There are important advances in the management of bacterial infection in patients with cystic fibrosis (CF), but there are many gaps in the field of fungal infections.
AimsThe aim of this study was to analyse whether chronic respiratory filamentous fungal colonization had clinical impact and whether antifungal treatment can change the disease.
MethodsThe prospective, bicentric and descriptive study was carried out within a 3-year follow-up period, with four-month periodicity medical controls. Adult patients from two CF units of tertiary hospitals were included. Clinical, microbiological, analytical and spirometric variables were collected. Quality of life was evaluated in a subgroup, using the Spanish version of the Revised Cystic Fibrosis Quality of Life Questionnaire (CFQ-R). To statistically analyze the evolution of forced expiratory along time (volume of air blown out in 1 second -FEV1-) and the forced vital capacity (FVC), mixed linear models were carried out.
ResultsFrom the ninety-eight patients under study, 40 suffered chronic filamentous fungal colonization. The presence of filamentous fungi in airway was associated to an annual fall of FEV1 and FVC of 0.029 and 0.017 litres, respectively (p<0.001). In addition, worse quality of life based on CFQ-R, significant when concerning physical condition and emotional state, was also linked with the fungal colonization. Protocolized antifungal therapy, nebulized or oral, improved FEV1 in 0.023 and 0.024 litres per year, respectively (p<0.001).
ConclusionsChronic filamentous fungal colonization in patients with CF is associated with a significant annual decline of lung function that persists over time. Chronic antifungal therapy slows down this progression, mainly in the patient with more advanced disease.
Existen avances importantes en el manejo de la infección bacteriana en pacientes con fibrosis quística (FQ), pero hay muchas lagunas en el campo de las infecciones fúngicas.
ObjetivosEl objetivo del estudio fue analizar si la colonización respiratoria crónica por hongos filamentosos tenía impacto clínico y si el tratamiento antifúngico modificaba la enfermedad.
MétodosEstudio prospectivo, bicéntrico y descriptivo con un seguimiento de 3 años y controles médicos cuatrimestrales. Se incluyeron pacientes adultos de dos Unidades de Fibrosis Quística de hospitales terciarios. Se recogieron datos de variables clínicas, microbiológicas, analíticas y espirométricas; en un subgrupo se evaluó la calidad de vida mediante el cuestionario específico para FQ (CFQ-R). Para analizar estadísticamente la evolución a lo largo del tiempo del volumen espiratorio máximo en el primer segundo (FEV1) y la capacidad vital forzada (FVC) se realizaron modelos lineales mixtos.
ResultadosDe los 98 pacientes incluidos en el estudio, 40 presentaron colonización crónica por hongos filamentosos. La presencia de hongos filamentosos en la vía aérea se asoció a una caída anual del FEV1 y FVC de 0,029 y 0,017 litros, respectivamente (p<0,001), además de a una peor calidad de vida según el CFQ-R, significativa en cuestiones como la capacidad física y el estado emocional. La terapia antifúngica protocolizada, nebulizada u oral, mejoraron el FEV1 0,023 y 0,024 litros al año, respectivamente (p<0,001).
ConclusionesLa colonización crónica por hongos filamentosos en pacientes con FQ se asocia a una caída anual de la función pulmonar significativa que persiste en el tiempo. El tratamiento antifúngico protocolizado ralentiza este deterioro funcional, fundamentalmente en el paciente con enfermedad más avanzada.
The presence of filamentous fungi in the airway of patients with cystic fibrosis (CF) is a common occurrence because this population group is subjected to many antimicrobial31 treatments and have a particular airway (hyperviscosity of mucus, alteration of both the mucociliary transport and the local immune response). Despite this, its role in the pathogenesis of the pulmonary disease is unknown. The fungi most commonly isolated in the patient with CF are yeasts, followed by filamentous fungi.30Aspergillus fumigatus is the most common of the filamentous fungi,9,19 followed by Aspergillus terreus, Scedosporium apiospermum and Lomentospora prolificans, all of which are related to infectious18 or allergic2,26 responses in the host with CF, but with no specific pathogenic pattern or specific patient profile.6 Yeasts, such as Candida albicans, are isolated in 50%-75% of respiratory samples and are usually considered colonizers; their involvement in lung function impairment is unknown.17 After Aspergillus, Scedosporium and L. prolificans are the most common etiological agents and are a reason for concern in CF, given its intrinsic resistance to most antifungal agents.8
The aim of this study was to analyse the impact of chronic respiratory filamentous fungal colonization using clinical and functional parameters, and to analyse the efficacy of the antifungal treatment. We also tried to determine whether there is a patient profile with CF more proned to suffer these infections and, therefore, a candidate for treatment.
Patients and methodsA prospective, bicentric and descriptive study was carried out within a 3-year protocolized follow-up period, with adult patients who attended the CF units of the University Hospital La Fe (Valencia) and the Hospital de La Princesa (Madrid). The project was approved by the Ethics Committee of Clinical Research of the University Hospital La Fe, registered number 2011/0159. The patients were recruited consecutively from 1st April 2012. Inclusion criteria were as follows: patients over 14 years of age suffering from CF, with no transplants, and who carried chronically a filamentous fungus (three or more isolations in sputum culture or other respiratory secretions per year). Exclusion criteria included being in treatment with CFTR modulators (cystic fibrosis transmembrane conductance regulator), solid organ transplant, and colonization by filamentous fungi when the study variables were not assessed at least twice a year during the three years of follow-up.
Clinical, microbiological, analytical and spirometric data were collected every 4 months throughout the 3 years of follow-up, together with information about the antifungal treatment received in those cases where it was prescribed.4The quality of life of a subgroup of patients was also quantified through a specific quality of life questionnaire for CF (CFQ-R).
The variables analyzed and evaluated in each consultation were age, sex, body mass index, associated comorbidities (hepatopathy and/or diabetes), identification of the filamentous fungus obtained in culture (species), concomitant infection/bronchial colonization (bacteria, mycobacteria, Nocardia) when the filamentous fungus was isolated, number of oral antibiotic cycles, number of hospital admissions, number of days of intravenous antimicrobial treatment during hospital admission, long-term treatment with azithromycin and/or ibuprofen, previous history of haemoptysis or pneumothorax, and allergic bronchopulmonary aspergillosis criteria at onset or developed during patient follow-up. The genetic study (mutation F508del in homozygosis) of all patients included in the study was obtained. For the evaluation of lung function, the following variables were recorded: absolute value and percentage of the maximum expiratory volume in the first second (FEV1), forced vital capacity (FVC) and FEV1/FVC index using a spirometry test performed in each consultation. Respiratory samples (direct sputum samples) were taken in sterile conditions from the patients with CF that were clinically stable at the time of consultation, and they were immediately processed using Sabouraud culture medium (in agar and liquid) with 2% chloramphenicol. The media were incubated at 37 ̊C. The yeasts and bacteria isolated were identified using mass spectrometry (MALDI-TOF, bioMérieux), whereas filamentous fungi were identified by macro and microscopic morphology. Antifungal susceptibility tests were also performed.
Antimicrobial therapy was applied in case of respiratory exacerbations. This treatment was oral in the case of mild to moderate episodes, or intravenous in the severe ones. The drugs and doses used in the oral cycles were ciprofloxacin 750 mg/12 h or sulfamethoxazole/trimethoprim 800/160 mg every 12 h for 14 days. In the intravenous cycles, drug combinations were used: one beta-lactam and one aminoglycoside, for example 4/0.5 g piperacillin/tazobactam every 8 h, 2 g/8 h ceftazidime or 2 g/8 h meropenem combined with 1 g/24 amikacin or 300 mg/24 h tobramicin (depending on plasma concentrations) for at least 14 days. In some cases linezolid (600 mg/12 h) was added.
Twenty six patients of the group with chronic filamentous fungus colonization (65%) received a protocolized long-term antifungal treatment during the three years of follow-up. The therapeutic protocol consisted in having inhaled amphotericin B lipid complex or azoles.15,23 The inhaled amphotericin B dose was 25 mg three times a week for two months, followed by a weekly dose for 6 months and, lastly, two doses per month until the end of the follow-up period. The voriconazole dose was 200 mg/12 h for 3 months each year throughout the 3 years of follow-up; the posaconazole dose was 300 mg/day, after a loading dose of 300 mg/12 h the first 24 h, for 3 three months each year throughout the 3 years of follow-up. Antifungal therapy selection was the choice of the physician responsible for the patient and the criterion to start it was the persistence of clinical and/or functional impairment even after the administration of the intravenous antibiotic therapy. Plasma concentrations of voriconazole were systematically monitored.7 Liver function was checked every six months through blood analyses in all patients, except for those under antifungal treatment (blood analyses were done monthly); blood analyses included alanine aminotransferasae, aspartate aminotransferase, alkaline phosphatase, gamma-glutamiltransferase, and direct and indirect billirubin.
Statistical analysis was performed using R software (version 3.6.0) and the R lmer (version 1.1-23) package. Data were summarized by median and standard deviation for quantitative variables and percentages for categorical variables. To evaluate the evolution of the FEV1 and the FVC over time in patients with different characteristics, lower-order interaction mixed lineal models were used. The information provided by the CFQ-R was also studied in each group using mixed lineal regression models for each of the 12 sections of the questionnaire. Statistical significance was considered at p<0.05.
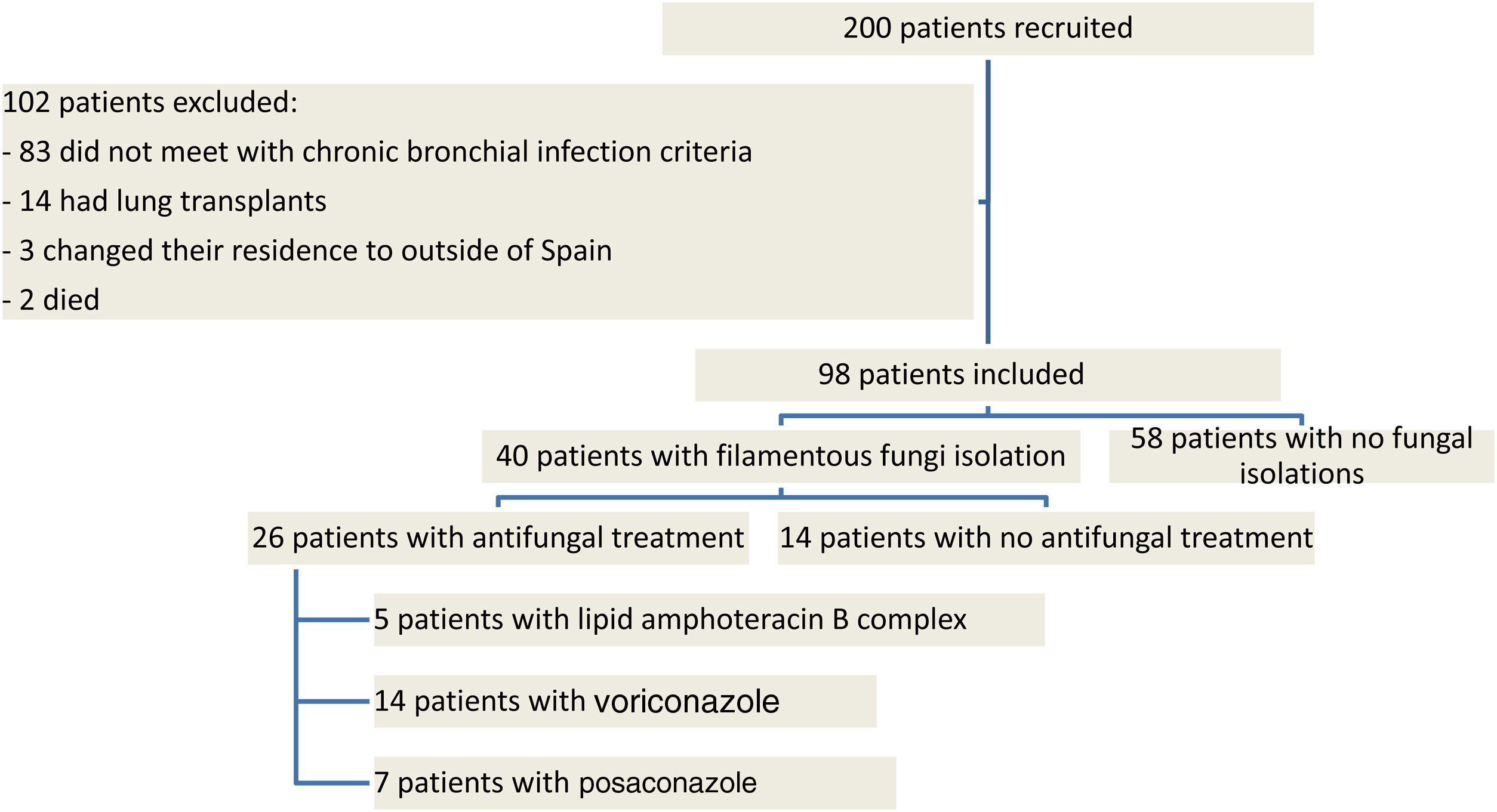
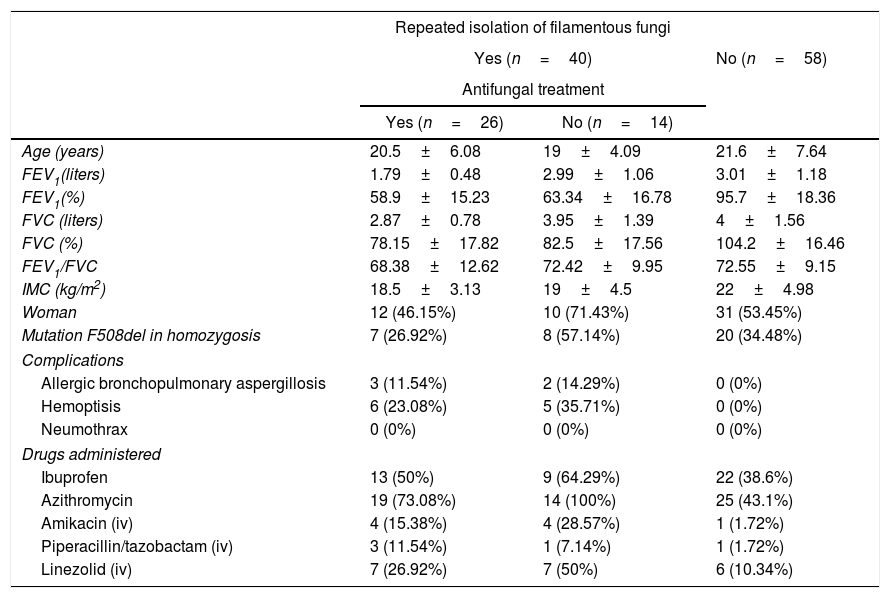
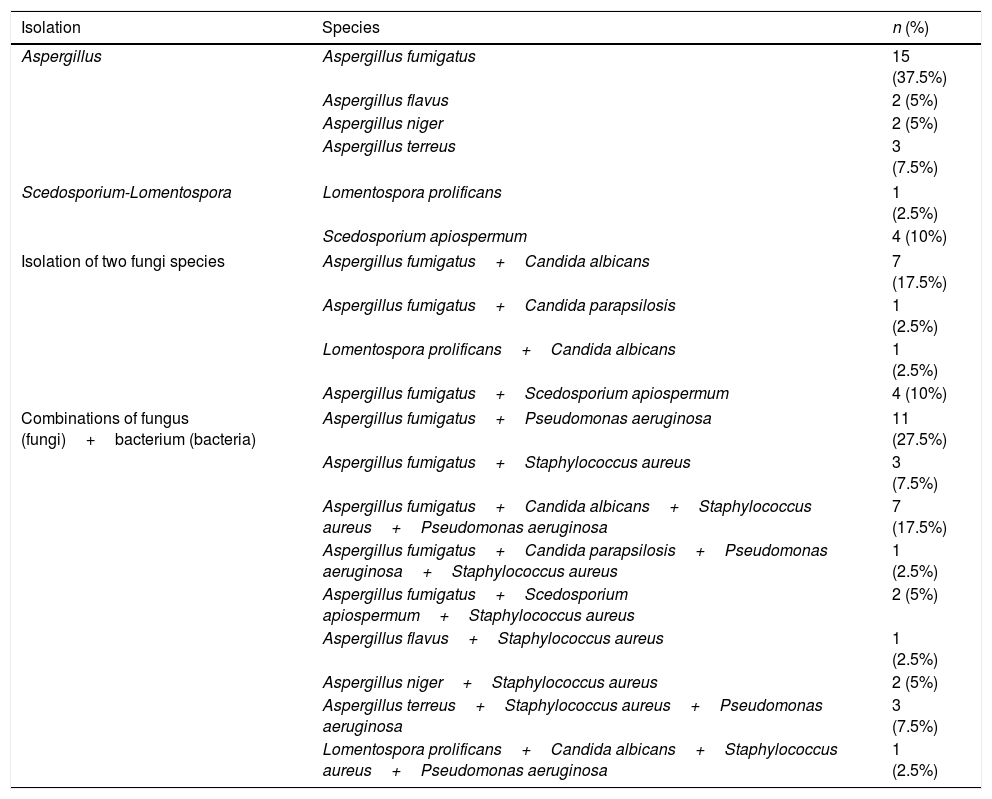
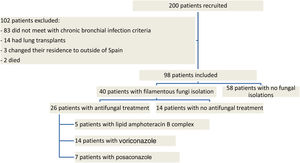
ResultsNinety eight patients out of 200 patients with CF were finally considered for the study once the exclusion criteria were applied (Fig. 1). Out of these 98 patients, 40 presented with chronic fungal colonization (with three or more isolations of filamentous fungi per year), whilst from the remaining 58 patients no filamentous fungi were isolated during follow-up (control group). Of the 40 patients with fungal isolations, 26 were treated with long-term antifungal therapy and 14 were not. The clinical characteristics of the patients are described in Table 1 and the isolated fungi species are listed in Table 2.
Baseline clinical characteristics of patients taken from their first consultation and the intravenous antimicrobial treatment received.
| Repeated isolation of filamentous fungi | |||
|---|---|---|---|
| Yes (n=40) | No (n=58) | ||
| Antifungal treatment | |||
| Yes (n=26) | No (n=14) | ||
| Age (years) | 20.5±6.08 | 19±4.09 | 21.6±7.64 |
| FEV1(liters) | 1.79±0.48 | 2.99±1.06 | 3.01±1.18 |
| FEV1(%) | 58.9±15.23 | 63.34±16.78 | 95.7±18.36 |
| FVC (liters) | 2.87±0.78 | 3.95±1.39 | 4±1.56 |
| FVC (%) | 78.15±17.82 | 82.5±17.56 | 104.2±16.46 |
| FEV1/FVC | 68.38±12.62 | 72.42±9.95 | 72.55±9.15 |
| IMC (kg/m2) | 18.5±3.13 | 19±4.5 | 22±4.98 |
| Woman | 12 (46.15%) | 10 (71.43%) | 31 (53.45%) |
| Mutation F508del in homozygosis | 7 (26.92%) | 8 (57.14%) | 20 (34.48%) |
| Complications | |||
| Allergic bronchopulmonary aspergillosis | 3 (11.54%) | 2 (14.29%) | 0 (0%) |
| Hemoptisis | 6 (23.08%) | 5 (35.71%) | 0 (0%) |
| Neumothrax | 0 (0%) | 0 (0%) | 0 (0%) |
| Drugs administered | |||
| Ibuprofen | 13 (50%) | 9 (64.29%) | 22 (38.6%) |
| Azithromycin | 19 (73.08%) | 14 (100%) | 25 (43.1%) |
| Amikacin (iv) | 4 (15.38%) | 4 (28.57%) | 1 (1.72%) |
| Piperacillin/tazobactam (iv) | 3 (11.54%) | 1 (7.14%) | 1 (1.72%) |
| Linezolid (iv) | 7 (26.92%) | 7 (50%) | 6 (10.34%) |
BMI: body mass index; FEV1: maximum expiratory volume in the first second; FVC: volume of air exhaled during maximal expiration; iv: intravenous.
Isolated species of fungi and bacteria.
| Isolation | Species | n (%) |
|---|---|---|
| Aspergillus | Aspergillus fumigatus | 15 (37.5%) |
| Aspergillus flavus | 2 (5%) | |
| Aspergillus niger | 2 (5%) | |
| Aspergillus terreus | 3 (7.5%) | |
| Scedosporium-Lomentospora | Lomentospora prolificans | 1 (2.5%) |
| Scedosporium apiospermum | 4 (10%) | |
| Isolation of two fungi species | Aspergillus fumigatus+Candida albicans | 7 (17.5%) |
| Aspergillus fumigatus+Candida parapsilosis | 1 (2.5%) | |
| Lomentospora prolificans+Candida albicans | 1 (2.5%) | |
| Aspergillus fumigatus+Scedosporium apiospermum | 4 (10%) | |
| Combinations of fungus (fungi)+bacterium (bacteria) | Aspergillus fumigatus+Pseudomonas aeruginosa | 11 (27.5%) |
| Aspergillus fumigatus+Staphylococcus aureus | 3 (7.5%) | |
| Aspergillus fumigatus+Candida albicans+Staphylococcus aureus+Pseudomonas aeruginosa | 7 (17.5%) | |
| Aspergillus fumigatus+Candida parapsilosis+Pseudomonas aeruginosa+Staphylococcus aureus | 1 (2.5%) | |
| Aspergillus fumigatus+Scedosporium apiospermum+Staphylococcus aureus | 2 (5%) | |
| Aspergillus flavus+Staphylococcus aureus | 1 (2.5%) | |
| Aspergillus niger+Staphylococcus aureus | 2 (5%) | |
| Aspergillus terreus+Staphylococcus aureus+Pseudomonas aeruginosa | 3 (7.5%) | |
| Lomentospora prolificans+Candida albicans+Staphylococcus aureus+Pseudomonas aeruginosa | 1 (2.5%) | |
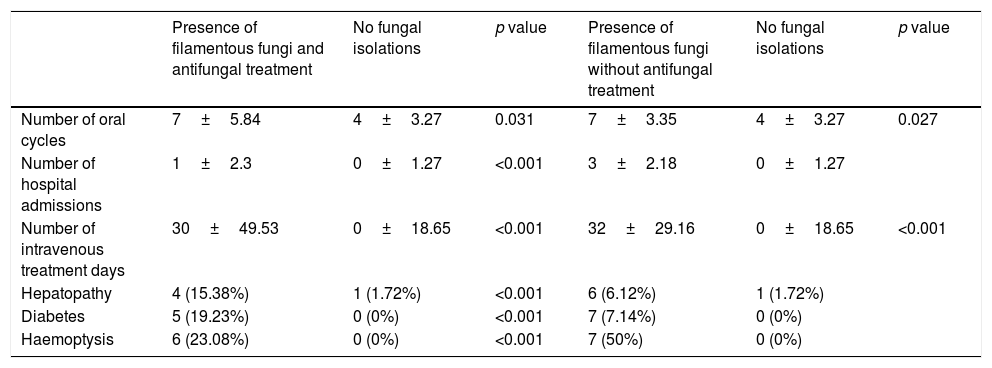
Regarding the concomitant bacterial infections, 23 patients (57.7%) from the group colonized by filamentous fungi presented with chronic infection by Pseudomonas aeruginosa, eight from the group with antifungal treatment and 15 from the group with no treatment, compared with eight patients (14.04%) from the control group. Chronic infection by Staphylococcus aureus was diagnosed in twenty patients (50%) from the group with fungal presence, in 10 patients from the group with antifungal treatment and in 10 from the group with no treatment, compared with 31 patients (53.45%) from the control group. Both the chronic concomitant infection by P. aeruginosa and by S. aureus had hardly any impact on the evolution of the FEV1 (annual loss of 0.03 and 0.01 litres), i.e. functional impairment was not linked to these bacterial infections. However, it is of note that the patients with a combination of A. fumigatus and P. aeruginosa required a larger number of oral cycles and a longer time of intravenous treatment compared with the other combinations. The patients with chronic colonization by filamentous fungi suffered a significantly higher number of exacerbations, hospital stays and days of intravenous antimicrobial treatment compared with the patients without isolations (Table 3), all linked with lower survival.12 Diagnoses of hepatopathy and diabetes related with CF and haemoptysis were more common in the group of patients with filamentous fungi than in the group without them.
Values recorded throughout the study in the group of patients with repeated filamentous fungi isolation (with or without antifungal treatment) and in the group without fungal isolations.
| Presence of filamentous fungi and antifungal treatment | No fungal isolations | p value | Presence of filamentous fungi without antifungal treatment | No fungal isolations | p value | |
|---|---|---|---|---|---|---|
| Number of oral cycles | 7±5.84 | 4±3.27 | 0.031 | 7±3.35 | 4±3.27 | 0.027 |
| Number of hospital admissions | 1±2.3 | 0±1.27 | <0.001 | 3±2.18 | 0±1.27 | |
| Number of intravenous treatment days | 30±49.53 | 0±18.65 | <0.001 | 32±29.16 | 0±18.65 | <0.001 |
| Hepatopathy | 4 (15.38%) | 1 (1.72%) | <0.001 | 6 (6.12%) | 1 (1.72%) | |
| Diabetes | 5 (19.23%) | 0 (0%) | <0.001 | 7 (7.14%) | 0 (0%) | |
| Haemoptysis | 6 (23.08%) | 0 (0%) | <0.001 | 7 (50%) | 0 (0%) |
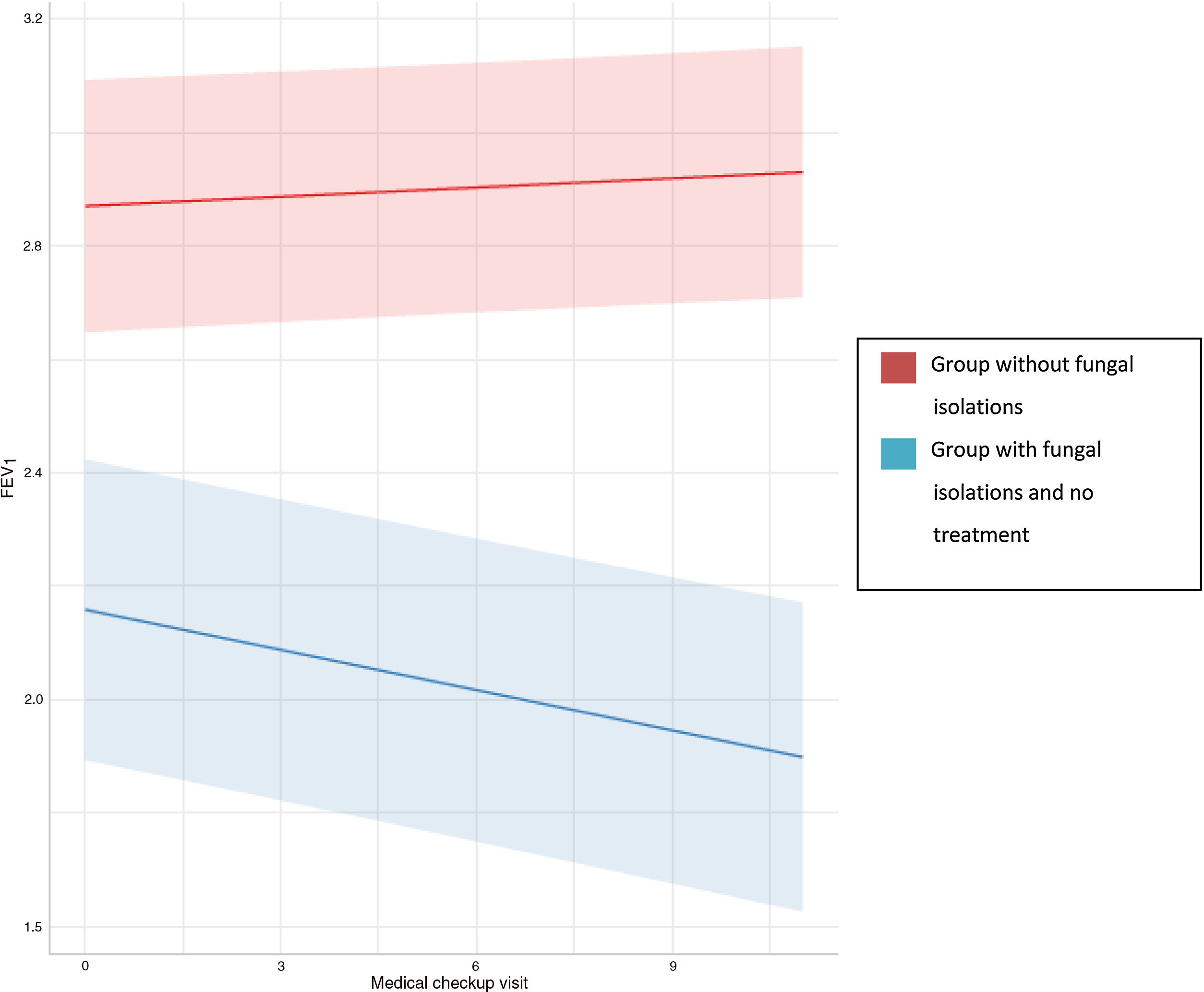
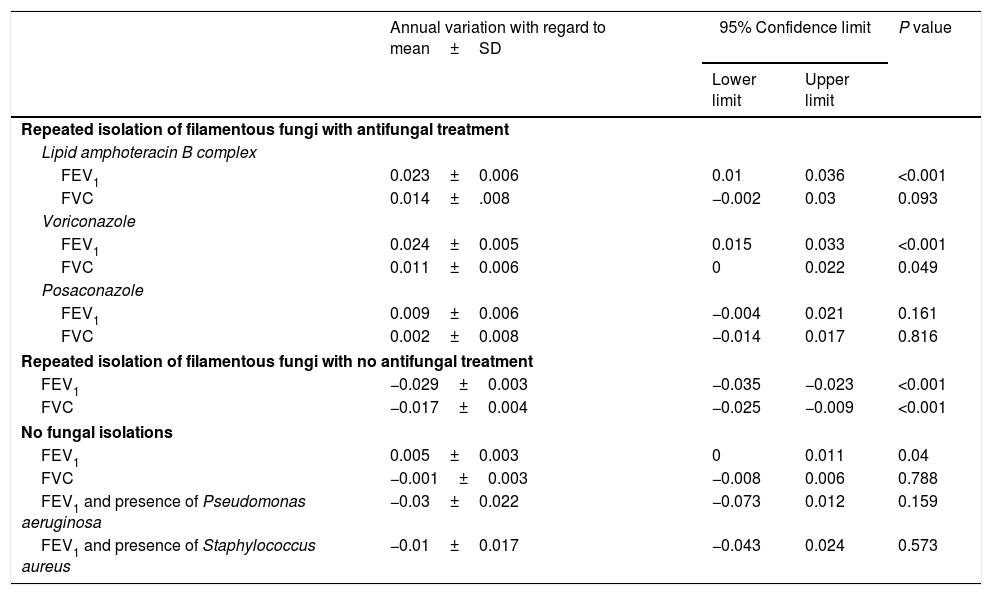
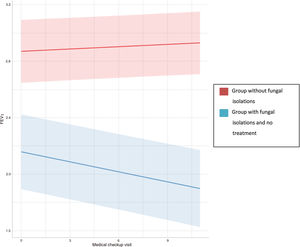
Regression analyses were used to study the evolution of FEV1 and FVC in the three groups (Table 4). In the group with no fungal colonization, we observed an annual increase in FEV1 of 0.005 litres, which was statistically significant, and an annual decrease of 0.001 litres of FVC, which was not statistically significant. In contrast, in patients with a presence of filamentous fungi in the airway who had not received antifungal treatment, an annual drop in FEV1 and FVC of 0.029 and 0.017 litres, respectively, was observed, which was statistically significant in both cases (Fig. 2). This means that the drop in FEV1 in patients colonised by filamentous fungi without antifungal treatment in our study was 1.34% per year, and 0.52% in the case of FVC. In the control group an increase of 0.18% per year in FEV1 and a drop in FVC of 0.027% was registered.
Impact of the different antifungal treatments and concomitant infections in functional respiratory evolution. FEV1: maximum expiratory volume in the first second (values in litres); FVC: volume of exhaled air during a maximal aspiration (values in litres).
| Annual variation with regard to mean±SD | 95% Confidence limit | P value | ||
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Repeated isolation of filamentous fungi with antifungal treatment | ||||
| Lipid amphoteracin B complex | ||||
| FEV1 | 0.023±0.006 | 0.01 | 0.036 | <0.001 |
| FVC | 0.014±.008 | −0.002 | 0.03 | 0.093 |
| Voriconazole | ||||
| FEV1 | 0.024±0.005 | 0.015 | 0.033 | <0.001 |
| FVC | 0.011±0.006 | 0 | 0.022 | 0.049 |
| Posaconazole | ||||
| FEV1 | 0.009±0.006 | −0.004 | 0.021 | 0.161 |
| FVC | 0.002±0.008 | −0.014 | 0.017 | 0.816 |
| Repeated isolation of filamentous fungi with no antifungal treatment | ||||
| FEV1 | −0.029±0.003 | −0.035 | −0.023 | <0.001 |
| FVC | −0.017±0.004 | −0.025 | −0.009 | <0.001 |
| No fungal isolations | ||||
| FEV1 | 0.005±0.003 | 0 | 0.011 | 0.04 |
| FVC | −0.001±0.003 | −0.008 | 0.006 | 0.788 |
| FEV1 and presence of Pseudomonas aeruginosa | −0.03±0.022 | −0.073 | 0.012 | 0.159 |
| FEV1 and presence of Staphylococcus aureus | −0.01±0.017 | −0.043 | 0.024 | 0.573 |
Regarding the group under antifungal treatment, five patients (12.5%) received inhaled amphotericin B lipid complex, 14 patients (35%) received oral voriconazole and seven patients (17.5%) oral posaconazole following the same therapeutic protocol schedule.28 In all treated cases a functional improvement was observed with regard to their baseline status, which was statistically significant in the cases treated with inhaled amphotericin B lipid complex and voriconazole (Table 3).
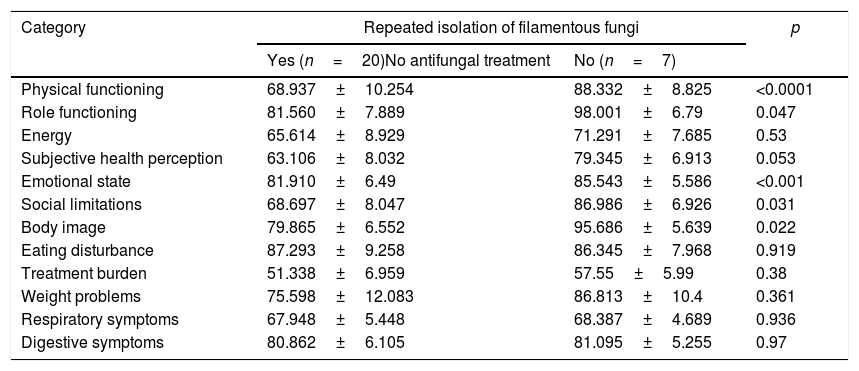
No relevant adverse effects took place during treatment administration, nor were there any major changes in liver function, and all the isolates were sensible to the antifungal treatment administered. The mean of plasma concentrations of voriconazole was 3.1 mg/ml. In 27 patients (20 from the group with chronic colonization by filamentous fungi who did not receive antifungal treatment and seven from the control group) the quality of life was measured through a questionnaire. A significant drop in quality of life was observed in the group of colonized patients in four sections out of the 12 in the questionnaire: “physical ability”, “limitations of role”, “emotional state” and “social isolation” (Table 5).
Scores on the CFQ-R and the statistical significance when comparing the group with repeated isolation of filamentous fungi that did not receive treatment and the group without fungal isolations. Each category is scored from 0 to 100, with higher scores corresponding to better quality of life.
| Category | Repeated isolation of filamentous fungi | p | |
|---|---|---|---|
| Yes (n=20)No antifungal treatment | No (n=7) | ||
| Physical functioning | 68.937±10.254 | 88.332±8.825 | <0.0001 |
| Role functioning | 81.560±7.889 | 98.001±6.79 | 0.047 |
| Energy | 65.614±8.929 | 71.291±7.685 | 0.53 |
| Subjective health perception | 63.106±8.032 | 79.345±6.913 | 0.053 |
| Emotional state | 81.910±6.49 | 85.543±5.586 | <0.001 |
| Social limitations | 68.697±8.047 | 86.986±6.926 | 0.031 |
| Body image | 79.865±6.552 | 95.686±5.639 | 0.022 |
| Eating disturbance | 87.293±9.258 | 86.345±7.968 | 0.919 |
| Treatment burden | 51.338±6.959 | 57.55±5.99 | 0.38 |
| Weight problems | 75.598±12.083 | 86.813±10.4 | 0.361 |
| Respiratory symptoms | 67.948±5.448 | 68.387±4.689 | 0.936 |
| Digestive symptoms | 80.862±6.105 | 81.095±5.255 | 0.97 |
The study of respiratory infections in patients with CF has been focused for decades on bacterial infections, mainly P. aeruginosa, and on their impact in the natural evolution of the disease16; fungi remained secondary, generally considered as mere spectators of the chronic bronchial infection. Thanks to the development of molecular techniques, mostly metagenomics, it is possible to quickly and simply identify multiple pathogens comprising the microbiota of the airways of patients with respiratory diseases such as CF,29 and this is helpful in the therapeutic management of these patients. This study is a prospective and long-to-medium term analysis of the impact of colonization by filamentous fungi in the evolution of CF. The presence of filamentous fungi in patients with CF is associated with a significant, persistent drop in lung function in follow-up time.
Previous studies have already demonstrated an association between the presence of yeasts in the airway of CF patients and some respiratory impairment.10,13,21,27 Amin et al. also demonstrated the relationship between A. fumigatus and poorer lung function, although the association between A. fumigatus and P. aeruginosa were related to low lung function.3 In our series, concomitant bacterial infections themselves do not justify the loss of annual lung function, although patients infected by P. aeruginosa presented with a FEV1 which was lower at the start of the study. In our study, patients chronically colonized by filamentous fungi had a poorer lung function, a greater number of pulmonary exacerbations, more hospital stays and more oral antibiotic cycles. Furthermore, they suffered, with a higher frequency, diabetes, hepatopathy and a poorer nutritional status, comorbidities which led to the impairment of their immune system and increased the risk of the presence of fungi in the airway, which was associated with a poorer prognosis in CF evolution.25 In contrast, in one prospective series focused on the role of Scedosporium, no significant differences were found in either FEV1 or in the number of annual exacerbations among patients infected by this fungus, compared with those who were not, although this study did not have a three-year follow-up period.22 Co-infection with Pseudomonas could account for the higher number of exacerbations, hospital admissions and more prolonged intravenous treatment times. In fact, in regular clinical practice, when there is a functional respiratory impairment or clinical impoverishment along with bacterial isolation in sputum, treatment is usually directed against bacteria, even although a fungus is also isolated in the respiratory sample.11 For this reason, these patients may receive a greater number of antibiotic cycles without the expected clinical and/or functional improvement, since no treatment against the fungus that could be involved in the pulmonary exacerbation process is administered. Furthermore, the quality of life of these patients is impaired in “physical ability” and “emotional state” aspects, and these concepts are directly related to the survival of these patients.1,5,24
One relevant fact in this study is that it proved that chronic antifungal treatment slows down the annual drop in the pulmonary function of these patients. In fact, antifungal therapy leads to slight improvement of the FEV1 in patients who are chronically colonized. It is important to point out that both inhaled amphotericin B and azoles had a positive impact on lung function. This beneficial effect was less evident in patients treated with oral posaconazole, possibly because they were in a more serious condition (higher number of pulmonary exacerbations and intravenous antibiotic cycles), but this data should be interpreted with caution, as antifungal concentrations in blood were not monitored during follow-up.7,20
The strength of our study comes from its prospective, highly homogenous design in therapeutic protocols and with a follow-up of three years. However, the small sample size analyzed prevents us from extrapolating our findings to all patients with CF, being more studies to confirm our data necessary. Based on our findings we may state that the presence of a chronic filamentous fungus in the airway of a patient with CF steers the disease towards a more torpid evolution. In fact it is associated with factors of severity, such as the need for a higher number of intravenous antimicrobial treatment cycles, an increase in the number of hospital admissions, greater impairment of pulmonary function and poorer quality of life, all of which are related, per se, with poorer prognosis and higher mortality. Furthermore, strict follow-up is particularly important if the patient colonized by filamentous fungi suffers from diabetes, hepatopathy, haemoptysis or undernutrition.14 This study is supportive of the little existing evidence on the validity of establishing an antifungal treatment to slow down the annual drop in pulmonary function, particularly in the patient with a more advanced CF.
FinancingThis study was partially financed by a grant awarded by the Spanish Society of Cystic Fibrosis in the year 2012.
Conflict of interestsThe authors have no conflict of interests to declare directly or indirectly related to the contents of this manuscript.