Alternaria alternata causes the Alternaria brown spot disease (ABS) in many tangerines and their hybrids worldwide. Plant extracts offer an alternative method for controlling this disease, which control is based on chemical fungicides.
AimsTo identify plant species with antifungal properties against A. alternata, the causal agent of the ABS.
MethodsPlant extracts prepared from leaves, barks, flowers, and stalks collected from 105 plant species in the State of Minas Gerais, Brazil, were tested for activity against the fungus A. alternata in vitro and in vivo.
ResultsThe most promising extract was obtained from Anadenanthera colubrina, which reduced the disease on Murcott tangor fruits to levels obtained with commercial fungicides. Artemisia annua, Cariniana estrelensis, Ficus carica, and Ruta graveolens presented moderate in vitro antifungal activity, but no effects were observed on the disease when the extracts were applied to fruits inoculated with the fungus. Besides, A. colubrina was the most active extract against A. alternata in the in vitro assay.
ConclusionsThe results obtained in the in vitro and in vivo assays suggested that the fungal growth test, which uses 96-well polypropylene plates, seems to be appropriate for selecting potential plant species for testing new methods to control ABS.
Alternaria alternata causa la mancha marrón en muchas mandarinas y en sus híbridos en todo el mundo. Extractos de plantas proporcionan un método alternativo para controlar esta enfermedad cuyo control se basa en fungicidas químicos.
ObjetivosIdentificar las especies de plantas con propiedades antifúngicas contra A. alternata, el agente causal de la mancha marrón.
MétodosExtractos de plantas preparados a partir de hojas, corteza, flores y tallos recogidos de 105 especies de plantas en el Estado de Minas Gerais, Brasil, fueron utilizados para estudiar su actividad contra el hongo A. alternata in vitro e in vivo.
ResultadosEl extracto más prometedor se obtuvo de Anadenanthera colubrina, que redujo la enfermedad en las frutas de tangor Murcott a los niveles obtenidos con fungicidas comerciales. Artemisia annua, Cariniana estrelensis, Ficus carica y Ruta graveolens presentaron moderada actividad antifúngica in vitro, pero no se observaron efectos sobre la enfermedad cuando los extractos fueron aplicados a los frutos inoculados con el hongo. Además, A. colubrina fue el más activo contra A. alternata en el ensayo in vitro.
ConclusionesLos resultados obtenidos en los ensayos in vitro e in vivo sugieren que el método de crecimiento de hongos, que utiliza placas de 96 pozos de polipropileno, parece apropiado para la selección de especies potenciales para testar nuevos métodos de control de la mancha marrón.
Alternaria brown spot (ABS) is an important disease of many tangerines and their hybrids in humid and semiarid areas worldwide.23 In Brazil, ABS was first found in the State of Rio de Janeiro and then became widespread in the States of São Paulo and Minas Gerais, the main citrus growing areas in Brazil.16,17 The disease has caused economical losses to Murcott tangor (probable Citrus sinensis [L.] Osb. x C. reticulata Blanco hybrid) producers.15 The causal agent of this disease is the fungus Alternaria alternata (Fr:Fr) Keissl f. sp. citri, which can attack both leaves and fruits, reducing the yield. Specifically on fruits, which are very susceptible to infections according to Vicent et al.,25 lesions varying from small dark necrotic spots to large sunken pockmarks turn them unmarketable, further increasing the producers losses.24
In order to produce fruits with good external quality in areas affected by ABS, the application of foliar fungicides is usually necessary to reduce the losses caused by this disease. When the environmental conditions are adequate to the development of the fungus, up to 15 fungicide applications might be necessary to control ABS.23 Thus, the production costs are increased and contamination of foods and environment with toxic substances also occur.11 Consequently, the development of more efficient, cheaper, and less toxic products active against A. alternata is necessary.3,10
One of the potentially useful alternatives to expensive and possibly toxic fungicides could be the use of plant extracts. They have presented very promising results on the control of plant pathogens.22 For example, Zimmu (Allium cepa L. x Allium sativum L.) extracts efficiently reduced the mycelial growth of Alternaria solani Sorauer.9 Tegengne et al21 conducted a screening program from which they selected plants with antifungal properties and the extract of Agapanthus africanus (L.) Hoffm was shown to be the most potent against Mycosphaerella pinodes, the causal agent of black spot in pea.
In the present study our goal was to identify plant species with antifungal properties against Alternaria alternata, the causal agent of the ABS disease in many tangerines and their hybrids worldwide. Therefore, we tested 105 plant species and 18 were selected for additional assays.
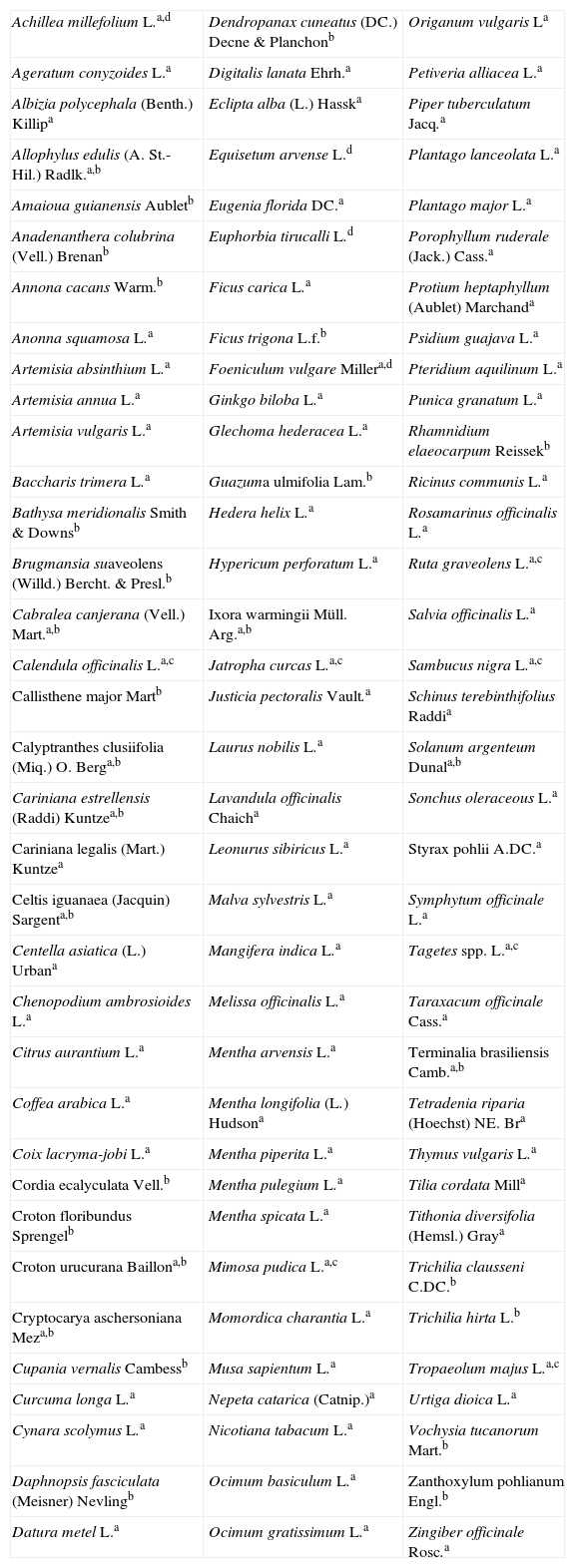
Materials and methodsPlant extractsThe 105 plant species were collected in Minas Gerais State, Brazil. They were identified by comparison with specimens available in the Herbarium ESAL-Universidade Federal de Lavras, during the year of 2005 (Table 1). Leaves, barks, flowers, and stalks were oven-dried for 48h at 40°C. After grounding plant materials to pieces smaller than 2mm, part (20g) of each sample was immersed into methanol (50ml) for 48h at room temperature (15 to 30°C). The resulting mixtures were filtrated through cotton wool plugs and the residues were extracted twice with more methanol. The three liquid phases obtained from each plant material were combined and concentrated to dryness in a rotary evaporator. The resulting 126 dry extracts were freeze-dried and stored at −10°C for future use in the experiments.
Plant species collected in Minas Gerais State (Brazil) and used in the present study.
| Achillea millefolium L.a,d | Dendropanax cuneatus (DC.) Decne & Planchonb | Origanum vulgaris La |
| Ageratum conyzoides L.a | Digitalis lanata Ehrh.a | Petiveria alliacea L.a |
| Albizia polycephala (Benth.) Killipa | Eclipta alba (L.) Hasska | Piper tuberculatum Jacq.a |
| Allophylus edulis (A. St.-Hil.) Radlk.a,b | Equisetum arvense L.d | Plantago lanceolata L.a |
| Amaioua guianensis Aubletb | Eugenia florida DC.a | Plantago major L.a |
| Anadenanthera colubrina (Vell.) Brenanb | Euphorbia tirucalli L.d | Porophyllum ruderale (Jack.) Cass.a |
| Annona cacans Warm.b | Ficus carica L.a | Protium heptaphyllum (Aublet) Marchanda |
| Anonna squamosa L.a | Ficus trigona L.f.b | Psidium guajava L.a |
| Artemisia absinthium L.a | Foeniculum vulgare Millera,d | Pteridium aquilinum L.a |
| Artemisia annua L.a | Ginkgo biloba L.a | Punica granatum L.a |
| Artemisia vulgaris L.a | Glechoma hederacea L.a | Rhamnidium elaeocarpum Reissekb |
| Baccharis trimera L.a | Guazuma ulmifolia Lam.b | Ricinus communis L.a |
| Bathysa meridionalis Smith & Downsb | Hedera helix L.a | Rosamarinus officinalis L.a |
| Brugmansia suaveolens (Willd.) Bercht. & Presl.b | Hypericum perforatum L.a | Ruta graveolens L.a,c |
| Cabralea canjerana (Vell.) Mart.a,b | Ixora warmingii Müll. Arg.a,b | Salvia officinalis L.a |
| Calendula officinalis L.a,c | Jatropha curcas L.a,c | Sambucus nigra L.a,c |
| Callisthene major Martb | Justicia pectoralis Vault.a | Schinus terebinthifolius Raddia |
| Calyptranthes clusiifolia (Miq.) O. Berga,b | Laurus nobilis L.a | Solanum argenteum Dunala,b |
| Cariniana estrellensis (Raddi) Kuntzea,b | Lavandula officinalis Chaicha | Sonchus oleraceous L.a |
| Cariniana legalis (Mart.) Kuntzea | Leonurus sibiricus L.a | Styrax pohlii A.DC.a |
| Celtis iguanaea (Jacquin) Sargenta,b | Malva sylvestris L.a | Symphytum officinale L.a |
| Centella asiatica (L.) Urbana | Mangifera indica L.a | Tagetes spp. L.a,c |
| Chenopodium ambrosioides L.a | Melissa officinalis L.a | Taraxacum officinale Cass.a |
| Citrus aurantium L.a | Mentha arvensis L.a | Terminalia brasiliensis Camb.a,b |
| Coffea arabica L.a | Mentha longifolia (L.) Hudsona | Tetradenia riparia (Hoechst) NE. Bra |
| Coix lacryma-jobi L.a | Mentha piperita L.a | Thymus vulgaris L.a |
| Cordia ecalyculata Vell.b | Mentha pulegium L.a | Tilia cordata Milla |
| Croton floribundus Sprengelb | Mentha spicata L.a | Tithonia diversifolia (Hemsl.) Graya |
| Croton urucurana Baillona,b | Mimosa pudica L.a,c | Trichilia clausseni C.DC.b |
| Cryptocarya aschersoniana Meza,b | Momordica charantia L.a | Trichilia hirta L.b |
| Cupania vernalis Cambessb | Musa sapientum L.a | Tropaeolum majus L.a,c |
| Curcuma longa L.a | Nepeta catarica (Catnip.)a | Urtiga dioica L.a |
| Cynara scolymus L.a | Nicotiana tabacum L.a | Vochysia tucanorum Mart.b |
| Daphnopsis fasciculata (Meisner) Nevlingb | Ocimum basiculum L.a | Zanthoxylum pohlianum Engl.b |
| Datura metel L.a | Ocimum gratissimum L.a | Zingiber officinale Rosc.a |
Part of plant: aleaves; bbarks; cflowers; dstalks.
Fruits exhibiting symptoms of ABS were submitted to the pathogen isolation protocol.5 Thus, pieces (10-25mm2) of washed fruit peels from Murcott tangor were subsequently immersed into 70% ethanol (30-60s), 2% sodium hypochlorite (30-60s) and distilled water (2 x 30s). Four fragments were placed in a Petri dish containing potato-dextrose-agar (PDA). After seven days at 25°C, under a 12h photoperiod, 9mm agar plugs of the medium containing the fungus mycelium were transferred to new PDA plates.18 Then, A. alternata (Fr:Fr) Keissl f. sp. citri was isolated and identified as described by Carvalho et al.5
Production of conidiaFungal plugs (9mm diameter) from the culture medium were transferred to PDA plates, which were maintained for seven days at 25°C under constant illumination provided by Philips daylight fluorescent lamps (20W, TLT, 75RS). The conidia were removed from the PDA plates by adding 10ml of 1% (v/v) Tween 80. The resulting mixture was filtered using sterilized cheesecloth.
Fungal growth assayDried plant extracts (2mg) were subsequently dissolved in 500μl of 1% (v/v) Tween 80 and mixed with 100μl of an A. alternata conidial suspension at 2.6-3.0 x 105 conidia/ml. Twenty microliters of the resulting conidia suspension were poured into each 300μl well of a 96-well polypropylene plate containing 130μl of PDA with terramicin-oxitetraciclin chlorhydrate 500mg (Pfizer, 0.55mg/ml PDA). After three days at 25°C, under a 12h photoperiod, plant extracts that prevented fungal growth were considered active. This experiment was carried out with four replicates, using 1% (v/v) Tween 80 as negative control. Aqueous 3.5mg/ml Dacobre WP (chlorotalonil 250g/kg and copper oxychloride 504g/kg, Iharabras S.A. Chemicals Industries) solution in 1% (v/v) Tween 80, and aqueous 0.16mg/ml Amistar 500 WG (Azoxistrobin 500g/kg, Syngenta Crop Protection) solution in 1% (v/v) Tween 80, were used as positive controls.
Scanning electron microscopyFungal disks treated with the most active extracts (A. colubrina, R. graveolens, and A. annua), and the positive (Dacobre WP, Amistar 500 WG) and the negative controls (1% Tween 80) during the fungal growth assay, were removed from the polypropylene plates and submitted to the procedure described by Bozzola and Russel4 with few adaptations. Each disk was fixed in a modified Karnovsky solution (2.5% glutaraldehyde, 2% paraformaldehyde in a 0.05M sodium cacodylate buffer at pH 7.2 containing 0.001M CaCl2) for 48h. The disks were washed three times with the same buffer for 30min, post-fixed for 2h in a 1% osmium tetroxide solution in 0.05M sodium cacodylate buffer at pH 7.2, and washed three times with distilled water. They were then dehydrated in a gradient series of acetone solutions (25, 50, 75, 90 and 100%) and dried with carbon dioxide in a critical point dryer (Bal-tec CPD 030). Finally, the disks were mounted on aluminum stubs with double-sided tape and coated with a 20nm gold layer by vacuum evaporation (Bal-tec SCD 050). All samples were observed in an Evo40 Leo scanning electron microscope.
Conidial germination assayBased on the fungal growth assay results, a total of 20 plant extracts were selected for the conidial germination assay. Four miligrams of each plant extract were dissolved in 1ml of 1% (v/v) Tween 80 and mixed with 100μl of an A. alternata conidia suspension at 2.6-3.0 x 105 conidia/ml. An aliquot of 520μl of each final suspension was added to 4.0ml of solidified water-agar (WA; 80g of agar and 555mg of tetracycline) medium contained in a 6.0cm Petri dish. After 12h at 25°C, under illumination, conidia were counted and those with the germinative tube length larger or equal to the smaller conidia diameter were considered germinated. This experiment was carried out with four replications (50 conidia each), using an aqueous 1% (v/v) Tween 80 solution as negative control and 3.5mg/ml Dacobre WP and 0.16mg/mL Amistar 500 WG solutions in 1% (v/v) Tween 80 as positive controls.
Mycelial growth assayPlant extracts (20 samples) dissolved (7mg/ml) in 1% (v/v) Tween 80 were poured into 9cm Petri dishes (0.5ml/dish) containing PDA (8ml/dish) with tetracycline (Bunker, 0.55mg/ml PDA). Subsequently, a 9mm PDA disk with a seven-day old A. alternata colony (1.8cm from the colony center) was placed upside down in the center of each Petri dish containing PDA impregnated with plant extract. After seven days at 25°C, under a 12h photoperiod, fungal colony diameters were measured and data were converted into percentage. This experiment was done with three replications, employing 1% (v/v) Tween 80 as negative control and 3.5mg/ml Dacobre WP and 0.16mg/ml Amistar 500 WG solutions in 1% (v/v) Tween 80 as positive controls.
pH measurementsThe pH of 20 plant extracts (2mg) dissolved in 600μl of 1% (v/v) Tween 80 1% were measured by dipping pH-indicator strips (Acilit® pH 0-6 and Neutralit® pH 5-10, Merck) into the solutions and comparing the resulting colors to the standard provided by the manufacturer.
Assay with Murcott tangor fruitsRipe and healthy Murcott tangor fruits were washed with sterilized water and then with 70% ethanol solution. They were let to dry for 60min in a biosafety hood. For injuring the fruits, four points were selected around the point of fruits insertion and, just after, four perforations (3mm deep) were made with a needle in each selected point.5 Each plant extract (2.0mg) was dissolved in 600μl of a 106 conidia/ml in 1% (v/v) Tween 80 and 20μl aliquots were deposited on each selected point. This experiment was carried out with four fruits per treatment. The conidia suspension in 1% (v/v) Tween 80 was used as negative control and mixtures of conidia suspension and the fungicides Dacobre WP (3.5mg/ml) and Amistar 500 WG (0.16mg/ml) as positive controls. Fruits with no conidia, but treated with 20μl of 1% (v/v) Tween 80, plant extract, or fungicide, were also used in this experiment. All fruits were kept in a growth chamber at 25°C under a 12/12 photoperiod. After 8 and 12 days, the lesion diameter around each selected location on fruits was measured with a ruler. Prior to the statistical analysis, data were converted into spots development rate (ABS dr) by dividing the mean value for each fruit by the mean value of all fruits treated only with conidia in 1% (v/v) Tween 80 (negative control). All experiments were repeated twice.
Statistical analysisConverted data (ABS dr) and data from the conidial germination and mycelial growth assays were submitted to analysis of variance, and average values were compared by Scott-Knott calculations (P≤0.05). Statistical analyses were done using SISVAR software.7
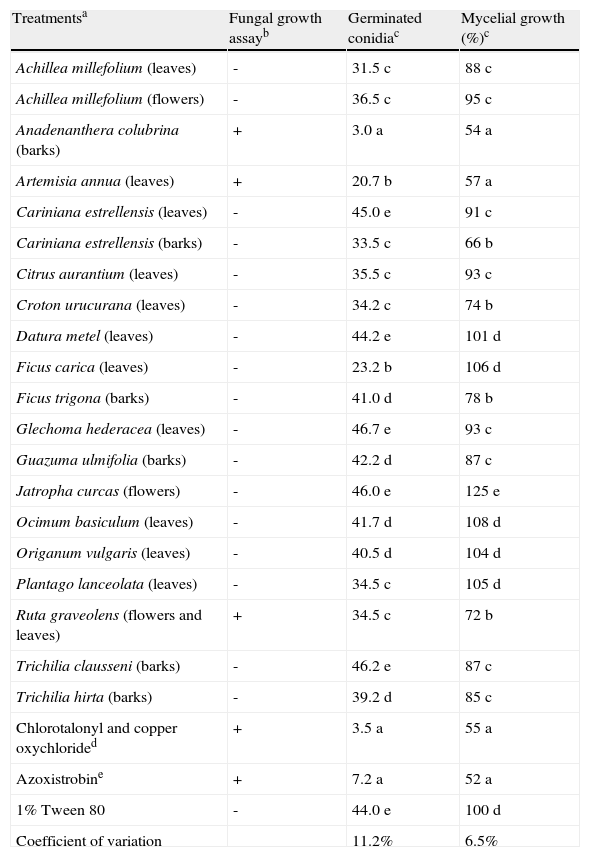
ResultsFungal growth, conidial germination and mycelial growth assaysAmong the 126 plant extracts tested in the fungal growth assay, only those from leaves of Artemisia annua, barks of Anadenanthera colubrina and a mixture of flowers and leaves of Ruta graveolens were active against A. alternata (Table 2). Consequently, such extracts, as well as 17 plant extracts that presented no activity in the fungus growth assay, were submitted to the conidial germination and mycelial growth assays.
Effect of plant extracts on Alternaria alternata in the fungal growth, conidia germination and mycelial growth assays.
| Treatmentsa | Fungal growth assayb | Germinated conidiac | Mycelial growth (%)c |
| Achillea millefolium (leaves) | - | 31.5 c | 88 c |
| Achillea millefolium (flowers) | - | 36.5 c | 95 c |
| Anadenanthera colubrina (barks) | + | 3.0 a | 54 a |
| Artemisia annua (leaves) | + | 20.7 b | 57 a |
| Cariniana estrellensis (leaves) | - | 45.0 e | 91 c |
| Cariniana estrellensis (barks) | - | 33.5 c | 66 b |
| Citrus aurantium (leaves) | - | 35.5 c | 93 c |
| Croton urucurana (leaves) | - | 34.2 c | 74 b |
| Datura metel (leaves) | - | 44.2 e | 101 d |
| Ficus carica (leaves) | - | 23.2 b | 106 d |
| Ficus trigona (barks) | - | 41.0 d | 78 b |
| Glechoma hederacea (leaves) | - | 46.7 e | 93 c |
| Guazuma ulmifolia (barks) | - | 42.2 d | 87 c |
| Jatropha curcas (flowers) | - | 46.0 e | 125 e |
| Ocimum basiculum (leaves) | - | 41.7 d | 108 d |
| Origanum vulgaris (leaves) | - | 40.5 d | 104 d |
| Plantago lanceolata (leaves) | - | 34.5 c | 105 d |
| Ruta graveolens (flowers and leaves) | + | 34.5 c | 72 b |
| Trichilia clausseni (barks) | - | 46.2 e | 87 c |
| Trichilia hirta (barks) | - | 39.2 d | 85 c |
| Chlorotalonyl and copper oxychlorided | + | 3.5 a | 55 a |
| Azoxistrobine | + | 7.2 a | 52 a |
| 1% Tween 80 | - | 44.0 e | 100 d |
| Coefficient of variation | 11.2% | 6.5% |
aExcept for Ficus trigona (pH 6.0), 3.3mg/ml plant extracts solutions in aqueous 1% (g/ml) Tween 80 presented pH 5.0.
b(+): Absence of mycelium growth, (-): Presence of mycelium growth.
cMeans of four replicates with the same letter in a column are not statistically significant (P≤0.05) according to the Scott-Knott method.
d0.87 and 1.76g/l, respectively.
e0.08g/l.
The results from the conidial germination assay (Table 2) showed that A. colubrina extract (3.0 germinated conidia) was not statistically different from the commercial fungicides Dacobre WP and Amistar 500 WG, which afforded values of 3.5 and 7.2 germinated conidia, respectively. Other extracts such as those from A. annua and F. carica also inhibited A. alternata conidia germination, but in a lesser extent (20.7 and 23.2 germinated conidia, respectively).
The mycelial growth assay (Table 2) also showed that A. colubrina extract was not statistically different from the commercial fungicides. Similarly to the conidial germination assay, other plant extracts, such as C. estrellensis (barks), also presented some antifungal activity in the mycelial growth (34% of inhibition). The best results were obtained with A. annua and A. colubrina (43 and 46% of inhibition, respectively), that were as efficient as the commercial fungicides (45-48% of inhibition).
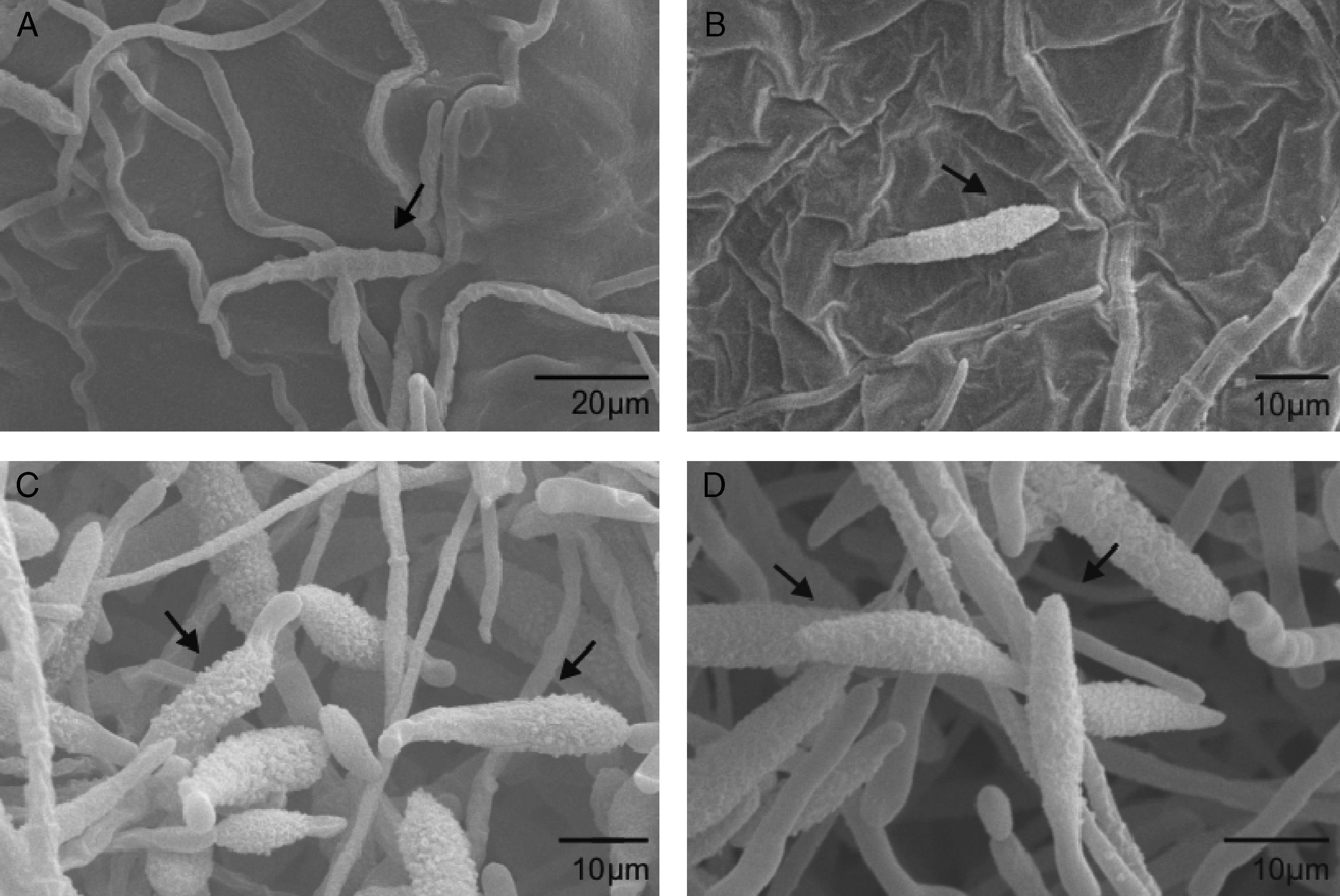
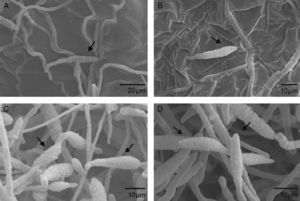
Scanning electron microscopyScanning electron microscopy (SEM) was used to further investigate the effects of extracts from A. colubrina, A. annua and R. graveolens on A. alternaria. The samples exposed to the A. colubrina extract revealed no conidia or mycelia. Regarding conidia exposed to A. annua extracts, shorter germ tubes than those exposed to Dacobre WP (chlorotalonil and copper oxychloride) were observed, while R. graveolens extracts and Amistar 500 WG caused shrinking in conidia (Fig. 1). The negative control (1% Tween 80) did not cause any spore shrinking.
Scanning electron micrograph details of Alternaria alternata conidia: A) Severe conidia shrinking caused by exposition to Ruta graveolens extract (3.3mg/ml). An arrow shows the rings (septa) enhanced by whither of cells. B) Conidia shrinking after treatment with Azoxistrobin (0.08g/l), but with less intensity than the observed for the R. graveolens extract. C and D) Conidia after exposition to 1% Tween 80. No deformity was observed.
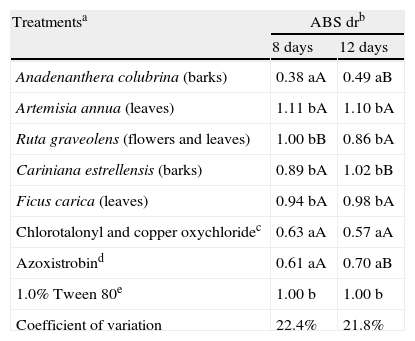
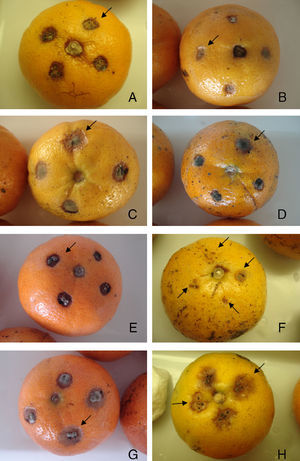
Based on the in vitro assays (fungal growth, conidial germination and mycelial growth assays), five plant extracts (A. colubrina, A. annua, C. estrelensis, F. carica and R. graveolens) were selected for assays with Murcott tangor fruits. Only that from A. colubrina showed suppression of lesions caused by A. alternata (Table 3; Fig. 2). Although the efficiency of this extract reduced over time (ABS dr was 0.38A at 8 days and 0.49B at 12 days), the results obtained with such plant extract were statistically similar to those of the commercial fungicides (ABS dr were 0.63 and 0.61 at 8 days and 0.57 and 0.70 at 12 days for Dacobre WP and Amistar 500 WG, respectively). A 16-day evaluation was also tried in this experiment, but except for the fungicides and A. colubrina treatments, all inoculated surfaces on fruits were completely deteriorated by fusion of all lesions. No disease was observed on fruits treated with only 1% (v/v) Tween 80 solution.
Plant extract and fungicide effects on Murcott tangor fruits inoculated with Alternaria alternata.
| Treatmentsa | ABS drb | |
| 8 days | 12 days | |
| Anadenanthera colubrina (barks) | 0.38 aA | 0.49 aB |
| Artemisia annua (leaves) | 1.11 bA | 1.10 bA |
| Ruta graveolens (flowers and leaves) | 1.00 bB | 0.86 bA |
| Cariniana estrellensis (barks) | 0.89 bA | 1.02 bB |
| Ficus carica (leaves) | 0.94 bA | 0.98 bA |
| Chlorotalonyl and copper oxychloridec | 0.63 aA | 0.57 aA |
| Azoxistrobind | 0.61 aA | 0.70 aB |
| 1.0% Tween 80e | 1.00 b | 1.00 b |
| Coefficient of variation | 22.4% | 21.8% |
a3.3mg/ml plant extracts solutions in aqueous 1% (g/ml) Tween 80.
bPreviously to the statistical analysis, data were converted into spots development rate (ABS dr) by dividing the mean value for each fruit by the mean value of all fruits treated only with conidia in 1% (v/v) Tween 80 (negative control). Means with the same lower case letter in a column and the same capital letter in a line are not statistically significant (P≤0.05) according to the Scott-Knott method.
c0.87 and 1.76g/l, respectively.
d0.08g/l.
e106 conidia/ml in a 1.0% (g/ml) Tween 80 solution.
Effects of plant extracts and fungicides on the development of Alternaria brown spots in Murcott tangor fruits eight days after inoculation (106Alternaria alternata conidia/ml): A) Chlorotalonyl and copper oxychloride at 0.87 and 1.76g/l, respectively. B) Azoxistrobin at 0.08g/l. C) Ficus carica. D) Cariniana estrellensis. E) Ruta graveolens. F) Anadenanthera colubrina. G) Artemisia annua extract at 3.3mg/ml. H) 106 conidia/ml resuspended in Tween 80 solution 1.0% (v/v).
The screening for plants with antifungal activity in the present study started with the fungal growth assay, during which some forms of action of antifungal substances can apparently be detected (Fig. 1). Although only three extracts were active against the fungus, 20 extracts (3 active and 17 inactive) were submitted to the other two in vitro assays to facilitate the comparison of results. Most of the inactive extracts during the initial screening (fungal growth assay) inhibited the conidia germination. Analogously, some inactive extracts during the fungal growth assay inhibited the microorganism largely in the mycelial growth assay, showing that antifungal activity of the metabolites produced by the same plant can vary according to the phase of fungal development.
Considering all the in vitro assays, the most active extract was that from A. colubrina, which is a native plant from Caatinga (Brazilian savanna), popularly known as angico, that occurs in the northeast region of Brazil.1 The ability of this extract to reduce mycelial growth of F. oxysporum f.sp. tracheiphlum when used alone or in combination with fungicides has recently been described by Silva et al.19 The authors suggested polyphenols as the active substances in the A. colubrina extract, since these compounds could form complexes with proteins and polysaccharides, inactivating enzymes essential for fungal growth.8 Furthermore, the barks of A. colubrina are rich in tannins, which may confer antimicrobial properties to the plant extract.6,13 The inexistence of conidia in the SEM image of the sample treated with the A. colubrina extract corroborates the strong inhibition of conidial germination by this plant, since without the germ tubes, conidia would be lost during the several washings or expositions to liquids used to prepare the sample for SEM analyses.
The inhibition of the fungus by the extracts of A. annua and R. graveolens in all in vitro assays is in accordance with the studies carried out by Soylu et al.20 and Meepagala et al.,12 respectively. These authors have reported the activity of A. annua against Sclerotinia sclerotiorum, Botrytis cinerea, Phytophthora infestans, and Verticillium dahliae, and also antifungal activity of R. graveolens extracts against Colletotrichum fragariae, C. gloeosporioides, C. acutatum, and B. cinerea.
Furthermore, SEM analyses showed shrinkage of those conidia exposed to R. graveolens, which may be attributed to irreversible ultra structural changes.14 Regarding A. annua, the shorter germ tubes observed by SEM analyses suggest that the plant extract was able to delay fungal germination.
Although less efficiently than the above-mentioned extracts, those obtained from C. estrellensis and F. carica also inhibited the fungus. Although no report about antifungal activity for the former plant is known, the later was reported by Aqil and Ahmad as inhibitor of Fusarium chlamydosporum2.
The results obtained in the test with fruits inoculated with A. alternata suggest consistency between in vitro and in vivo assays, since the extract of A. colubrina was the most active in all experiments, reducing the effects of the fungus on fruits to levels comparable to those observed for the commercial fungicides. Regarding the other extracts employed in the experiments with fruits, their inactivity against the disease seems to correlate with their low to moderate antifungal activity observed during the in vitro assays. Thus, the fungal growth assay is a good protocol to start a screening program to select plant extracts active against A. alternata.
The results obtained in the present study suggests the fungal growth assay as a good method to be used in an initial step of a screening program aimed to select plant extracts for the control of ABS in Murcott tangor fruits. Among the plants studied, the most promising was A. colubrina, the extract of which reduced the development of A. alternata on such fruits to levels statistically equal to those observed for commercial fungicides. Consequently, A. colubrina seems to present a great potential for the development of new products for ABS control.
FinancingThe authors gratefully acknowledge CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), CAPES (Coordenadoria de Aperfeiçoamento de Pessoal de Nível Superior) and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais) for financial support and fellowships.
Conflict of interestThe authors report no conflict of interest.
The authors also express their sincere thanks to Dr. Paulo César Lima (Departamento de Ciências Exatas, Universidade Federal de Lavras) for helping with the statistical analysis.