β-1,3-Glucan is important for infective forms (mycelial phase) of Histoplasma capsulatum and shares many features allotted to pathogen-associated molecular patterns. These cell wall carbohydrates interact with phagocytes by binding to Toll and lectin-like receptors, present on cell surfaces of macrophages, neutrophils, and dendritic cells. This review focuses on recent findings of the major H. capsulatum and host carbohydrate-driven interactions that account for internalization of fungal infective forms into phagocytes, and its subsequent avoidance of intracellular elimination. The yeast phase of H. capsulatum possesses different modulating factors of the macrophagic-anti-fungal mechanisms, mainly α-1,3-glucan, which is considered relevant for virulence.
This manuscript is part of the series of works presented at the “V International Workshop: Molecular genetic approaches to the study of human pathogenic fungi” (Oaxaca, Mexico, 2012).
El β-1,3-glucano es importante para las formas infectivas (fase micelial) de Histoplasma capsulatum y comparte varias características asignadas a los patrones moleculares asociados con patógenos. Estos hidratos de carbono de la pared celular interaccionan con los fagocitos uniéndose a receptores tipo Toll y tipo lectina, que están presentes en las superficies celulares de macrófagos, neutrófilos y células dendríticas. En esta revisión se presta atención a los hallazgos recientes sobre las principales interacciones entre H. capsulatum y las células del huésped mediadas por hidratos de carbono, que permiten la internalización de las formas infectivas del hongo por los fagocitos, así como la posterior evitación de su eliminación intracelular. Se discuten los datos experimentales relevantes publicados recientemente. La fase de levadura de H. capsulatum incluye distintos factores moduladores de los mecanismos de macrófagos y antifúngicos, sobre todo el α-1,3-glucano, que se considera relevante para la virulencia.
Este artículo forma parte de una serie de estudios presentados en el «V International Workshop: Molecular genetic approaches to the study of human pathogenic fungi» (Oaxaca, México, 2012).
Pathogens recognition is commonly based on the identification of molecular patterns by distinct receptors and subsequent activation of signaling pathways, which allow the defense reaction to fend off invading microorganisms. Pathogen fungi have several molecular components in the cell wall that play a critical role in their pathogenicity mechanisms. Indeed, cell wall contains adhesins and a broad number of receptors interacting with environmental factors that, after activation, start a complex network of downstream intracellular signals.14 The dimorphic fungal pathogen Histoplasma capsulatum var. capsulatum causes the respiratory and systemic disease, histoplasmosis.27 Like most systemic-disease-causing fungal pathogens, this infection is acquired via the respiratory tract by inhalation of infective propagules, mainly microconidia, hypha fragments, and spores produced during the mycelial phase of this fungus.29,59 Target organs are lungs, where alveolar macrophages and dendritic cells (DCs) are responsible for phagocytosis of infective forms, establishing the link with the adaptive immune response.13,32 Dimorphic switching from the mycelial phase (M-morphotype) to yeast phase (Y-morphotype), occurs within alveolar macrophages.25 Infective M-morphotype is considered a non-virulent phase, whereas Y-morphotype is the parasitic-virulent phase of H. capsulatum. Extensive changes in gene expression between these two phases are directly responsible for the behavior of each morphotype.63 Cell wall undergoes drastic changes in carbohydrate polymers (polysaccharides) composition by switching from one to the other morphotype.24,39
Polysaccharides constitute about 80% of H. capsulatum cell wall components, and they play an important role in biology and pathogenicity of the fungus.28,38 Polysaccharides of the cell wall of H. capsulatum can be classified as glucans, they are d-glucose polymers linked by α- or β-glycosidic bonds. Both α- and β-glucans are present in cell walls of M- and Y-morphotypes. Chitin is a lineal glycan composed of β-1,4 N-acetyl-d-glucosamine and mannans are lineal polymers of mannose usually linked to proteins (mannoproteins), and both can be either homo- or co-polymers.10,17,18,20,35 It has been suggested that glucans and mannans interfere with the innate immune response. Glucans and mannans are recognized by lectin-like receptors of phagocytic cells, and they induce beneficial signals that can promote fungus survival and favor infection; although the specific mechanisms involved in this process are not yet completely defined.48
Chemical and ultra-structural studies of cell walls of the Y-morphotype have revealed higher amounts of α-glucan than β-glucan, chitin, and co-polymer galactomannan. In contrast, the M-morphotype contains higher amounts of β-glucan and galactomannan, and has less α-glucan content.29,35
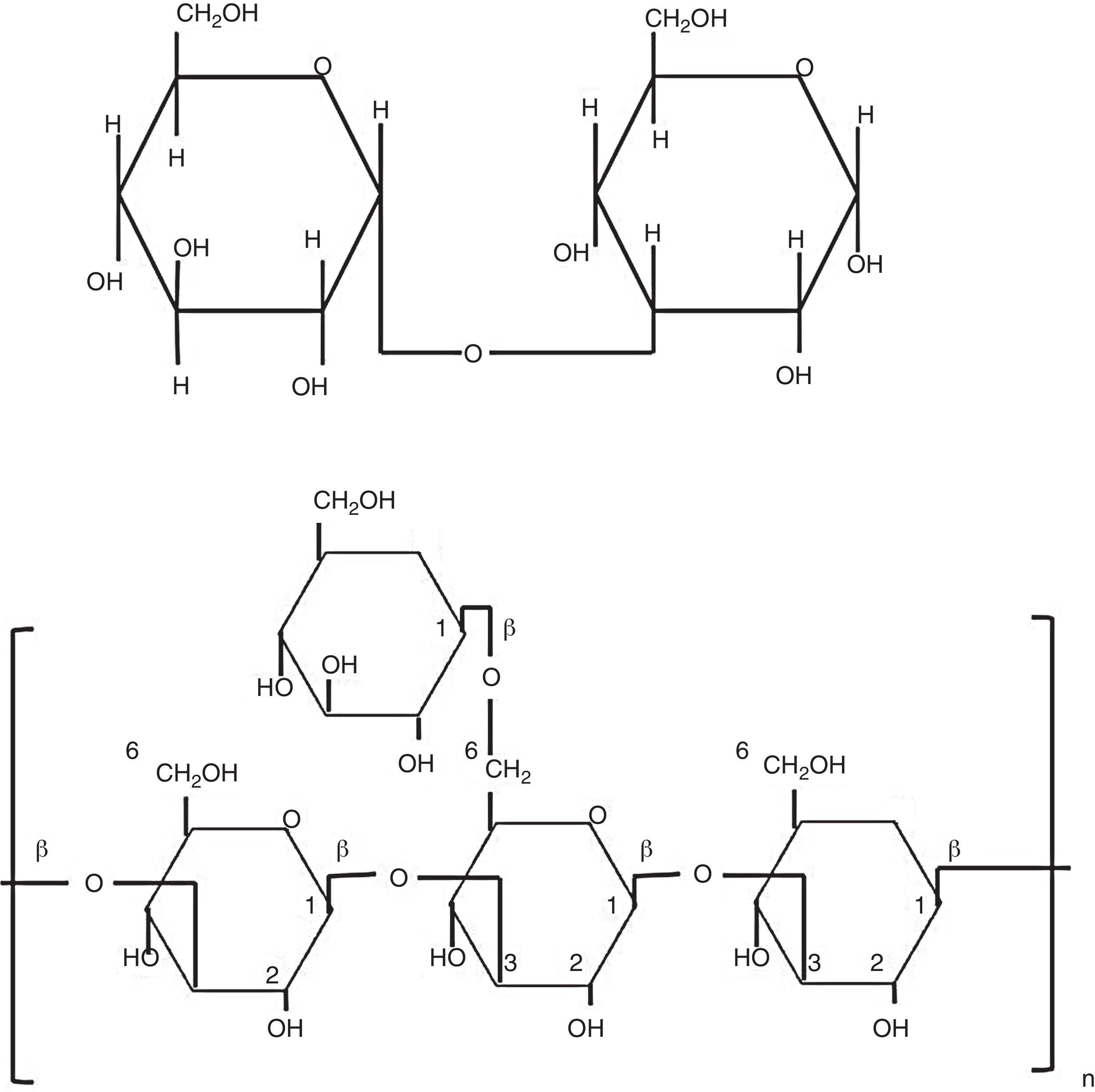
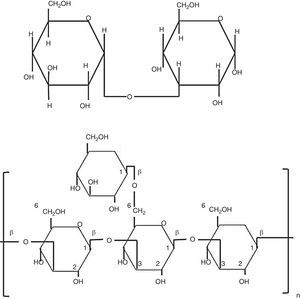
Biological properties of glucansCarbohydrates perform diverse and important biological functions, such as energy storage molecules or as structural components of the cell wall. Polysaccharides can reach a high degree of polymerization. Glucans are d-glucose polymers linked by glycosidic bonds involving the anomeric hydroxyl group (OH), rendering α- or β-configurations (Fig. 1).10 Differences between β-glucan bonds and the chemical structure related to their solubility and biological activities are very important. Fungal β-glucans are composed by glucose units connected by β-1,3 or β-1,6 linkages (Fig. 1). Spatial arrangement of β-1,4-glucan is present in structural polysaccharides of some fungal cell walls.2,5 These straight chains are transversally linked by hydrogen bonds and turn into parallel structures. On the other hand, α-1,4-glucan brings on helicoidal assemblies, and both α- and β-1,6 bonds, generate flexible and relaxed structures.5,10
Glycosidic β-1,3 and β-1,6 bonds yield insoluble molecules, thus, they exhibit higher biological activity than soluble β-1,4-glucan.60 Biological and immunological characteristics of β-glucans are sustained on molecular and structural features, such as polymer length, branching degree, and kind of glycosidic bond. As long as β-glucans have more β-1,3 chains, branched with d-glucose attached to position β-1,6, they reach the most active form. Interestingly, length of side chains, more than length of main chain, determines the β-glucans activity on the immune system,60 and some pathogen fungi, such as Pneumocystis carinii, Saccharomyces cerevisiae, and Candida albicans, have both type of glycosidic bonds, β-1,3 and β-1,6.30,41,61 Commercially available β-glucans from different biological sources have been used as adjuvant to stimulate innate and adaptive immune responses and in the control of some diseases.55
Insoluble β-glucans derived from yeasts activate DCs and macrophages through the Dectin-1 receptor, promoting TH1, CD4+ T-lymphocytes response. This type of response is characterized by activation of cytotoxic effectors against intracellular infections or neoplastic cells; thus, these molecules have been used widely in anti-tumoral therapy. In spite that soluble β-glucans can bind to macrophages and DCs, they interact with receptors other than the Dectin-1, in such way, they do not activate the immune response and, hence, they lack any therapeutic effect.52
Insoluble forms of glucans, mainly in α-1,4 or α-1,6 bonds, activate pro-inflammatory cytokines and hydrogen peroxide production in macrophages.9 α-Glucans are crucial for yeast cell wall function, and they play an important role in the virulence of several pathogenic fungi, like Aspergillus fumigatus, H. capsulatum, Cryptococcus neoformans, and Blastomyces dermatitidis.2,4,12 It is clear now that α-glucan of B. dermatitidis is vital for the yeast-phagocyte interaction.33 In this sense, Paracoccidioides brasiliensis shows a change from β- to α-glucan when cells convert from mycelial into yeast phase.4,57
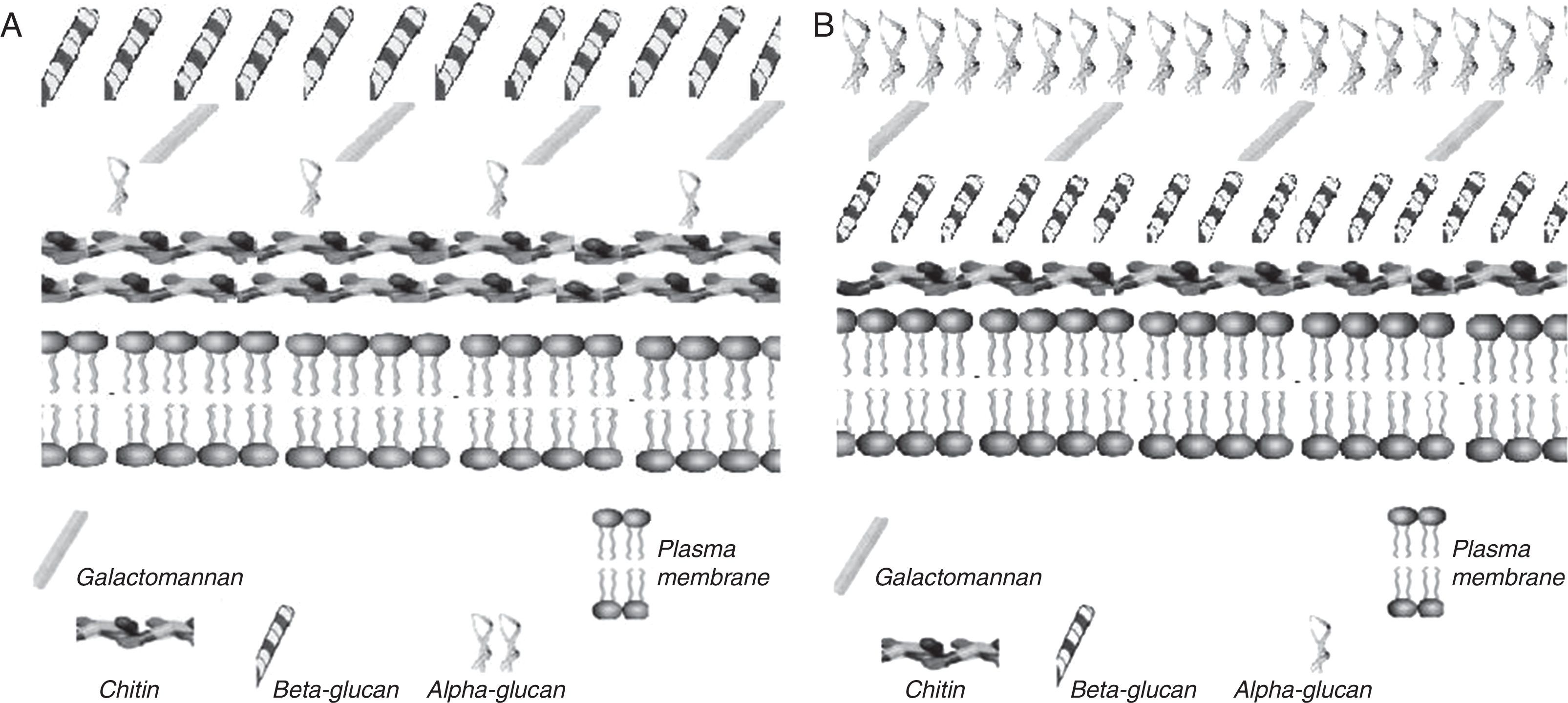
Several important aspects are related with α-1,3-glucan in the cell wall of H. capsulatum, this glucan is associated with virulence,21–23,34 because it contributes to intracellular latency of the fungus, probably by protecting yeasts inside phagolysosomes,21 and regulating proliferation of Histoplasma within macrophages38; furthermore, it reduces tumor necrosis factor alpha (TNF-α) production by infected cells.53 Unlike the α-1,3-glucan of H. capsulatum, the insoluble α-glucans activate macrophages to produce pro-inflammatory cytokines such as interleukin-8 (IL-8) and TNF-α, which participate in the pathogenesis of disease.48 Polysaccharides in the cell wall of H. capsulatum have different arrangements depending on the morphotype. According to soluble polysaccharides and chitin content, the Y-morphotype of H. capsulatum is classified as chemotype I and II.18,19 Chemotype I cell walls are characterized by lower content of soluble material and higher chitin content than chemotype II. On the other hand, the Y-morphotype has an external coat of α-1,3-glucan, masking an internal envelope rich in immunostimulatory β-glucan and chitin (Fig. 2).20,24 In general, for H. capsulatum chemotype II, the M-morphotype contains β-1,3-glucan, higher proportions of mannose, and lower chitin content when compared to yeast phase.18,19 The molecular organization of cell walls of H. capsulatum is shown in Fig. 2. Finally, an interesting feature of H. capsulatum is that it can regulate synthesis of α-1,3-glucan, depending on the environmental conditions.38
A schematic view of H. capsulatum cell wall. (A) M-morphotype; (B) Y-morphotype, chemotype II. Note important disproportion in α-, β-glucans, and chitin contents, as well as their differential distribution within the cell wall. The figure was constructed based on data from Guimaraes et al.,28 in accordance with major H. capsulatum cell wall components.
Success of pathogenic fungi depends on the capacity of the host innate immune response to recognize fungal cell wall components, like glucans. The host innate immune system responds through the pattern recognition receptors. Structurally, β-glucans are greatly conserved among fungi, and share many of the characteristics assigned to pathogen-associated molecular patterns (PAMPs). It is known that some PAMPs located on the cell wall of fungi interact with macrophages and DCs, by binding to Toll-like receptors (TLR), for example TLR2 and TLR4.44,45,54 Even though, these receptors do not participate in phagocytosis, they can trigger different cellular signals. PAMPs are also recognized by diverse carbohydrate receptors known as lectin-like receptors, located on the cell surfaces of macrophages, neutrophils, and DCs. After activation, they stimulate the inflammatory immune response. The most important lectin-like receptors recognizing β-glucan of H. capsulatum are: complement receptor 3 (CR3 or CD11b/CD18) and the lectin-like receptor associated to dendritic cells type1 (Dectin-1).6,7,16,26,33,52 CR3 has two recognition domains, one is for C3bi binding and the other binds carbohydrates like β-glucans. When microorganisms are opsonized with C3bi, C3bi binds to the CD11b domain, while β-glucan binds to the C-terminal of the CD18 domain; afterwards, fungal cells are phagocytized. Macrophages kill these microorganisms through usual microbicidal mechanisms.62
The function of Dectin-1 is still controversial in host defense against pathogenic fungi. This receptor can mediate interactions with different fungi, such as Candida, Aspergillus, Pneumocystis, and Coccidioides.28 Different ligands have been reported for Dectin-1, one of them is the fungal β-glucan. When this polysaccharide activates immune system cells, reactive oxygen species (ROIs) are produced, and transcriptional factor NF-κB is translocated to the nucleus and increases expression of pro-inflammatory cytokines.41
Dectin-1 has extracellular domains for carbohydrates recognition, mainly β-glucans, and a transducer domain facing the cytoplasm, which shares an activation motif with the tyrosine immune receptors.56
In general, macrophages stimulated by fungal β-glucans, secrete pro-inflammatory cytokines, such IL-1 and IL-6, TNF-α, macrophage inflammatory protein-2 (MIP-2), ROIs, and eicosanoids.31,55 In addition, β-glucans directly participate in the inflammatory process by recruiting leucocytes.44 Other important properties of β-glucans include: stimulation of hematopoietic stem cells, activation of alternative complement pathway, and also the activation of B and T lymphocyte, macrophages, DCs, and NK cells. In this sense, the involvement of β-glucans in stimulation of anti-tumor responses has been reported recently, which is an interesting topic for further investigation.46,51 The Y-morphotype of H. capsulatum is well adapted to the intracellular environment, and it has different ways to avoid macrophagic anti-fungal mechanisms; one of the most important is the α-glucan of the cell wall.53 As we mentioned before, there are important differences between M- and Y-morphotypes of H. capsulatum regarding its capacity to induce or not an inflammatory response, and these differences can be explained, in part, by the type and amounts of glucans in the fungal cell wall.8,58 α-1,3-Glucan, is the less abundant form of α-glucans in microorganisms. In H. capsulatum, virulence is multifactorial; however, there are some virulent strains of H. capsulatum chemotype I that lack α-1,3-glucan in their cell walls.20 Yeast chemotype II has the α-1,3-glucan located in the most external face of the cell wall, and this molecule is associated with pathogenicity mechanisms not well-determined yet.20,54 Nevertheless, in the Y-morphotype of H. capsulatum, evidence has been found regarding the role of α-1,3-glucan in virulence by avoiding β-1,3-glucan recognition by a β-glucan receptor (Dectin-1).52,53 In fact, this mechanism could explain blockade of β-glucan interaction with Dectin-1 and, consequently, abrogation of the host innate immune response.
So far, a specific receptor for α-1,3-glucan has not been identified. However, interestingly, Bittencourt et al.3 demonstrated that α-glucan from Pseudallescheria boydii induces TNF secretion by cells of the innate immune system in a mechanism involving TLR2, MyD88, and CD14. Morphotypes of H. capsulatum have an impact on the particular type of immune response developed by the host. Histoplasmosis depends on the balance between responses mediated by T-helper lymphocytes Th1 characterized by high IFN-γ production, which reinforces a cellular immune response associated to control the infection process, and Th2 linked to IL-4 production together with elevated antibodies production, and this correlates with the severity of the infection.1,25,43,47
In murine models, IL-17 favored elimination of H. capsulatum. In contrast, IL-23 could extend mice survival in absence of IL-12.13–15 Th17 response has been associated to protective mechanisms against several microorganisms, including fungi.11,36 Receptors implicated in this response include lectin-like receptors, such as Dectin-1 and Dectin-2, DC-SIGN, CLEC-1, and CD161.12,56 Th17 cells show ability to produce IL-17 and a number of pro-inflammatory cytokines, such as TGFβ, IL-1β, IL-6, IL-21, IL-22, and IL-23. It is possible that IL-17, which also displays pro-inflammatory attributes,40,49 regulate the equilibrium of Th1 along with Th2 responses,37,42,50 favoring elimination of the intracellular infection by H. capsulatum.
Concluding remarksMajor polysaccharides of the fungal cell wall are chitin, β-glucans, and α-glucans. Chitin and β-glucans seem to participate in keeping structural integrity of fungi5; however, the precise role of α-glucan is still controversial.
The most virulent yeast phase of H. capsulatum contains high amounts of α-glucans corresponding to 35–46% of total carbohydrates of the cell wall, whereas in the M-morphotype and in some Y-morphotype cells, the amount of this polysaccharide is minimal. Nevertheless, controversial results have been reported in P. brasiliensis strains, because virulence increases with α-glucan content.58 Pathogenicity of H. capsulatum does not depend solely on any alteration of the immunologic status of the host, as Candida and other opportunistic fungi do. Instead, H. capsulatum exerts virulence factors endorsing survival, by avoiding the immune-based antifungal mechanisms. In part, this successful performance is due to properties related to the polysaccharide α-1,3-glucan. This molecule can modulate the host immune response developed against H. capsulatum, and is associated also to the ability of yeasts to survive inside immune cells. Structural changes of the cell wall of H. capsulatum in the course of infection are a critical aspect during host-cells invasion process and the whole host–parasite relationship. More precise characterization of the role of fungal glucans in the clinical course of histoplasmosis is a central subject of future research.
Conflict of interestsThe authors declare that they have no conflict of interests.