Data regarding yeast microbiota in goat milk is scarce.
AimsTo isolate and identify species of the genus Candida in milk samples from clinically healthy goats, and evaluate their enzymatic activity and biofilm formation.
Methods1092 milk samples from clinically healthy goats were collected and processed. The yeast isolates were identified by phenotypic, methods and their enzymatic activity (phospholipase, hemolysin and protease) and biofilm formation evaluated.
ResultsWe obtained 221 Candida isolates belonging to six species: Candida kefyr (35.7%), Candida guilliermondii (33%), Candida famata (23.5%), Candida glabrata (5.9%), Candida albicans (1.35%) and Candida parapsilosissensu lato (0.45%). Protease activity was detected in all Candida species while hemolysin activity was only present in C. kefyr, C. guilliermondii, C. famata and C. albicans. Only C. albicans showed phospholipase activity. With the exception of C. parapsilosis sensu lato, all Candida species formed biofilm, with 60.19% of the isolates being poor producers, 9.93% moderate producers, and 1.35% strong producers.
ConclusionsThe milk of clinically healthy goats contains several species of the genus Candida that could play a role as opportunistic pathogens in mastitis.
El conocimiento de la microbiota levaduriforme presente en la leche de cabra es escaso.
ObjetivosAislar e identificar especies del género Candida en muestras de leche de cabras clínicamente sanas, evaluar su actividad enzimática y su capacidad de formar biopelículas.
MétodosSe recogieron y procesaron 1092 muestras de leche de cabras clínicamente sanas. Las levaduras aisladas fueron identificadas mediante métodos fenotípicos, evaluándose posteriormente su actividad enzimática (producción de fosfolipasas, hemolisinas y proteasas) y la formación de biopelículas.
ResultadosSe obtuvieron 221 aislamientos de Candida de seis especies: Candida kefyr (35,7%), Candida guilliermondii (33%), Candida famata (23,5%), Candida glabrata (5,9%), Candida albicans (1,35%) y Candida parapsilosis sensu lato (0,45%). En todas las especies de Candida se detectó actividad proteolítica, y únicamente C. kefyr, C. guilliermondii, C. famata y C. albicans presentaron actividad hemolítica. Por su parte, C. albicans fue la única especie con actividad fosfolipasa. Con excepción de C. parapsilosissensu lato, todas las especies de Candida formaron biopelícula, con el 60,19% de los aislamientos poco formadores de biopelícula, el 9,93% moderadamente formadores y el 1,35% altamente formadores.
ConclusionesLa leche de cabras clínicamente sanas presenta diversas especies del género Candida que podrían actuar como patógenos oportunistas de mastitis.
The mammary glands in ruminants are known to harbor diverse bacteria; little is known, however, about their fungal microbiota. In dairy cattle, studies report the presence of different species of the genus Candida.7,18,21,25,34,37 A variety of enzymatic activities, mainly proteases, hemolysins and phospholipases, allow different species of Candida to adhere, colonize and invade host tissues.8,10 In addition, biofilm production enables Candida infection and resistance to antimicrobials.13,33 The objective of this study was to isolate and identify Candida species in milk samples from clinically healthy goats, and to evaluate their enzymatic activity and biofilm formation.
We collected 1092 milk samples during a six month-period from a farm with Alpina goats clinically healthy (n = 100) in Querétaro, Mexico. The samples were centrifuged (4000rpm, 10min), the pellets resuspended into 2ml of yeast extract peptone dextrose (YEPD) broth (casein peptone 2%, dextrose 2%, yeast extract 1%), and incubated 48h at 37°C. Yeasts were isolated by plating 50μl from each suspension on Sabouraud dextrose agar (SDA) supplemented with 50mg/l chloramphenicol. Conventional phenotypic methods were used for yeast identification: Gram staining, germ tube production, pseudohyphae formation, sensitivity to 0.1% cycloheximide, film formation in liquid culture, urease production, carbohydrates assimilation and fermentation, and development in Biggy medium and CHROMagar Candida. 2,5,6,14,24,27,28,30,32,40 Each yeast isolate was evaluated for enzymatic activity and biofilm production. Phospholipase and protease activity were performed according to Kantarcioglu and Yücel,20 and hemolysin activity according to Luo et al.26 Briefly, 6mm diameter sterile filter discs were embedded with 10μl yeast suspension (1×106–5×106CFU/ml). These were placed onto SDA agar supplemented with 4% egg yolk, 0.2% bovine serum albumine or 7% sheep blood for phospholipase, protease and hemolysin activity evaluation, respectively. After incubation (37°C, 7 days), the yeast colony and the corresponding clear halo diameters were measured. The enzymatic activity was determined through the enzyme activity index according to Williamson's formula.41Candida biofilm formation was evaluated using the Gokce et al. safranine method.15 For each isolate a 1×108CFU/ml yeast suspension was prepared in yeast nitrogen base medium (YNBG) with 8% glucose. From this stock suspension a 1:100 dilution was made, and 96-well polystyrene flat-bottom microplates were filled (200μl/well) and incubated 48h at 37°C. Afterwards, the plates were washed three times with sterile phosphate-buffered saline (PBS, pH 7.4). The biofilms formed were fixed with methanol for 15min (200μl/well); methanol was discarded and the microplates were allowed to dry for 10min at 37°C. Thereafter, 1% safranin solution was added (200μl/well), and after 20min at room temperature a washing with PBS was done. Immediately after, 95% ethanol was added (200μl/well, 20min at room temperature). Finally, the optical density (OD) was determined at 490nm using a Biotek ELISA reader. Categorization of biofilm production was established through OD intervals as described by Stepanovic et al.16,39 Descriptive statistics were used to analyze the results.
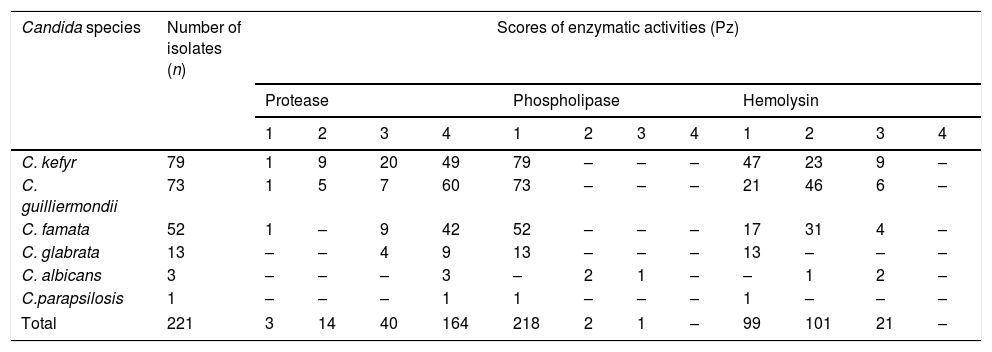
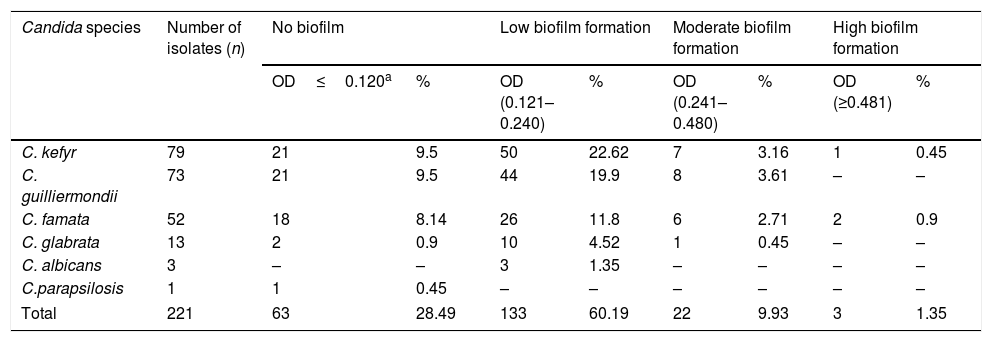
Candida was the solely yeast genus found in the goat milk samples analyzed. We obtained 221 Candida isolates, further identified as Candida kefyr 35.74%, Candida guilliermondii 33%, Candida famata 23.53%, Candida glabrata 5.9%, Candida albicans 1.35%, and Candida parapsilosis(sensu lato)44 0.45% (Table 1). Concerning enzymatic activity, 98.65% of the isolates showed protease activity, 55.2% hemolytic activity, and 1.36% phospholipase activity. Protease activity varied among the species and within the isolates of a given species. C. kefyr, C. guilliermondii, and C. famata showed the whole range of protease activity, having more than 87% of them medium and high activity. All C. glabrata presented protease activity, circa 70% of them showing high activity. C. albicans and C. parapsilosis isolates exhibited only high protease activity. Hemolysin activity was observed in C. albicans (100%), C. guilliermondii (71.23%), C. famata (67.30%) and C. kefyr (40.50%). Phospholipase activity was just detected in C. albicans (Table 2). Regarding biofilm formation, four categories were determined: no biofilm formation, low biofilm formation, moderate biofilm formation, and high biofilm formation. Biofilm was produced in various degrees by 71.49% of all Candida isolates but C. parapsilosis (sensu lato) (Table 3).
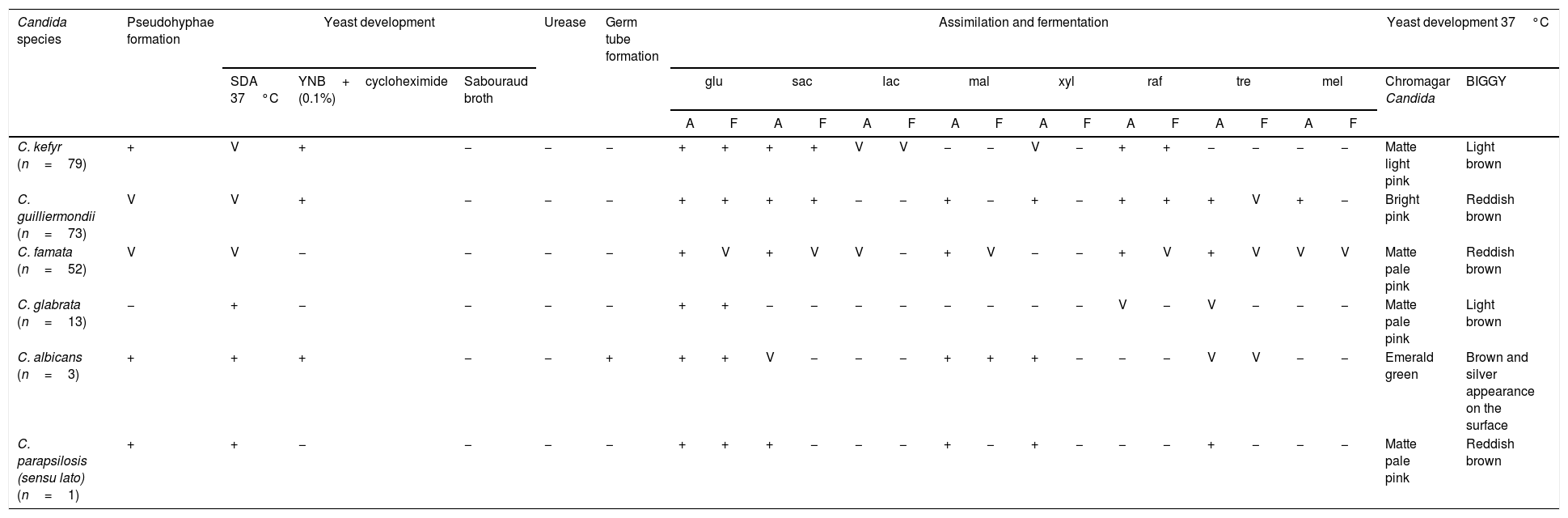
Identification of Candida isolates from the milk of clinically healthy goats.
| Candida species | Pseudohyphae formation | Yeast development | Urease | Germ tube formation | Assimilation and fermentation | Yeast development 37°C | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SDA 37°C | YNB+cycloheximide (0.1%) | Sabouraud broth | glu | sac | lac | mal | xyl | raf | tre | mel | Chromagar Candida | BIGGY | ||||||||||||
| A | F | A | F | A | F | A | F | A | F | A | F | A | F | A | F | |||||||||
| C. kefyr (n=79) | + | V | + | − | − | − | + | + | + | + | V | V | − | − | V | − | + | + | − | − | − | − | Matte light pink | Light brown |
| C. guilliermondii (n=73) | V | V | + | − | − | − | + | + | + | + | − | − | + | − | + | − | + | + | + | V | + | − | Bright pink | Reddish brown |
| C. famata (n=52) | V | V | − | − | − | − | + | V | + | V | V | − | + | V | − | − | + | V | + | V | V | V | Matte pale pink | Reddish brown |
| C. glabrata (n=13) | − | + | − | − | − | − | + | + | − | − | − | − | − | − | − | − | V | − | V | − | − | − | Matte pale pink | Light brown |
| C. albicans (n=3) | + | + | + | − | − | + | + | + | V | − | − | − | + | + | + | − | − | − | V | V | − | − | Emerald green | Brown and silver appearance on the surface |
| C. parapsilosis (sensu lato) (n=1) | + | + | − | − | − | − | + | + | + | − | − | − | + | − | + | − | − | − | + | − | − | − | Matte pale pink | Reddish brown |
A: assimilation; F: fermentation; SDA: Sabouraud dextrose agar; YNB: yeast nitrogen base; glu: glucose; sac: saccharose; lac: lactose; mal: maltose; xyl: xylose; raf: raffinose; tre: trehalose; mel: melezitose; V: variable strains.
Enzymatic activity of Candida isolates from the milk of clinically healthy goats.
| Candida species | Number of isolates (n) | Scores of enzymatic activities (Pz) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protease | Phospholipase | Hemolysin | |||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | ||
| C. kefyr | 79 | 1 | 9 | 20 | 49 | 79 | – | – | – | 47 | 23 | 9 | – |
| C. guilliermondii | 73 | 1 | 5 | 7 | 60 | 73 | – | – | – | 21 | 46 | 6 | – |
| C. famata | 52 | 1 | – | 9 | 42 | 52 | – | – | – | 17 | 31 | 4 | – |
| C. glabrata | 13 | – | – | 4 | 9 | 13 | – | – | – | 13 | – | – | – |
| C. albicans | 3 | – | – | – | 3 | – | 2 | 1 | – | – | 1 | 2 | – |
| C.parapsilosis | 1 | – | – | – | 1 | 1 | – | – | – | 1 | – | – | – |
| Total | 221 | 3 | 14 | 40 | 164 | 218 | 2 | 1 | – | 99 | 101 | 21 | – |
Enzyme activity: 1=null (Pz=1); 2=low (Pz=0.61–0.99); 3=medium (Pz=0.41–0.60); 4=high (Pz≤0.40).
Pz =Diameter of the colonyDiameter of the halo
Biofilm formation of Candida isolates from the milk of clinically healthy goats.
| Candida species | Number of isolates (n) | No biofilm | Low biofilm formation | Moderate biofilm formation | High biofilm formation | ||||
|---|---|---|---|---|---|---|---|---|---|
| OD≤0.120a | % | OD (0.121–0.240) | % | OD (0.241–0.480) | % | OD (≥0.481) | % | ||
| C. kefyr | 79 | 21 | 9.5 | 50 | 22.62 | 7 | 3.16 | 1 | 0.45 |
| C. guilliermondii | 73 | 21 | 9.5 | 44 | 19.9 | 8 | 3.61 | – | – |
| C. famata | 52 | 18 | 8.14 | 26 | 11.8 | 6 | 2.71 | 2 | 0.9 |
| C. glabrata | 13 | 2 | 0.9 | 10 | 4.52 | 1 | 0.45 | – | – |
| C. albicans | 3 | – | – | 3 | 1.35 | – | – | – | – |
| C.parapsilosis | 1 | 1 | 0.45 | – | – | – | – | – | – |
| Total | 221 | 63 | 28.49 | 133 | 60.19 | 22 | 9.93 | 3 | 1.35 |
OD: optical density.
Yeast identification was performed by conventional methods, which are the routine diagnostic methods used in our laboratory. Although known to be less sensitive and more time consuming than molecular methodologies, they are considered the gold Candida identification standard.1,43 Predominance of non-C. albicansCandida species in milk from clinically healthy goats, and from other ruminants with and without mastitis have been reported.9,17,19,21,22,25 Our findings support this since, out of the six Candida species found, C. albicans accounted only for 1.35% of the isolates. Little is known about the association of Candida spp. enzymatic production and biofilm formation with the yeasts virulence. Both proteases and hemolysins were detected in C. albicans, C. famata, C. kefyr, C. guilliermondii and C. parapsilosis, whereas phospholipases were only detected in C. albicans. These features are in agreement with previous studies.3,4,12,15,26,29,36In vitro production of these virulence factors are strain-dependent and may differ according to the anatomical site infected or the yeasts involvement in a pathological process.4,33 Biofilm formation has been described in C. albicans, C. tropicalis, C. parapsilosis and C. glabrata, and correlation between yeast virulence and high biofilm production has been demonstrated in human beings.11,13,15,23,31,33,42 In animals, a research studying milk from buffaloes with mastitis have shown high biofilm production in Candida zeylanoides, Candida rugosa and Candida kefyr.35 In this study, C. albicans, C. kefyr, C. guilliermondii, and C. famata isolates mostly showed a low production of biofilms. This might be related to the samples origin, clinically healthy animals, where formation of high density biofilms might not be crucial to yeasts persistence. Nevertheless, all these microorganisms could be opportunistic pathogens in mastitis.35,38
More research on Candida presence, both in milk of clinically healthy and diseased goats, and their virulence factors is needed to determine their relationship with the development of fungal mastitis in this animal species. Our study is the first of its kind in Mexico, demonstrating the presence of different species of the genus Candida in goat milk from clinically healthy animals; the isolates exhibited various virulence factors under laboratory conditions.
FinancingProgram for Support to Projects for the Innovation and Improvement of Teaching (PAPIME) grant PE206819. DGAPA-UNAM. México.
Conflict of interestsThe authors declare that they have no conflict of interest.
The authors are grateful to Dr.Cristina Escalante Ochoa for her valuable review of this manuscript.