The Malassezia genus includes mainly lipophilic yeasts belonging to the cutaneous microbiota of man and other mammals. Some Malassezia species have been associated with various dermatological diseases. The factors permitting the transformation of yeasts of the Malassezia genus from a commensal organism to a pathogenic agent are still little known but the production of various enzymes such as lipase, phospholipase and lipoxygenase could contribute to the pathogenic activity of these yeasts.
AimsHere we have determined and compared the extracellular phospholipase activity of sixty human isolates of Malassezia so as to relate this feature to the species of Malassezia and to the origin (from dermatological diseases or not) of the strains examined.
MethodsPhospholipase production was determined using the semi-quantitative egg-yolk plate method.
Results and conclusionsMalassezia obtusa, Malassezia slooffiae, Malassezia globosa, Malassezia restricta had difficulty developing in the chosen culture medium so that it was not possible to measure phospholipasic activity. Malassezia pachydermatis showed the highest phospholipase activity. Twenty-nine Malassezia sympodialis strains produced phospholipase; the isolates from patients with pityriasis versicolor had significantly higher phospholipasic activity than those isolated from healthy individuals. This observation suggests that the phospholipasic activity of Malassezia may play a role in the onset of skin lesions, especially in the case of pityriasis versicolor.
El género Malassezia incluye numerosas levaduras lipofílicas que pertenecen a la microbiota cutánea del hombre y de otros mamíferos. Algunas especies se han asociado con diversas dermatosis. Apenas se conocen los factores que permiten la transformación de las levaduras del género Malassezia de un microorganismo comensal a uno patógeno, pero la producción de diversas enzimas, como lipasas, fosfolipasas y lipooxigenasa, podría contribuir a la actividad patogénica de estas levaduras.
ObjetivosEn la presente investigación hemos determinado y comparado la actividad de la fosfolipasa extracelular a partir de 60 aislamientos de Malassezia en seres humanos para relacionar esta característica con las especies del género y con el origen (dermatosis o no) de las cepas examinadas.
MétodosLa producción de fosfolipasa se determinó utilizando el método semicuantitativo de la placa de yema de huevo.
Resultados y conclusionesMalassezia obtusa, Malassezia slooffiae, Malassezia globosa y Malassezia restricta crecieron mal en el medio de cultivo seleccionado, por lo que no fue posible determinar la actividad fosfolipasa. Malassezia pachydermatis mostró la mayor actividad enzimática. Produjeron fosfolipasa 29 cepas de Malassezia sympodialis; los aislamientos de pacientes con pitiriasis versicolor se caracterizaron por una actividad fosfolipasa significativamente mayor que los aislamientos de individuos sanos. Esta observación sugiere que la actividad fosfolipasa de Malassezia podría desempeñar un papel en el inicio de las lesiones cutáneas, en particular en el caso de la mencionada micosis.
The Malassezia genus includes mainly lipophilic yeasts belonging to the cutaneous microbiota of man and other mammals. These yeasts were first described by Eichstedt in 1846 as being associated with lesions of pityriasis versicolor.
The taxonomy and nomenclature of the Malassezia genus has, in recent years, been revised several times. In fact, up until 1995 only three species had been identified: Malassezia furfur, Malassezia pachydermatis and Malassezia sympodialis. The species Malassezia globosa, Malassezia restricta, Malassezia obtusa, and Malassezia slooffiae were described in 1995 on the basis of their morphology, ultrastructure, physiology and genomic features.11,13 In the last few years, the new lipid-dependent species Malassezia dermatis,31Malassezia japonica,30 and Malassezia yamatoensis29 were isolated from human skin. Other species were found on animals: Malassezia nana15 on cats and dogs, Malassezia equina, Malassezia caprae and Malassezia cuniculi,3,4 found mainly on horses, goats and rabbits respectively.
Yeasts belonging to the Malassezia genus have been associated with various dermatological diseases: pityriasis versicolor (PV), dandruff, seborrheic dermatitis (SD), atopic dermatitis, folliculitis, psoriasis, onychomycosis, and blepharitis. The pathogenic role of Malassezia in PV has now been universally accepted although authors disagree about which species is most widely associated with the disease. Some of them suggest that M. globosa is the causal agent of PV.8,9,14
As well as these diseases, cases of fungemia caused by M. pachydermatis and M. furfur have been reported in premature newborns and immunocompromised patients artificially fed with lipid emulsions.32
Most Malassezia species are lipid-dependent; as a result these yeasts probably require lipolytic enzymes such as lipases and phospholipases to use the environmental lipids essential for their growth. In a recent work, Xu35 described the genome and secretory proteome of two species of Malassezia, suggesting that the lipid-dependency of these species is associated with the apparent absence of the gene for fatty acid synthase. Further studies have demonstrated extracellular production of phospholipase and lipase.7,17
Phospholipases are a heterogeneous group of enzymes which hydrolyse one or more ester bonds of glycerol-phospholipids. Phospholipids and proteins are the main chemical constituents of the cellular membranes of the host so that the phospholipases and proteases are involved in the destruction processes of such cellular membranes. Many fungal species, such as Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus can produce enzymes belonging to the phospholipases group.2,20,34 Researchers have associated some extracellular phospholipases with the virulence of C. albicans.16,23
The factors permitting the transformation of yeasts of the Malassezia genus from a commensal organism to a pathogenic agent are still little known, but the production of various enzymes such as lipase, phospholipase and lipoxygenase could contribute to the pathogenic activity of these yeasts too.
Little information is available in literature regarding the phospholipasic activity of species of the Malassezia genus. Using the Price method (egg yolk medium),26 extracellular phospholipasic activity was demonstrated in vitro on strains of M. furfur isolated from skin lesions in humans.22,27 These works were prior to the classification introduced by Guého et al. so, bearing in mind the current classification, some strains included in these studies could be reclassified as different species.
The phospholipasic activity of M. pachydermatis has also been associated with skin lesions in dogs.5,6
In this work we have analysed and compared the extracellular phospholipasic activity of Malassezia isolates belonging to different species, identified according to the current classification so as to verify whether the various species of Malassezia have different phospholipasic activity and to relate this feature to the origin (from dermatological diseases or not) of the strains examined.
Materials and methodsThe strains included in this study were isolated from patients with PV or SD, both from skin lesions and from healthy skin, and from individuals without any dermatological disease. Fourteen reference strains from the Pasteur Institute (IP) and Central Bureau voor Schimmelcultures (CBS) (Table 1) were also examined.
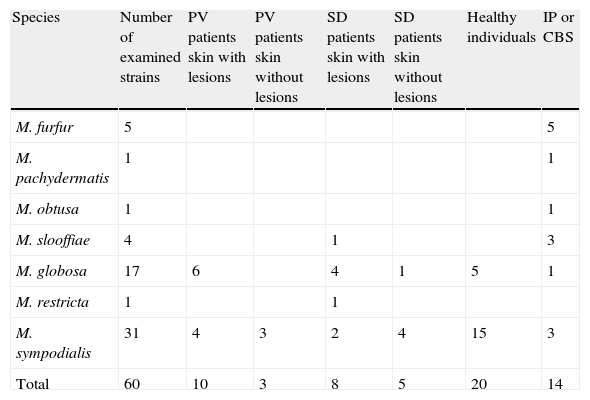
Number, species and origin of the strains examined.
| Species | Number of examined strains | PV patients skin with lesions | PV patients skin without lesions | SD patients skin with lesions | SD patients skin without lesions | Healthy individuals | IP or CBS |
| M. furfur | 5 | 5 | |||||
| M. pachydermatis | 1 | 1 | |||||
| M. obtusa | 1 | 1 | |||||
| M. slooffiae | 4 | 1 | 3 | ||||
| M. globosa | 17 | 6 | 4 | 1 | 5 | 1 | |
| M. restricta | 1 | 1 | |||||
| M. sympodialis | 31 | 4 | 3 | 2 | 4 | 15 | 3 |
| Total | 60 | 10 | 3 | 8 | 5 | 20 | 14 |
CBS: Central Bureau voor Schimmelcultures; IP: Pasteur Institute; PV: pityriasis versicolor; SD: seborrheic dermatitis.
Our strains were isolated from scaly skin. The samples were inoculated on Dixon modified (m-Dixon) agar,12 supplemented with 0.05g chloramphenicol pH 6.0.
The inoculated plates were incubated aerobically at 32°C for 2 weeks in plastic boxes with a layer of paper soaked in water on the bottom to prevent desiccation. The culture was considered positive when at least one Malassezia colony was observed. Malassezia yeasts growing on m-Dixon agar were selected and subcultured for species identification. Malassezia species were identified by their morphological characteristics, catalase test, and growth in the presence of Tween 20, 40, 60 and 80 as unique lipid supplementation, using the scheme established by Guillot et al.12 To improve the differential identification of M. furfur, M. sympodialis and M. slooffiae, the splitting of esculin (β-glucosidase activity), the assimilation of Cremophor EL and tryptophan described by Mayser et al. were also assayed.19,21
Phospholipase production was determined using the semi-quantitative egg-yolk plate method previously reported.25,26 The egg-yolk plates were inoculated with 5μl of a fresh culture containing 108cells/ml. The inoculated egg-yolk plates were incubated at 32°C in plastic boxes as described above, and readings were taken daily from day 7 to day 20. The formation of precipitation zones around the colony was considered indicative of enzyme production.
The production of phospholipase (Pz) was expressed as a ratio of colony diameter to total diameter of the colonies plus the precipitation zone.25 When the Pz was equal to 1.0 the samples were considered negative, and when the Pz was less than 1.0, phospholipase activity was considered positive. Each strain was tested in duplicate and the Pz value represents an average of the two Pz values reported. The Student t test was used for statistical analysis of the Pz value; a P value ≤ 0.05 was considered significant.
ResultsThe phospholipasic activity determined by the Price method is a simple technique applicable to a large number of strains. However, the measurement of the precipitation zone was not easy; in fact, the colony was already visible after 48hours of incubation, while the precipitation zone around the colony was not observed until 8-10 days later, appearing extremely dense but clearly distinguishable from the colony. Up until day 15 of incubation both the diameter of the colony and that of the precipitation zone increased, whereas from day 15 to day 20 only the precipitation zone increased. Measurements of the precipitation zone taken at the day 15 and at day 20 from incubation were therefore deemed significant.
The ability of the species belonging to the Malassezia genus to produce phospholipase is shown in Table 2.
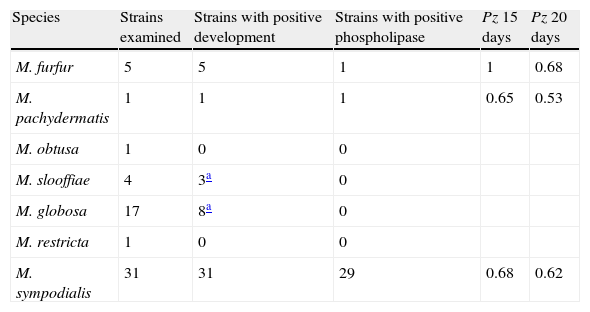
Phospholipasic activity of the Malassezia species expressed as a Pz value mean after 15 and 20 days of culture.
| Species | Strains examined | Strains with positive development | Strains with positive phospholipase | Pz 15 days | Pz 20 days |
| M. furfur | 5 | 5 | 1 | 1 | 0.68 |
| M. pachydermatis | 1 | 1 | 1 | 0.65 | 0.53 |
| M. obtusa | 1 | 0 | 0 | ||
| M. slooffiae | 4 | 3a | 0 | ||
| M. globosa | 17 | 8a | 0 | ||
| M. restricta | 1 | 0 | 0 | ||
| M. sympodialis | 31 | 31 | 29 | 0.68 | 0.62 |
Pz: ratio of colony diameter to total diameter of the colonies plus the precipitation zone.
M. furfur grew well on this medium but only for one strain it was possible to distinguish a late (day 20) precipitation zone around the colony. M. pachydermatis grew well and showed phospholipasic activity. M. sympodialis grew well and showed phospholipasic activity with the exception of two strains, one isolated from healthy subjects, the other supplied by IP, ex human normal skin.
M. obtusa, M. slooffiae, M. globosa, M. restricta, showed poor growth and did not produce a precipitate.
The greatest phospholipasic activity (Pz value) was shown by the strain M. pachydermatis after 20 days of incubation.
For M. sympodialis we compared the phospholipasic activity expressed as a Pz value mean with the dermatological disease presented by the patient.
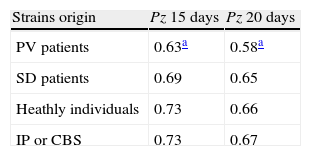
Table 3 shows the Pz mean value of the strains isolated from patients with PV or SD, from individuals without any dermatological disease and from the reference strains of IP and CBS.
Phospholipasic activity of M. sympodialis expressed as a Pz mean value after 15 and 20 days of culture.
| Strains origin | Pz 15 days | Pz 20 days |
| PV patients | 0.63a | 0.58a |
| SD patients | 0.69 | 0.65 |
| Heathly individuals | 0.73 | 0.66 |
| IP or CBS | 0.73 | 0.67 |
Pz: ratio of colony diameter to total diameter of the colonies plus the precipitation zone; CBS: Central Bureau voor Schimmelcultures; IP: Pasteur Institute; PV: pityriasis versicolor; SD: seborrheic dermatitis.
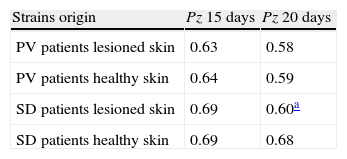
The Student t test highlighted significant differences between the Pz values of strains isolated from patients with PV and those of strains isolated from healthy individuals, both for readings on day 15 and day 20 subsequent to incubation (P=0.04 and 0.05 respectively), while such difference was not significant for strains isolated from patients with SD (P=0.08 and 0.4). The CBS and IP reference strains were isolated from individuals without any dermatological disease and showed a mean Pz value the same as that of the healthy individuals included in our research.Table 4 shows the phospholipase activity of M. sympodialis strains isolates from patients with PV or SD, from sites with and without evident skin lesions.
Phospholipasic activity of M. sympodialis with reference to strains origin.
| Strains origin | Pz 15 days | Pz 20 days |
| PV patients lesioned skin | 0.63 | 0.58 |
| PV patients healthy skin | 0.64 | 0.59 |
| SD patients lesioned skin | 0.69 | 0.60a |
| SD patients healthy skin | 0.69 | 0.68 |
Pz: ratio of colony diameter to total diameter of the colonies plus the precipitation zone; PV: pityriasis versicolor; SD: seborrheic dermatitis.
After 15 days of incubation, the strains isolated from disease (PV and SD patients), both from healthy skin and lesioned skin, had the same Pz value, while, after 20 days, the strains isolated from the lesioned skin of patients with SD showed higher phospholipasic activity than the strains isolated from healthy skin (but not significantly P=0.1) (Table 4).
DiscussionExtracellular phospholipases are considered virulence factors for many pathogenic bacteria and protozoa such as Clostridium species, Listeria monocytogenes, Pseudomonas species, Staphylococcus aureus, Mycobacterium tuberculosis, Toxoplasma gondii and Entamoeba histolytica and for some fungi such as Candida albicans, Aspergillus fumigatus or Cryptococcus neoformans.10
Barret-Bee et al.1 in particular, were the first to correlate the production of phospholipase by C. albicans with its pathogenic nature, demonstrating that isolates with a high pathogenic potential (high level of adhesion to oral epithelial cells and greater pathogenicity for the mouse) had higher phospholipasic activity than yeasts with a low pathogenic potential. In addition, C. albicans blood isolates have shown greater in vitro phospholipasic activity than oral isolates from healthy patients.16 Similarly, it has been shown that isolates of C. neoformans taken from infections in AIDS patients show a higher level of phospholipasic activity than isolates taken from non AIDS patients or from bird droppings.33,34
For the Malassezia genus some authors have observed the production of different extracellular enzymes such as lipase and phospholipase.7,17,18,24
Using the Price method, extra-cellular phospholipasic activity was shown in vitro on strains of M. furfur isolated from human skin lesions.22,27 In addition, higher phospholipasic activity was observed in isolates of M. pachydermatis from skin lesions in dogs than in those from healthy skin.5,6
The method used in this study to highlight the production of phospholipase by yeasts belonging to the Malassezia genus proved simple to apply and produced valid results for the species M. furfur, M. pachydermatis and M. sympodialis, while the strains belonging to the species M. obtusa, M. slooffiae, M. globosa, M. restricta, had difficulty developing in the chosen medium so that it was not possible to measure phospholipasic activity. To measure the phospholipasic activity of Malassezia,ten days at least of incubation at 32°C were needed, unlike the two or six days needed for C. albicans and C. neoformans, respectively.28,34
These results confirm those of other authors7 and indicate that the yeasts of the Malassezia genus produce phospholipase more slowly than C. albicans and C. neoformans.
For M. sympodialis it was possible to compare the production of phospholipase (Pz value) with the origin of the strains. Isolates of M. sympodialis taken from patients with PV showed significantly higher phospholipasic activity (P=0.04) than those isolated from healthy individuals. Strains isolated from patients with SD and from healthy individuals did not show significant differences (P=0.08) in the production of phospholipase. Strains isolated from the same patient, from healthy skin and from lesions, had the same Pz on average, both in the case of pityriasis versicolor and of seborrheic dermatitis. It is probable that the same strain colonises the entire individual but producing lesions is only able in some parts of the body, probably in relation to specific local conditions connected with the production of cutaneous lipids and/or alterations of the skin “ecosystem”.
These results are only partially comparable with previous works since only a limited amount of data in the literature refers to new species of Malassezia.
However, because egg-yolk contains substrates for both phospholipases (phospholipids) and lipases (triglycerides), the egg-yolk-based assay is not entirely specific (although the Pz value correlates with hydrolysis of phosphatidylcholine26) and should be considered as an initial screening which requires further confirmation using more specific enquiry such as radiometric or colorimetric methods.
This study suggests however that the phospholipasic activity of Malassezia may play a role in the onset of skin lesions, especially in the case of PV, even though phospholipases should be considered as only one of the many factors involved in the complex interaction between the yeast and its host leading to the development of skin lesions. More in-depth studies will be needed to understand the pathogenic role played by the enzymes secreted by Malassezia spp.
Conflict of interestThe authors report no conflict of interest.