Since Pall-Gelman stopped manufacturing ITLC-SG, it has become necessary to validate alternative stationary phases.
ObjectiveTo validate different stationary phases versus ITLC-SG Pall-Gelman in the determination of the radiochemical purity (RCP) of 111In-pentetreotide (111In-Octreoscan) by planar chromatography.
Material and methodsWe conducted a case–control study, which included 66 111In-pentetreotide preparations. We determined the RCP by planar chromatography, using a freshly prepared solution of 0.1M sodium citrate (pH 5) and the following stationary phases: ITLC-SG (Pall-Gelman) (reference method), iTLC-SG (Varian), HPTLC silica gel 60 (Merck), Whatman 1, Whatman 3MM and Whatman 17. For each of the methods, we calculated: PRQ, relative front values (RF) of the radiopharmaceutical and free 111In, chromatographic development time, resolution between peaks. We compared the results obtained with the reference method. The statistical analysis was performed using the SPSS program. The p value was calculated for the study of statistical significance.
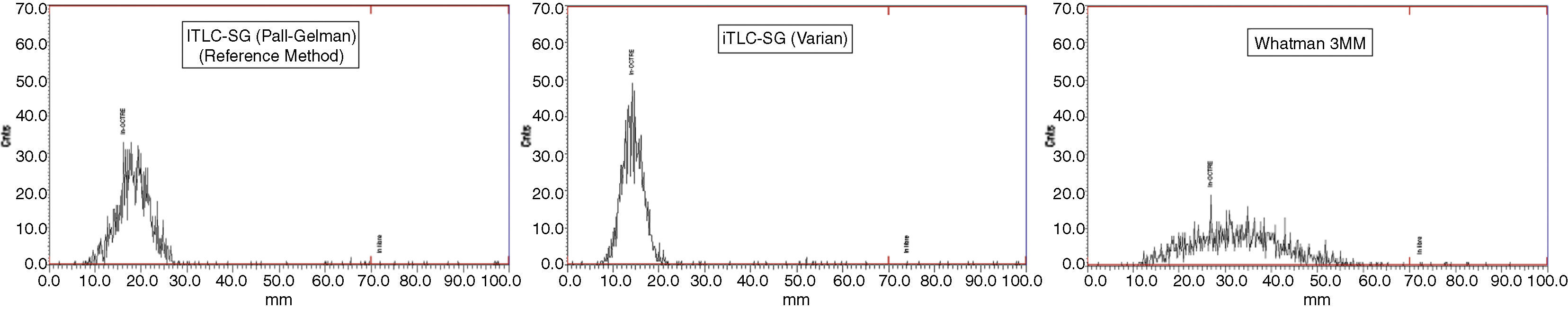
ResultsThe highest resolution is obtained with HPTLC silica gel 60 (Merck). However, the chromatographic development time is too long (mean=33.62min). Greater resolution is obtained with iTLC-SG (Varian) than with the reference method, with lower chromatographic development time (mean=3.61min). Very low resolutions are obtained with Whatman paper, essentially with Whatman 1 and 3MM. Therefore, we do not recommend their use.
ConclusionsAlthough iTLC-SG (Varian) and HPTLC silica gel 60 (Merck) are suitable alternatives to ITLC-SG (Pall-Gelman) in determining the RCP of 111In-pentetreotide, iTLC-SG (Varian) is the method of choice due to its lower chromatographic development time.
Tras el cese en la fabricación de ITLC-SG por Pall-Gelman, es necesaria la validación de fases estacionarias alternativas.
ObjetivoValidar diferentes fases estacionarias frente a ITLC-SG de Pall-Gelman en la determinación de la pureza radioquímica (PRQ) de 111In-pentetreótida (111In-Octreoscan) por cromatografía planar.
Material y métodosRealizamos un estudio caso-control en el que incluimos 66 preparaciones de 111In-pentetreótida. Determinamos la PRQ mediante cromatografía plana, empleando una solución recién preparada de citrato de sodio de 0,1M (pH 5) y las siguientes fases estacionarias: ITLC-SG (Pall-Gelman) (método de referencia), iTLC-SG (Varian), Silicagel 60 HPTLC (Merck), Whatman 1, Whatman 3MM y Whatman 17. Para cada uno de los métodos calculamos: PRQ, factores de retención (RF) del radiofármaco e 111In libre, tiempo de desarrollo cromatográfico y resolución entre picos. Comparamos los resultados obtenidos con los del método de referencia. Para el análisis estadístico de datos utilizamos el programa SPSS. Para el estudio de significación estadística calculamos el valor de p.
ResultadosCon Silicagel 60 HPTLC (Merck) se obtiene la mayor resolución, aunque el tiempo de desarrollo cromatográfico es prolongado (media=33,62min). Con iTLC-SG (Varian) se obtiene una resolución mayor que con el método de referencia, siendo menor el tiempo de desarrollo cromatográfico (media=3,61min). Con papel Whatman se obtienen resoluciones muy bajas, fundamentalmente con Whatman 1 y 3MM, por lo que no recomendamos su utilización.
ConclusionesAunque iTLC-SG (Varian) y Silicagel 60 HPTLC (Merck) son alternativas adecuadas a ITLC-SG (Pall-Gelman) en la determinación de la PRQ de 111In-pentetreótida, iTLC-SG (Varian) es el método de elección por su menor tiempo de desarrollo cromatográfico.
Article
Revista Española de Medicina Nuclear e Imagen Molecular (English Edition)