118
When such results are presented as testimony regarding the potential effect of a substance on the death, illness, mental, or physical impairment of a person, or in family custody matters, the evidentiary impact is significant. Accurate compilation and meticulous, objective interpretation of investigation results are therefore critical to any responsible forensic toxicological assessment.
In this article, cases of complex toxicological interpretation have been presented for which toxicokinetic and toxicodynamic knowledge is required. These cases involve a number of factors, and in some cases numerous factors, that impact interpretations and establish associated limitations. Communication of these limitations to the judicial authority is essential to help determine the meaning of the results in the context of the litigation.
Some factors have been carefully studied and their influence clarified. In other cases, challenges that have opened doors to other scientific fields such as genetics or metabolomics remain unresolved. Compared to other experimental methods, metabolomics is presented in forensic toxicology as a tool that will impact the development and growth of data provision to optimise result interpretation.
Although further research is necessary to determine the appropriate biomarkers, there is marked interest by the scientific community to conduct studies that promote and progress in knowledge on limitations, enabling a deeper understanding of what really happened in the facts investigated. It is vitally important that these limitations be properly recognised in any expert statement or testimony when determining the contribution of results obtained in court cases.
FundingNone.
Please cite this article as: Soria ML. The improvements in forensic toxicology and its role in the forensic process. The interpretation of results (II). Revista Española de Medicina Legal. 2024. https://doi.org/10.1016/j.remle.2024.05.002.