Scedosporiasis is an emerging mycosis that has gained importance in recent years due to its worldwide prevalence. It is caused by species of the Scedosporium apiospermum complex. These species can cause opportunistic infections in immunocompromised patients and, occasionally, in immunocompetent patients as well. The high intrinsic antifungal resistance make these infections difficult to manage.
AimsThe objective of this study was to interpret the mycological findings in a transplant patient, together with the images obtained in the radiological studies, in order to provide an early and effective antifungal therapy.
MethodsThe mycological analysis of samples taken from a heart transplant patient with radiological images suggesting a fungal infection was performed. Computed tomography scan of the head and thorax showed space-occupying lesions in both the frontal lobe and cerebellum, and multiple pulmonary nodules. The nodules were punctured and the samples obtained were analyzed according to the procedures for mycological analysis. The identity of the isolates was confirmed by nucleotide sequencing. Eventually, the antifungal susceptibility was studied.
ResultsThe fungal isolates obtained, whose identity was confirmed by sequencing, belonged to the species Scedosporium boydii. Injured tissues were surgically removed and a treatment with amphotericin B and voriconazole-minimum inhibitory concentration (MIC) 0.5μg/mL and ≥0.5μg/mL respectively – was administered.
ConclusionsAlthough the patient died due to complications of a Klebsiella pneumoniae sepsis refractory to treatment, the progression of the fungal disease, although slow, was favourable in the early phases of the treatment due to a correct diagnosis and the antifungal susceptibility test carried out. Clinical cases of this nature highlight the need to increase the epidemiological study of these microorganisms, as well as the proper treatment of the diseases caused, in order to achieve early diagnoses that reduce the morbidity and mortality of patients.
La escedosporiasis es una micosis emergente de relevancia en los últimos años por su prevalencia mundial. Es causada por especies del complejo Scedosporium apiospermum (S. apiospermum), que pueden provocar infecciones oportunistas de difícil tratamiento en pacientes inmunocomprometidos y, ocasionalmente, en inmunocompetentes. El alto grado de resistencia intrínseca de las especies de este complejo dificulta el manejo de las infecciones.
ObjetivosInterpretar los hallazgos micológicos en un paciente trasplantado, en conjunción con los estudios radiológicos, a fin de instaurar una terapia antifúngica precoz y efectiva.
MétodosSe realizó el estudio micológico de muestras de un paciente con trasplante cardiaco, cuyos exámenes radiológicos eran compatibles con una infección fúngica. La tomografía axial computarizada de cabeza y tórax mostró masas ocupantes en el lóbulo frontal y el cerebelo, así como múltiples nódulos pulmonares. Se punzaron las mismas y se procesó de acuerdo con el protocolo de análisis micológico de rutina; la identidad de los aislamientos se confirmó por secuenciación nucleotídica. Finalmente se evaluó la sensibilidad antifúngica.
ResultadosLa identidad de los aislamientos fúngicos obtenidos fue Scedosporium boydii (S. boydii). Se procedió a la remoción quirúrgica del tejido afectado y se puso un tratamiento con anfotericina B y voriconazol, para los cuales los valores de concentración inhibitoria mínima del aislamiento fueron 0,5 μg/mL y ≥ 0,5 μg/mL, respectivamente.
ConclusionesSi bien el paciente falleció por complicaciones asociadas a sepsis por Klebsiella pneumoniae (K. pneumoniae) refractaria al tratamiento, la evolución del cuadro micológico, aun siendo lenta, progresó favorablemente en las primeras fases del tratamiento. Esto se atribuye a un correcto diagnóstico y evaluación de la sensibilidad antifúngica del hongo aislado. Casos clínicos de este tenor muestran la necesidad de profundizar tanto en el estudio epidemiológico de estos microorganismos como en el tratamiento de las enfermedades que provocan, para lograr así un diagnóstico temprano que disminuya la morbimortalidad de los pacientes.
Scedosporiasis is an emerging fungal infection caused by species belonging to the Scedosporium apiospermum species complex, made up of cryptic species in continuous evaluation and reclassification. Its identification requires the sequencing of the intergenic regions (ITS). This complex has acquired relevance in recent years due to its prevalence worldwide, as well as the severity of the consequences of the infections it produces, described mainly in immunocompromised patients, such as neutropenic individuals with haematological malignancies or solid organ transplants,4,7,27,49,62 but also in immunocompetent patients50,60 with certain risk factors such as type I diabetes.30,60 Among the conditions associated with this infection there are cutaneous forms, soft tissue conditions and even disseminated forms. An example of dissemination is Scedosporiasis/Lomentosporiasis, an emerging, devastating invasive mycosis, with high mortality rates6,54 caused by Scedosporium spp. and Lomentospora prolificans, previously known as Scedosporium prolificans.13,14,28,38,44,63 Invasive Scedosporiasis/Lomentosporiasis can also occur in patients with cystic fibrosis, especially in cases of lung transplant.6
The nomenclature and taxonomy of the genus Scedosporium has been revised on several occasions.15,22,36,37 In the first one, S. apiospermum and Pseudallescheria boydii (which was traditionally considered a sexual form of Scedosporium) were divided into two different species.22,21 More recently, Scedosporium was adopted as a generic name over Pseudallescheria, and S. prolificans was renamed L. prolificans.22,37 Currently, the genus Scedosporium comprises 11 different species: Scedosporium aurantiacum, Scedosporium minutisporum, Scedosporium dehoogii, Scedosporium desertorum, Scedosporium americanum, Scedosporium cereisporum, S. apiospermum, Scedosporium boydii, Scedosporium ellipsoideum, Scedosporium angustum and Scedosporium fusoideum. Finally, Lackner et al. proposed to group the last five species into the S. apiospermum complex.1,10,21,36,37
In general, few studies consider these taxonomic changes in the clinical description of scedosporiasis cases. Furthermore, even taxonomists question the relevance of species-level identification within the S. apiospermum complex due to the potential recombination events between species and the lack of clinical data supporting its differentiation.6
The ubiquitously located Scedosporium genus can colonize and cause infections in the skin, paranasal sinuses, lung or disseminated infections, and even affects the central nervous system (CNS).51 In the case of disseminated infections, S. apiospermum occurs more frequently in cutaneous locations, while S. boydii is more involved in brain infection and fungaemia.19 In Argentina, among the species of the S. apiospermum complex, 43.75% corresponds to S. apiospermum, 31.25% to S. aurantiacum and 25% to S. boydii.17 They are the most commonly recovered fungi from respiratory tract secretions of patients with chronic lung diseases such as cystic fibrosis.17 Pulmonary colonization of patients with cystic fibrosis by Scedosporium is well established and the rate ranges between 0% and 21%. It is the most common genus after Aspergillus.32,33,61
The diagnosis of a Scedosporium infection is a real challenge because the clinical features and histopathology are similar to those of Aspergillus, Fusarium and other relatively common hyaline hyphomycetes.24,34,41Scedosporium grows well on Sabouraud agar medium with glucose, on which it forms velvety grey colonies with short aerial hyphae. The observation of asexual reproduction in culture is necessary for a correct diagnosis, but it is also possible that there is confusion with other morphologically similar species belonging to the same complex,23 as well as with other fungal genera that cause hyalohyphomycosis.
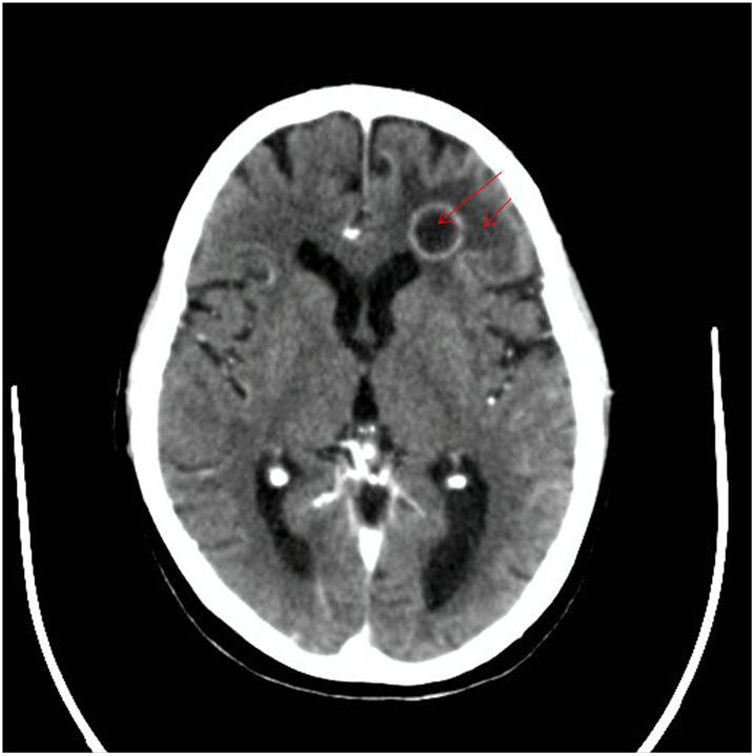
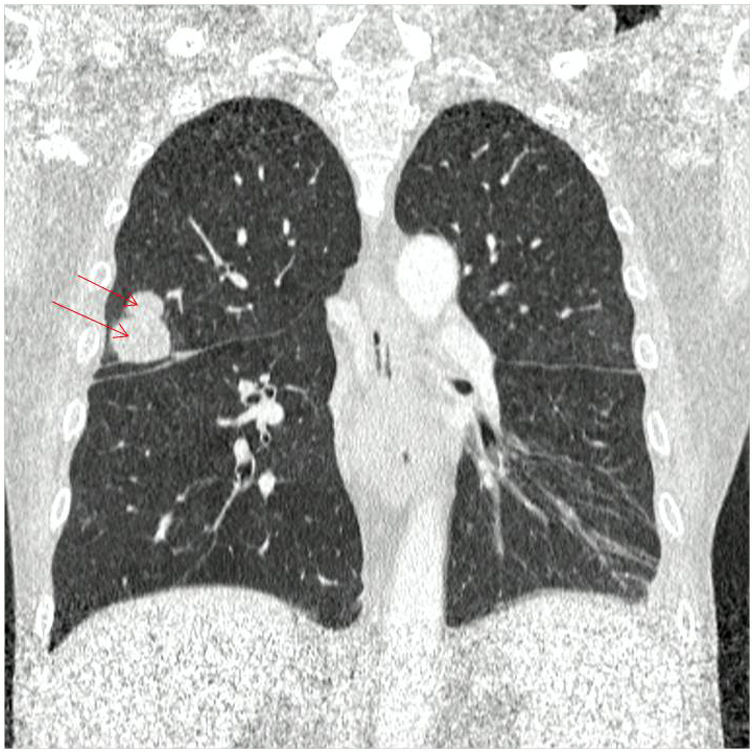
Materials and methodsThe samples studied in this research come from a 58-year-old male patient with a history of chronic obstructive pulmonary disease, diagnosed more than 20 years ago, without treatment at the time of consultation. In 2007, he suffered an acute myocardial infarction and two vascular stents were placed, to which another three were added in 2014. In 2021, he was hospitalized 8 months after an orthotopic double heart transplant for ischaemic heart disease in December 2020. The patient received immunosuppressant treatment with mycophenolate, tacrolimus and prednisone, and was undergoing outpatient treatment with ertapenem for epididymo-orchitis caused by Klebsiella pneumoniae and spondylodiscitis of the same aetiology. In July 2021, the patient was admitted to the hospital again during the on-call service due to hypoglycaemia associated with incoherent speech. With the initial suspicion of secondary relative adrenal insufficiency, due to the abrupt decrease in corticosteroid therapy, treatment with hydrocortisone at stress doses was initiated. Given the lack of improvement, a computed axial tomography (CT) of the head, thorax and abdomen was performed, which showed an occupying mass in the frontal lobe and the left area of the cerebellum, in addition to pulmonary nodules (Figs. 1 and 2). CT-guided puncture of a lung nodule and complete excision of the frontal lobe abscess was performed. The cerebellar abscess was also excised, but when the persistence of the lesion was observed in the control image, a reintervention was performed, without complications, with material taken for culture.
The mycological analysis of the samples was carried out at the Mycology Reference Center (CEREMIC, acronym of the name in Spanish), Faculty of Biochemical and Pharmaceutical Sciences, National University of Rosario. The microscopic analysis of the clinical samples consisted of direct examinations with lactophenol blue and 20% potassium hydroxide, and May-Grünwald Giemsa, Gram Nicolle and Ziehl Nielsen stains. The cultures were incubated at 28°C in Sabouraud glucose agar, Sabouraud-chloramphenicol agar and Mycosel media, and at 37°C in blood agar and Sabouraud glucose agar. After 7 days, microcultures were carried out for the micromorphological study of the isolates.
The molecular identification of the isolate obtained was carried out at the Mycology Center, Institute of Research in Microbiology and Medical Parasitology (IMPaM, UBA-CONICET). To do this, amplification was carried out by PCR (Polymerase Chain Reaction) and subsequent bidirectional nucleotide sequencing of the ITS region to identify the species complex, and the nucleotide sequences of a specific region of the gene of the β-tubulin for species identification. To do so, conidia of each strain were inoculated in 6mL of YPD (yeast peptone dextrose: yeast extract, peptone, dextrose) medium and cultured for 24–72h at 37°C. The mycelium was recovered and DNA extraction was performed according to the protocol of Tang et al.61 The quality and quantity of genomic DNA was estimated with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA) and stored at −20°C until use. Primers BT2a/BT2b were used to partially amplify the β-tubulin gene, while the ITS region was amplified using universal fungal primers ITS1/ITS4.6,57 Amplifications were performed in a 25μL reaction volume containing 0.2μM of each primer and 20–50ng of the fungal genomic DNA. PCR amplification was carried out in a thermocycler (Perkin-Elmer, USA) with an amplification programme that consisted of an initial denaturation step at 94°C for 5min, followed by 35 cycles at 95°C for 30s, 52°C (Bt2) or 54°C (ITS) for 30s, and 72°C for 2min; the amplification ended with a final extension step of 72°C for 10min.
Nucleotide sequencing was performed by the chain termination method using dideoxynucleotides in the presence of a chain terminator labelled with fluorescent reagents (BigDyeTerminator V.3.1, AppliedBiosystems) (Macrogen Inc., South Korea), after purification of the PCR products by Macrogen Inc. The resulting nucleotide sequences were edited with the Codon Code Aligner v7.1.2 programme DEMO (CodonCodeCorporation, www.codoncode.com) and were aligned with the MUSCLE algorithm contained in the Unipro UGENE v.44 programme. Subsequently, with the BLAST tool (Basic Local Alignment Search Tool), a local alignment was carried out to compare each of the sequences obtained with those found in the NCBI GenBank database and the Centre for Fungal Biodiversity of the Dutch Royal Academy of Sciences to find those with the greatest similarity. A phylogenetic analysis was then carried out to identify the cryptic species of the complex. For phylogenetic analysis, the sequence data obtained were aligned with the MUSCLE algorithm and with the Unipro UGENE v.44 programme.48 The multiple sequence alignment (MSA) obtained was evaluated with the ProtTest 3.0 programme16 to obtain the parameters of the phylogenetic model that best fitted it. Finally, the PhyML 3.2.0 program25 was used with the parameters obtained previously, the maximum likelihood trees optimized by SubtreePruning and Regrafting (SPR) and Nearest-Neighbour Interchange (NNI) were calculated, with a support of 1000 bootstraps. Finally, the trees obtained were visualized with the FigTree v1.4.4 me.53
The analysis of in vitro sensitivity to antifungals was determined by the microdilution method in liquid medium according to document M38-Ed3 of the Clinical and Laboratory Standards Institute (CLSI).12 The antifungals amphotericin B, caspofungin, isavuconazole, itraconazole, posaconazole and voriconazole, all purchased from Sigma–Aldrich (Stockholm, Sweden), were tested. All azoles and amphotericin B were tested at concentrations between 0.016 and 16μg/mL, and minimum inhibitory concentration (MIC) values were determined visually. Caspofungin was evaluated between 0.008 and 8μg/mL, and minimum effective concentration (MEC) values were determined microscopically.
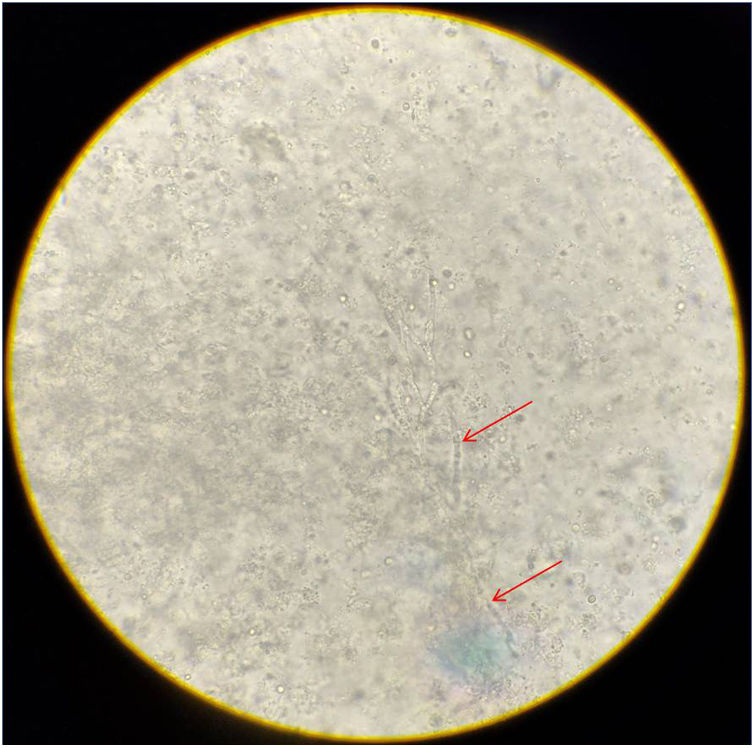
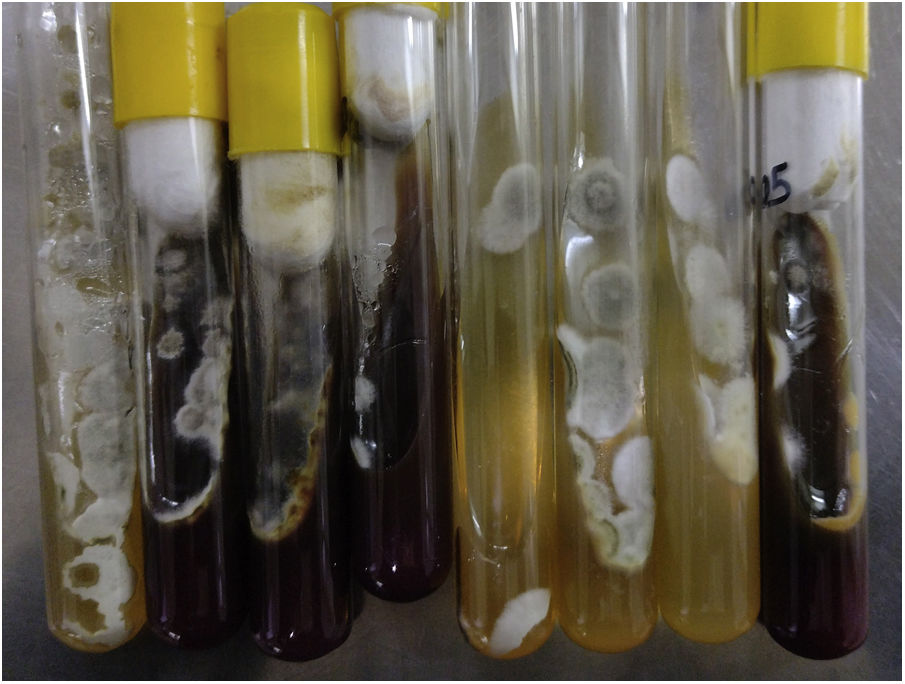
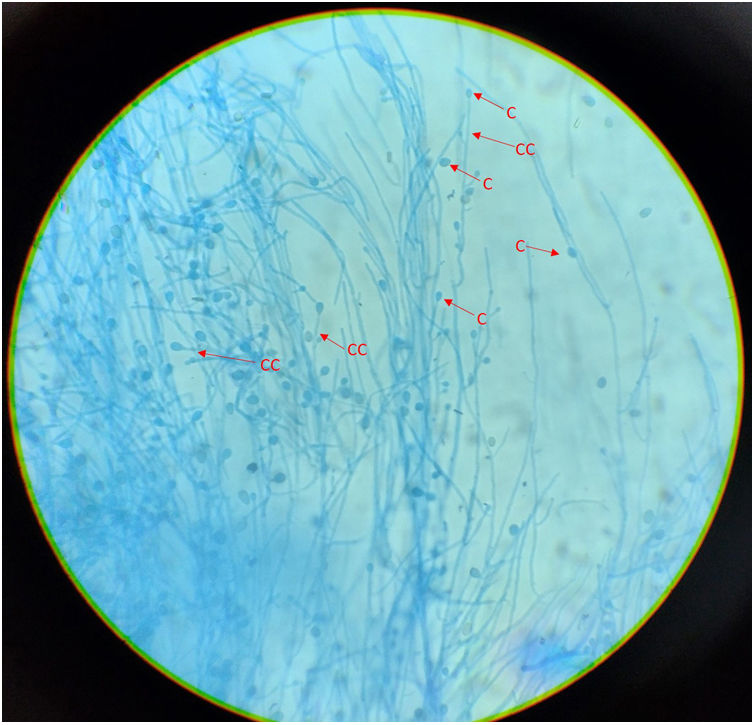
ResultsMicroscopic studies of all processed materials revealed the presence of fine, hyaline and septate filaments (Fig. 3). In the cultures at both temperatures, after 7 days, the development of a filamentous fungus with cottony colonies, white at the beginning in colour and then turning to a grey-brown colour on the obverse and greyish brown on the reverse, was obtained (Fig. 4). In the morphological study by microculture, thin hyaline, septate filaments were observed, with undifferentiated conidiogenous cells from which single conidia re with a smooth wall and a subspherical to elongated shape emerged. The width and length of 100 conidia were measured under a microscope, and an arithmetic mean of 7μm×4μm was obtained. According to the morphological characteristics observed, we were able to identify the isolate as compatible with fungi belonging to the S. apiospermum complex (Fig. 5).
Microculture of the isolation obtained. Thin, hyaline, septate filaments are observed, with undifferentiated conidiogenous cells from which single smooth-walled conidia emerge, subspherical to elongated in shape. Lactophenol blue stain, 400×. CC: undifferentiated conidiogenous cell; C: conidium.
Molecular identification through nucleotide sequencing of the ITS and β-tubulin regions confirmed the presence of S. boydii.
By determining the in vitro sensitivity to antifungals, the following MIC or MEC values were obtained, as appropriate: amphotericin B (MIC 0.5μg/mL), caspofungin (MEC≥8μg/mL), isavuconazole (MIC≥16μg/mL), itraconazole (MIC≥16μg/mL), posaconazole (MIC 0.5μg/mL), and voriconazole (MIC≥0.5μg/mL). The patient was treated with liposomal amphotericin B and voriconazole, and resection of the affected tissue was performed. Progress was slow but encouraging in the early phases of treatment. Finally, and after suffering complications, the patient died from sepsis due to treatment-resistent K. pneumoniae.
DiscussionInfections due to species of the Scedosporium complex are reported with increasing frequency in the world medical literature.10 This type of mycosis is included in the so-called mycoses caused by emerging fungi, which, although less frequent than the usual mycoses, caused by the genera Candida, Aspergillus or Cryptococcus, are not exempt from high morbidity and mortality.18,45 Infections by the S. apiospermum complex can occur in both immunosuppressed (mostly) and immunocompetent patients. In the latter, the subgroup of patients with cystic fibrosis may suffer from mycetoma as a frequent presentation.10 Clinical manifestations include endophthalmitis, pneumonia, endocarditis, lung abscess, disseminated disease and CNS disease in the form of brain abscesses, meningitis, and mycotic aneurysms.9,10
The predisposing conditions for the S. apiospermum complex to give rise to invasive infections include diabetes mellitus, insulin dependence, transplants, leukaemia, systemic lupus erythematosus, lymphoma, etc. Regarding patients with a solid organ transplant, if we look at the incidence of infection by the S. apiospermum complex in relation to the transplanted organ, the highest occurs in lung transplants (0.07%), followed by heart transplants. (0.22%), liver (0.07%) and kidney (0.05%).18 Haematopoietic stem cell transplant patients also represent a proportion of patients at high risk of invasive Scedosporium/Lomentospora infections.62 The clinical manifestations in this type of patient are very variable, as is the presentation of the disease: disseminated (as a more fatal form), pneumonia, skin infection, brain abscess, endophthalmitis, meningitis, ocular involvement, or fungal aneurysm, among others.2,20,31,46 These clinical forms occur after haematogenous dissemination from the lungs, skin, or any area of localized infection. In transplant patients, it must be considered that systemic infection is favoured by forced immunosuppression in the context of transplantation.9 The possibility of prior colonization by Scedosporium must also be taken into account.54
Rodriguez-Tudela et al.56 presented 10 cases of brain abscess caused by the S. apiospermumy complex and documented that the average time to diagnosis was 43 days. Furthermore, evidence of pulmonary infiltrates was found on the initial chest radiographs of all of these cases, and abscesses were found in the majority, suggesting haematogenous spread to the CNS. It has also been described that the average time of onset of infection after transplantation is usually 4 months.18 In the case presented, this period was approximately 8 months, together with other infections of bacterial origin.
The most relevant and dangerous expression of a disseminated infection by Scedosporium species is their ability to invade blood vessels and, in some cases, form mycelium and conidia in the tissue. In patients with acute leukaemia or allogeneic haematopoietic stem cell transplantation, Scedosporium produces a fatal massive infection in the setting of severe aplasia or neutropenia. Clinical features include fever, dyspnoea, pulmonary infiltrates, signs and symptoms of meningoencephalitis, skin lesions, and other manifestations that are the result of multiple organs involved. In these cases, the S. apiospermum complex, as well as L. prolificans, are isolated from blood culture in a high percentage.24
If we take into account that the diagnosis of an infection by species of the Scedosporium complex is difficult because the clinical and histopathological characteristics are similar to those of infections by Aspergillus, Fusarium and other hyaline hyphomycetes,11,34 an accurate diagnosis with the help of isolation of the fungus from cultures is of utmost importance. Another characteristic to consider is that, although the high recovery rate of these fungi in blood culture is described in the literature, especially in patients with disseminated infections, the majority of blood cultures become positive very close to the patient's death, resulting in limited diagnostic usefulness.56
Analysis based on the nucleotide sequence is the gold standard technique for the identification of fungi, which acquires particular relevance in the case of cryptic species that make up complexes. These species do not always have the same behaviour, in their role as aetiological agents of mycoses, and also their sensitivity to antifungals. ITS rDNA sequencing acceptably identifies the major species in Scedosporium/Lomentospora, but partial sequencing of the β-tubulin (BT2) gene must be performed to differentiate closely related or cryptic species.62
CNS infections due to species of the S. apiospermum complex are difficult to treat due, on the one hand, to the resistance of S. apiospermum to many of the available antifungals, including amphotericin B, itraconazole and ketoconazole, and on the other hand to the low penetration of these compounds into the CNS. The most effective alternative is surgical removal,56 which can be planned in association with the provision of appropriate antifungal medications such as voriconazole, which has been shown to be effective in the treatment of a disseminated infection.47 There are currently no specific epidemiological cut-off points of species, nor clinical cut-off points for the genus Scedosporium. However, the literature has studies designed to assess these values.35 Various case reports and therapeutic protocol guidelines currently available propose voriconazole (with or without surgery) as the first-line treatment for scedosporiasis.31 Furthermore, voriconazole is the antifungal of choice for this CNS pathology and is associated with a significant improvement and increased survival, compared to the use of amphotericin B.27,52,59
Scedosporium species are intrinsically resistant to fluconazole, some are resistant to polyenes and demonstrate reduced sensitivity to echinocandins.64 Triazoles, especially voriconazole, are the most suitable against these microorganisms.9,42,45,58 Voriconazole can be administered intravenously or orally (its bioavailability by this route is 90%) and has a much greater penetration into the CNS than other agents, with concentrations close to 50% of serum concentrations. In our patient, who presented with a localized infection, the removal of the affected tissue together with a combined antifungal therapy achieved an improvement in the mycosis, as reported in the international literature, with this work protocol being associated with a better prognosis.18,52
The high degrees of intrinsic antifungal resistance and inter- and intraspecies variations make these infections difficult to treat, hence it is recommended to evaluate sensitivity to available antifungals.26 The technical difficulties and the lack of standardization of the methods used make it necessary for these tests to be carried out in reference centres for each isolation.43
Nearly 100% of patients with disseminated disease die, while those with localized disease have the best survival.52 Considering the frequent progression to fatal forms of these clinical conditions, some therapeutic indications suggest that treatment for a possible infection due to the S. apiospermum species complex should be started as soon as its presence is suspected.10 Likewise, it is essential to perform a differential diagnosis with Aspergillus infections, since while species of this genus are usually sensitive to amphotericin B, the S. apiospermum species complex has limited sensitivity to it.47,3,8,40,39,55
Although, as we detailed before, there are no epidemiological cut-off points for this genus, a MIC value of less than 2μg/mL for voriconazole could predict a favourable outcome in the treatment of Scedosporium infections. Combination antifungal therapy allows the therapeutic effect to be achieved at lower concentrations and therefore reduces toxic side effects, improves safety and tolerability, decreases treatment time and prevents therapeutic failure when antimicrobial resistance is suspected. Bhat et al.5 reported the success of treatment with voriconazole and terbinafine in a patient with CNS disease. Jenks et al.29 reported an association between survival and the use of combination therapy (amphotericin B and posaconazole). They also documented that terbinafine-based regimens were significantly associated with treatment success and increased survival. The choice of therapeutic protocol should be determined according to the type of infection, whether it is disseminated, and its source.29
Reports of mycoses produced by this complex in Argentina are rare, so we believe that it is essential to carry out collaborative studies to achieve an accurate and early diagnosis that allows adopting the most effective therapeutic approach, especially when it comes to patients receiving transplants where iatrogenic immunosuppression is chronic.
Conflict of interestsThe authors have no conflict of interests to declare.