The genus Microthecium contains 31 species worldwide distributed. Most of them are saprobic on soil and plant debris, but a few have been reported as mycoparasites on hypocrealean fungi. By contrast, this genus has never been reported as phytopathogenic, nor endophytic.
AimsTo isolate and identify endophytic fungi from Mediterranean herbaceous plants and trees in order to contribute to the knowledge of the hosts and their geographical location. The present work has been focused on the study of endophytic fungi of hawthorn (Crataegus monogyna).
MethodsThe following steps were taken: i, isolation of the fungal strain from living stems of C. monogyna; ii, cultural and micro-morphological study, and iii, sequence comparison of different genetic markers by BLAST search with sequences deposited in GenBank.
ResultsAt the present work we describe a new species of the genus, Microthecium pleomorphosporum, isolated from living stems of C. monogyna in Mallorca (Balearic Islands, Spain). This fungus is characterized by the production of non-ostiolate perithecia and two sorts of ascospores (some smooth-walled, others delicately reticulated) bearing a germ pore at each end which are frequently ornamented by a surrounding donut-like structures, and a phialidic asexual morph and bulbils. The morphologically closest related species is Microthecium tenuissimum, which has bigger ascospores and lacks asexual reproduction. Phylogenetically, M pleomorphosporum is close-related to other species of the genus, although no genetic marker that discriminates this new species from other phylogenetically closer ones could be elucidated as a gold standard.
ConclusionsM. pleomorphosporum, order Melanosporales, is reported here as the first endophytic species of C. monogyna.
El género Microthecium incluye 31 especies de distribución cosmopolita. La gran mayoría de ellas son saprobias en el suelo y se encuentran sobre detritos vegetales, y tan solo unas pocas se han descrito como micoparásitas de hongos hipocreales. Por el contrario, nunca se había descrito a este género como fitopatógeno ni endofítico.
ObjetivosAislar e identificar hongos endófitos de plantas herbáceas y árboles mediterráneos con el fin de contribuir al conocimiento de los hospederos y su situación geográfica. El presente trabajo se ha centrado en el estudio de los hongos endófitos del espino albar (Crataegus monogyna).
MétodosSe incluyeron diferentes procesos: (1) aislamiento de la cepa fúngica a partir de tallos vivos de Crataegus monogyna; (2) estudio cultural y micromorfológico y (3) comparación de las secuencias nucleotídicas de diferentes marcadores filogenéticamente informativos con secuencias de taxones conocidos depositadas en el GenBank mediante búsqueda BLAST.
ResultadosEn el presente trabajo describimos una nueva especie para el género, Microthecium pleomorphosporum, aislada de tallos vivos de C. monogyna en Mallorca (Islas Baleares, España). Este hongo se caracteriza por la producción de peritecios no ostiolados y de dos tipos de ascosporas (unas con paredes lisas y otras con paredes delicadamente reticuladas), con un poro germinativo en cada extremo frecuentemente ornamentado por estructuras circundantes en forma de rosquilla, y un estado asexual que produce conidios fialídicos y bulbillos. La especie morfológicamente más cercana es Microthecium tenuissimum, que tiene ascosporas más grandes y carece de multiplicación asexual. Filogenéticamente, Microthecium pleomorphosporum está estrechamente emparentada con otras especies del género y no se ha podido establecer ningún marcador genético de referencia (gold standard) para discriminar esta nueva especie de otras filogenéticamente más próximas.
ConclusionesMicrothecium pleomorphosporum, del orden de los Melanosporales, puede ser la primera especie endofítica de C. monogyna.
The genus Microthecium Corda, recently resurrected by Marin-Felix et al.,9 contains species mostly producing yellowish-orange, orange-brown or reddish globose ascomata, brown, citriform to plataniform ascospores with smooth, reticulate, pitted or wrinkled walls, bearing a terminal apiculate or depressed germ pore at each end, and almost producing an asexual phialidic morph and bulbils in vitro. Currently, there are 31 accepted species in that genus,9 usually found on dung, plant debris, soil, monocotyledonous bulbs and Pinophyta, frequently as parasites of other fungal taxa2 and never been previously described as endophytes.
During a survey on endophytic fungi of the Mediterranean flora, we isolated in pure culture a fungus morphologically similar to the genus Microthecium. After a molecular study and morphological comparison with the accepted species of the genus, we suggest erecting the new species M. pleomorphosporum.
Materials and methodsFungal isolationA fungus belonging to the order Melanosporales was isolated from living healthy stems of Crataegus monogyna Jacp. sampled in Menut (Mallorca, Balearic Islands, Spain). C. monogyna is known as “hawthorn” in English, receiving the common names of espino albar and majuelo in Spain, arç blanc in Catalonia and cirerer de pastor in the Balearic Islands. It is a spiny deciduous tree distributed mainly in the Mediterranean region, growing on all sorts of soils, from almost sea level to 2200masl, 4–8m in height, with a trunk not always unique, blackish-brown to grayish-brown bark. The dark greenish leaves, pedunculated 2–4cm long, are lobed, denticulate, and alternate along the stem; the white flowers are grouped in corymbs, later producing red pommel fruits with a single seed.
The fungus was isolated from the stems after surface sterilization according to Crous et al.3 Briefly, the stems were cut in fragments of 2–4cm long, submerged in 70% ethanol for 1min, treated with NaOCl 2% for 2min and, finally, with ethanol 70% for 1min. After that, the sterilized stems were rinsed twice with sterile water, plated onto malt extract agar (MEA; Difco Inc., Detroit, USA) into 90mm diameter disposable Petri dishes, and incubated at 25°C. After two weeks some yellowish ascomata were observed under the stereomicroscope, and some of them were transferred, using a sterile needle, to 55mm diam. Petri dishes, ones containing oatmeal agar (OA; oatmeal flakes, 30g; agar-agar, 20g; distilled water, 1L) and others with potato dextrose agar (PDA; Pronadisa, Madrid, Spain). The Petri dishes were incubated at 15, 25 and 35°C.
Morphological characterizationFor cultural characterization, the isolate was grown for up to 30 days on OA, potato carrot agar (PCA; grated potatoes, 20g; grated carrot, 20g; agar-agar, 20g; L-chloramphenicol, 100mg; distilled water, 1L), and PDA at 5, 10, 15, 20, 25, 30, 35 and 40°C.9 Color notations in parentheses are from Kornerup and Wanscher.7 Vegetative and reproductive structures were examined under an Olympus BH-1 bright field microscope by direct mounting in lactic acid and water of the ascomata and/or microcultures grown on OA and PDA. Pictures were obtained with a Zeiss Axio Imager M1 brightfield microscope.
Molecular studyThe DNA of our fungal isolate was extracted and purified directly from the colonies by means of the Fast DNA Kit protocol (MP Biomedicals, Solon, Ohio). The amplification of the internal transcribed spacer region (ITS) of the nuclear rDNA was carried out according to Magaña-Dueñas et al.8; genes’ fragments encoging actin (act), translation elongation factor 1-α (tef1), RNA polymerase II subunit 2 (rpb2) and beta-tubulin (tub2) were also amplified according to Voigt and Wöstemeyer14 (act), Houbraken et al.5 (tef1), Sung et al.13 (rpb2) and Woudenberg et al.15 (tub2). BigDye Terminator 3.1 cycle sequencing kit (Applied Biosystems Inc., Foster City, California) was used to sequence both strands with a combination of the same primers used in the amplification. PCR products were purified and sequenced at Macrogen Europe (Amsterdam, The Netherlands) with a 3730XL DNA analyzer (Applied Biosystems), and the consensus sequences were obtained using SeqMan (version 7.0.0; DNASTAR, Madison, WI, USA). The sequences obtained were compared with other fungal sequences deposited at the National Center for Biotechnology Information (NCBI) database using the Basic Local Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi). A maximum level of identity (MLI) ≥98% was used for species-level identification.
ResultsMolecular studyBased on the BLAST search in NCBIs GenBank nucleotide database using a fragment of the ITS nucleotide sequence of our strain FMR 18380 (272bp, accession number OX383416.1), it was displayed a percentage identity greater than 98.78% for Microthecium tenuissimum (D. García, Stchigel & Guarro) Y. Marín, Stchigel, Guarro & Cano (the morphologically nearest species) (CBS 112764, GenBank NR_161011.1; Identities=266/269 (98.88%), 0 gaps), Microthecium fimicola (E.C. Hansen) Y. Marín, Stchigel, Guarro & Cano (CBS 967.97, GenBank MK926777.1; Identities=266/170 (98.52%), one gap) and Microthecium quadrangulatum (D. García, Stchigel & Guarro) Y. Marín, Stchigel, Guarro & Cano (CBS 112763, GenBank MK926778.1; Identities=266/270 (98.52%), one gap). When using the act sequence (820bp, accession number OX383416.1), percentage identities were greater than 99% for Microthecium compresum Udagawa & Cain (NBRC 8627, GenBank KP981525.1; Identities=772/772 (100%), 0 gaps), M. fimicola (NBRC 8354, GenBank KP981528.1; Identities=771/772 (99.87%), 0 gaps) and Microthecium zobelii Corda (NBRC 9442, GenBank KP981542.1; Identities=770/772 (99.74%), 0 gaps); with the tub2 sequence (476bp, accession number OX383417.1) the species displayed were M. tenuissimum (CBS 112764, GenBank MK926880.1; Identities=417/417 (100%), 0 gaps), M. fimicola (CBS 967.97, GenBank MK926877.1; Identities=417/417 (100%), 0 gaps) and M. zobelii (CBS 341.73, GenBank MK926882.1; Identities=416/417 (99.76%), 0 gaps); using rpb2 sequence (640 pb, accession number OX383419.1) the species were M. tenuissimum (CBS 112764, GenBank MK876742.1; Identities=527/527 (100%), 0 gaps), M. fimicola (CBS 967.97, GenBank MK876739.1; Identities=526/527 (99.81%), 0 gaps) and M. zobelii (CBS 341.73, GenBank MK876744.1; Identities=523/527 (99.24%), 0 gaps). Finally, with the tef1 sequence (759bp, accession number OX383418.1) the species displayed were M. fimicola (FMR 13148, GenBank KP981593.1; Identities=748/750 (99.73%), 0 gaps), Microthecium levitum Udagawa & Cain (FMR 13884, GenBank KP981598.1; Identities=742/750 (99.73%), 0 gaps) and M. quadrangulatum (CBS 112763, GenBank MK981599.1; Identities=742/750 (99.33%), 0 gaps).
TaxonomyMicrothecium pleomorphosporum Stchigel, Pintos & Cano, sp. nov. Mycobank MB 841012 (Fig. 1).
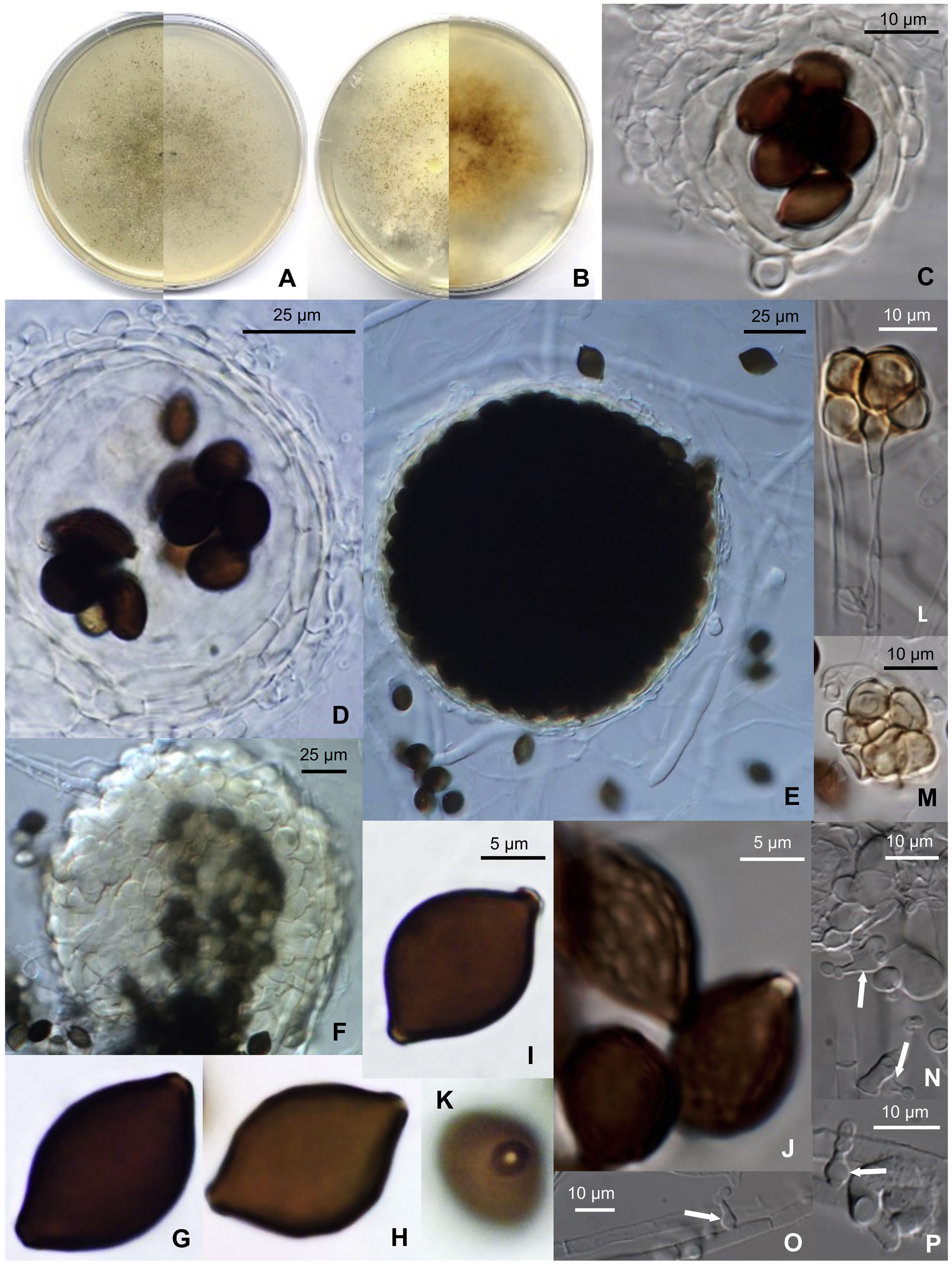
Microthecium pleomorphosporum (CBS 148652). (A) Colony after 14 days at 25°C on PCA (front and reverse). (B) Colony after 14 days at 25°C on PDA (front and reverse). (C and D) Translucent ascomata showing the peridial layers and eight ascospores within the asci. (E) Broad ascoma. (F) Detail of the peridial wall. (G–I) Smooth-walled ascospores. (J) Reticulate ascospores. (K) Donut-like structure surrounding the germ pore of an ascospore. (L and M) Bulbils. (N and P) Phialidic conidiogenous cells (white arrows) with catenate conidia.
Etymology: From Greek πλέω-, numerous, -μορφή-, form, and -σπóρια, spore, due to the variable shape of the ascospores.
Diagnosis: M. pleomorphosporum might be a new species withing the genus as it differs from the rest in producing two sorts of ascospores, smooth-walled and inconspicuously reticulated ones, plus a phialidic asexual morph and bulbils.
Morphological description: Hyphae are septate, hyaline, smooth- and thin-walled, anastomosing, frequently moniliform, 1–8 (–15)μm wide, that produce cylindrical, pale-orange arthroconidia in short chains when cultures are old. Sexual morph: ascomata perithecial, non-ostiolate, superficial to immersed and scattered on the culture medium, glabrous, translucent, pale-yellow to yellowish-orange at first, later becoming dark-brown due to the production of ascospores, opening when old by one or two undetermined sites at the ascomata wall, globose, 50–200μm diam.; ascomata wall translucent, pale-yellow to yellowish-orange, 5–15μm thick, composed by 2–6 layers of smooth- and thin-walled, flattened, pale-yellow to pale-orange cells of 5–30μm diam., with textura angularis to textura globulosa; asci 8-spored, unitunicate, broadly ellipsoidal to sacciform, 32–35×15–20μm, soon evanescent, non-stipitate; unicellular ascospores, chocolate-brown, smooth-walled or delicately reticulate in part or in all their surface, citriform but flattened at one side, (13–) 15–18×10–13μm×8–9μm, with a protruding germ pore surrounded by a donut-like structure derived from the outer wall of the ascospore. Asexual morph: conidiophores absent; phialides hyaline, smooth- and thin-walled, flask-shaped, 5–10×2–3μm, ventricose at the base or at the middle part, rarely at the upper part of the cell, arising laterally on the vegetative hyphae, delimitated by a basal septum; conidia enteroblastic, unicellular, hyaline, smooth- and thin-walled, produced in short basipetal chains, globose to ellipsoidal, 2–3×2μm. Bulbils present, 4– to multicelled, yellowish to orange, up 30μm diam, individual cells smooth- and thin-walled, more or less globose.
Cultural characteristics: Colonies on PCA grow rapidly, filling 90mm diam. plates in 14 days at room temperature (22–25°C). They have a submerged mycelium and aerial hyphae, granulose and brown (M. 6E6) due to the production of abundant ascomata; the reverse is grayish-brown (M. 6D4). Colonies on OA and PDA grow rapidly, filling 90mm diam. plates in 14 days at room temperature; the color is dark-brown due to the production of abundant ascomata (M. 6E7). At 15°C the colonies grow fast on all media, filling 65–90mm diam. plates in 14 days, with abundant aerial mycelia, and ascomata abundantly produced. At 35°C no growth was observed.
Holotype: Spain, Balearic Islands, Malla, Serra de la Tramuntana, Lluc, Menut, recovered from healthy stems of C. monogyna Jacq., 39°50′30.86″N, 2°54′03.63″E, altitude 522msl, 05/IX/2020, collected by A. Pintos Amengual, identified by A. M. Stchigel, J. Cano and A. Pintos; holotype CBS H-27905, cultures ex-type FMR 18380=AP5920=CBS 148652.
Notes: M. pleomorphosporum is morphologically related to M. tenuissimum (D. García, Stchigel & Guarro) Y. Marín, Stchigel, Guarro & Cano.4 Both species produce two sorts of ascospores, smooth-walled and reticulate ones. However, they can be differentiated by the size of the ascospores [(13–) 15–18×10–13μm×8–9μm in M. pleomorphosporum vs. 19–23×(12–) 14–15 (–17)×10–13μm in M. tenuissimum]. M. pleomorphosporum produce, as well, the typical asexual morph of the genus (hyaline, flask-shaped single phialides arising laterally on the vegetative hyphae) and bulbils, which are absent in M. tenuissimum, and glabrous ascomata (bearing few short setae in M. tenuissimum).
Key to the species of Microthecium (based on Marin-Felix et al.9).
| 1. | Sexual morph absent, only producing bulbils …… | M. sepedonioides (Preuss) Y. Marín, Stchigel, Guarro & Cano |
| Sexual morph present …… | 2 | |
| 2. | Ascomata non-ostiolate …… | 3 |
| Ascomata ostiolate …… | 14 | |
| 3. | Ascospores with an ornamented wall …… | 4 |
| Ascospores smooth-walled or nearly so …… | 8 | |
| 4. | Ascospores pitted, with wing-like ridges …… | M. foveolatum Udagawa & Y. Horie |
| Ascospores coarsely reticulate …… | 5 | |
| 5. | Asci 4-spored …… | 6 |
| Asci 8-spored …… | 7 | |
| 6. | Ascospores (25–)28–34(–40)μm long …… | M. beatonii D. Hawksw. |
| Ascospores 22–28μm long …… | M. perplexum D. Hawksw. | |
| 7. | Ascospores 25–34μm long …… | M. episphaerium (W. Phillips & Plowr.) Höhn |
| Ascospores 17–20μm long …… | M. retisporum Udagawa & Cain | |
| 8. | Ascomata always bigger than 120μm diam …… | 9 |
| Ascomata from 50μm diam …… | 13 | |
| 9. | Ascospores shorter than 20μm …… | 10 |
| Ascospores longer than 20μm …… | 11 | |
| 10. | Ascospores 15–19×11–13×8–9μm, with the narrow faces coarsely reticulate and the others smooth …… | M. compressum Udagawa & Cain |
| Ascospores 10–17×8–12×9–10μm, entirely smooth-walled …… | M. levitum Udagawa & Cain | |
| 11. | Ascospores fusiform …… | M. hypomyces (Höhn.) Höhn. |
| Ascospores citriform …… | 12 | |
| 12. | Ascospores 28–30×12–13(–15)μm …… | M. geoporae (W. Oberm.) Höhn. |
| Ascospores 18–25×8.5–12×6–9μm …… | M. zobelii Corda | |
| 13. | Ascospores 19–23×(12–)14–15(–17)×10–13μm; asexual morph absent …… | M. tenuissimum (D. García, Stchigel & Guarro) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores (13–)15–18×10–13μm×8–9μm; asexual morph phialidic; bulbils produced …… | M. pleomorphosporum Stchigel, Pintos & Cano | |
| 14. | Ascospores with wing-like appendages …… | 15 |
| Ascospores otherwise …… | 16 | |
| 15. | Ascospores wrinkled, (12–)13–18×(7–)8–10μm …… | M. ciliatum Udagawa & Takada |
| Ascospores pitted, (17–)20–22(–24)×12–14×10–12μm …… | M. lenticulare (Udagawa & T. Muroi) Y. Marín, Stchigel, Guarro & Cano | |
| 16. | Ascospores ornamented …… | 17 |
| Ascospores smooth-walled …… | 24 | |
| 17. | Ascospores punctate or punctate-reticulate …… | 18 |
| Ascospores reticulate or striate-reticulate …… | 20 | |
| 18. | Ascospores punctate, ellipsoidal …… | M. africanum (J.C. Krug) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores punctate-reticulate, ellipsoidal-fusiform …… | 19 | |
| 19. | Ascospores delicately punctate; asexual morph and bulbils present …… | M. japonicum (Y. Horie, Udagawa & P.F. Cannon) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores coarsely punctate; asexual morph and bulbils absent …… | M. moreaui (P.F. Cannon & D. Hawksw.) Y. Marín, Stchigel, Guarro & Cano | |
| 20. | Ascospores striate-reticulate …… | 21 |
| Ascospores reticulate …… | 22 | |
| 21. | Ascospores with inconspicuous ridges forming a very coarse reticulum, 18–22(–28)×9.5–11(–13)×8–9μm …… | M. micropertusum (Y. Horie, Udagawa & P.F. Cannon) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores without ridges or reticulum, 26–36×13–17μm …… | M. mansonii (Kirschst.) Y. Marín, Stchigel, Guarro & Cano | |
| 22. | Ascospores with 4–6 prominent longitudinal ribs …… | M. quadrangulare (Dania García, Stchigel & Guarro) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores without longitudinal ribs …… | 23 | |
| 23. | Ascospores spindle-shaped, 19.5–22×8.5–11μm …… | M. internum (Tehon & G.L. Stout) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores citriform to fusiform, 14–20×10–17μm …… | M. fimicola (E.C. Hansen) Y. Marín, Stchigel, Guarro & Cano | |
| 24. | Crown of setae absent …… | M. nectrioides (Marchal) Y. Marín, Stchigel, Guarro & Cano |
| Crown of setae present …… | 25 | |
| 25. | Ascospores citriform …… | M. marchicum (Lindau) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores otherwise …… | 26 | |
| 26. | Ascospores ellipsoid, often somewhat plataniform …… | 27 |
| Ascospores otherwise …… | 29 | |
| 27. | Bulbils present …… | M. fallax (Zukal) Y. Marín, Stchigel, Guarro & Cano |
| Bulbils absent …… | 28 | |
| 28. | Ascospores 21–34×11–17μm …… | M. brevirostre (Fuckel) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores 18–22×9–11μm …… | M. fimbriatum (Rostr.) Y. Marín, Stchigel, Guarro & Cano | |
| 29. | Ascospores ellipsoid to fusiform …… | M. fusisporum (Petch) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores ellipsoid to navicular …… | 30 | |
| 30. | Ascospores (9.5–)11–12(–13)×4–4.5μm …… | M. pegleri (D. Hawksw. & A. Henrici) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores longer than 15μm …… | 31 | |
| 31. | Ascospores 16–24×8–12μm …… | M. fayodi (Vuill.) Y. Marín, Stchigel, Guarro & Cano |
| Ascospores 25–30×11–15μm …… | M. brevirostratum (Moreau) Y. Marín, Stchigel, Guarro & Cano |
Endophytic fungi live inside plants without causing disease, growing within the roots, stems and/or leaves. Some of them are capable of establishing a symbiotic relationship with the host, and have the ability to boost the plant growth and nutrient acquisition, increasing the host's resistance to drouth and salinity, but also to the deleterious action of animals (insects, herbivorous mammals) and microbial pathogens.11 Despite the fact that hundreds of articles have been published on this topic, and the increasing interest by the scientific community in the production of bioactive compounds by endophytic fungi,1,6,12 only a few studies have been carried out to know the biodiversity of endophytic fungi of the autochthonous Mediterranean plants. For this reason, it is not surprising that only Aureobasidium sp., Chaetomium sp., Hormonema sp. and Torula herbarum have been reported as endophytic fungi in C. monogyna.10
Most of the species of the genus Microthecium are saprobic on plant debris and soil, but few of them have been reported as mycoparasitic on Fusarium spp., i.e., M. moreaui (formerly Persiciospora moreaui), M. mycoparasiticum (syn. Sphaerodes mycoparasitica), M. quadrangulatum (syn. Sphaerodes quadrangularis) and M. retisporum (syn. Sphaerodes retispora).9 On the other hand, none of the species within the genus have been reported as plant pathogens or as endophytes. Consequently, M. pleomorphosporum is not only a new species, but the first endophytic species within its genus. Morphologically, M. pleomorphosporum is, very alike to the nearest species, M. tenuissimum. However, both species are easily discriminated, because M. pleomorphosporum produces glabrous ascomata (these are sparsely setose in M. tenuissimum), which reach up to 200μm in diam. (and up to 125μm high in M. tenuissimum), ascospores smaller in size than M. tenuissimum, and has a phialidic asexual morph and bulbils, absent in M. tenuissimum.
ConclusionsM. pleomorphosporum is a new species of the genus, the first reported as a plant endophyte. Probably, new studies on endophytes in Mediterranean flora can increase the number of fungal taxa (including more members of the order Melanosporales) living into healthy plants, as well to know the role of these endophytes in preventing the development of plant diseases, due to the well known biotrophic activity of Melanosporales on plant pathogenic fungi. Like all other Microthecium species, M. pleomorphosporum is impossible to discriminate/circumscribe at the phylogenetic level, but on the other hand it is easily recognizable by its strictly glabrous ascomata, the ornamentation of the ascospores (smooth-walled ones, and slightly reticulated others), and by the production of phialoconidia and bulbils.
Conflicts of interestThe authors declare having no conflict of interest.