The latest published guideline on the diagnosis and management of rare mycoses in humans caused by moulds highlights the high antifungal resistance of some of the species that cause them and provides medical recommendations for their treatment.3 These fungi may cause severe infections, with a high mortality rate, in people with underlying diseases.
Among these super moulds, Lomentospora prolificans (formerly Scedosporium prolificans), a soil-borne saprophytic fungus that causes lomentosporiosis, must be highlighted. Most cases are reported from Australia and the south-west of the USA, and to a lesser extent from Spain, Germany and Japan. However, prevalence and incidence data of this mycosis are largely unknown. This species seems to be intrinsically resistant to most of the antifungals used, showing high MIC values for amphotericin B, itraconazole, voriconazole, posaconazole, terbinafine, caspofungin, micafungin and anidulafungin. The aforementioned guidelines recommend combination antifungal therapy to treat these infections, in particular voriconazole and terbinafine, among others. The identification of L. prolificans is achieved by macroscopic and microscopic examination of its colonies. The colonies are usually blackish with a wet appearance and have characteristic anellated, flask-shaped conidiogenous cells that give rise to obovoidal conidia. However, the species must be confirmed by subsequent sequencing of the ITS1-5.8S-ITS2 region of the rDNA.
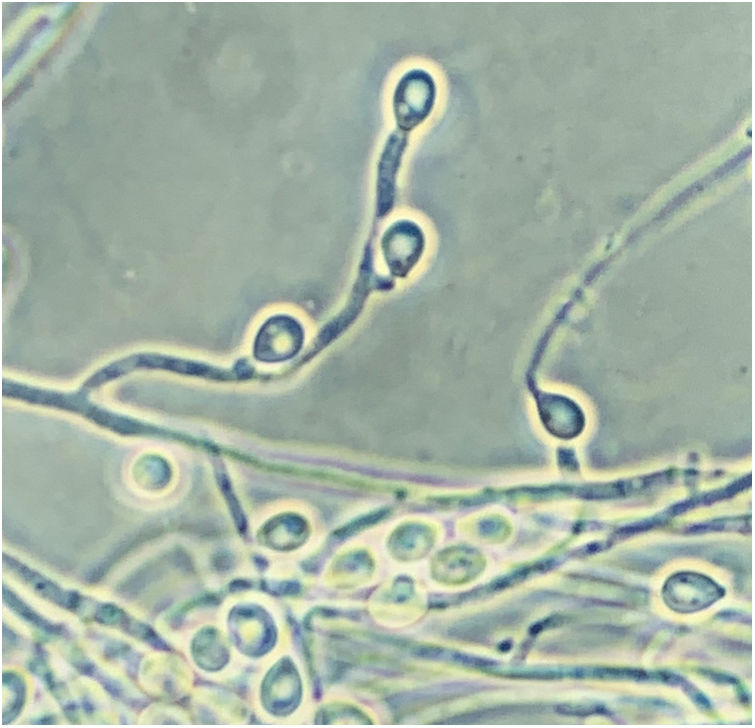
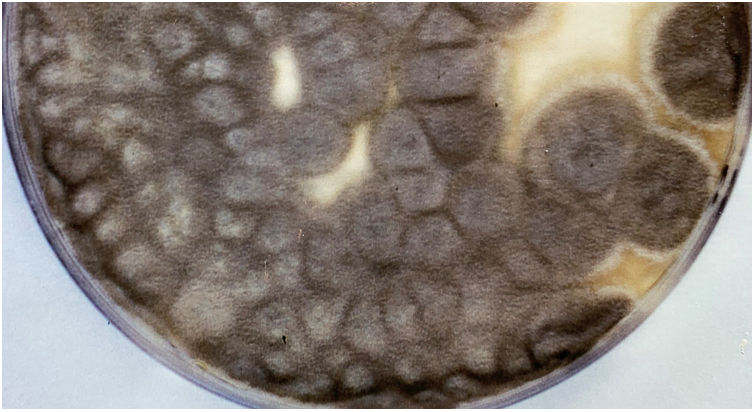
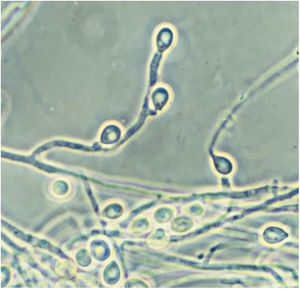
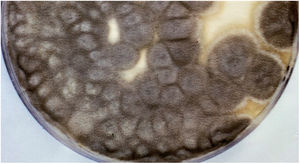
Scedosporidiosis causative agents are also noteworthy as this disease is caused by different species of the genus Scedosporium, and mainly by some of those included in the Scedosporium apiospermum complex, such as S. apiospermum sensu stricto and Scedosporium boydii. These two are the most commonly isolated worldwide among patients in clinical settings. Unlike L. prolificans, they may exhibit two asexual reproductive forms (Scedosporium-like [Fig. 1] and Graphium-like synanamorphs) with non-inflated conidiogenous cells, which mostly produce obovoidal or ellipsoidal conidia. Colonies are usually hairy or cottony and greyish-brown coloured (Fig. 2). To confirm their identification to the species level, sequencing of both ITS and β-tubulin genes is required.
Pure culture of Scedosporium apiospermum growing on a Sabouraud glucose agar plate supplemented with chloramphenicol and inoculated with nasal biopsy material from a dog with scedosporiosis.2 Note the cottony appearance of the colonies.
Most cases of scedosporiosis have been reported from the USA, Australia, Germany, India, Spain and Japan. Scedosporium species have also high MIC values for amphotericin B, isavuconazole, itraconazole and fluconazole. The lowest MIC values observed are for voriconazole, posaconazole and the echinocandins. The consensus guidelines recommend voriconazole to treat these mycoses along with surgical debridement, when appropriate. In animals, these mycoses are also very rare. Cases in dogs are the most frequently published, but only about twenty cases have been described up to now (Pubmed search using the following descriptors: “Scedosporium” AND “dogs” and “Pseudallescheria” AND “dogs”; 28 April 2021).
Lomentosporiosis is the least common mycoses and only disseminated infections have been reported in immunocompromised dogs, with a poor prognosis. Most of the few described cases are also localised in Australia. In one of these cases the animal was receiving an immunosuppressive drug treatment for immune-mediated haemolytic anaemia.4 The L. prolificans strain isolated was resistant to all the antifungals tested: amphotericin B, 5-fluorocytosine, itraconazole, fluconazole, voriconazole, posaconazole, caspofungin, micafungin and anidulafungin. Despite discontinuing the immunosuppressive medication and having an initial clinical response to voriconazole and terbinafine, the disease progressively developed with neurological involvement that led to euthanize the dog six months after diagnosis.
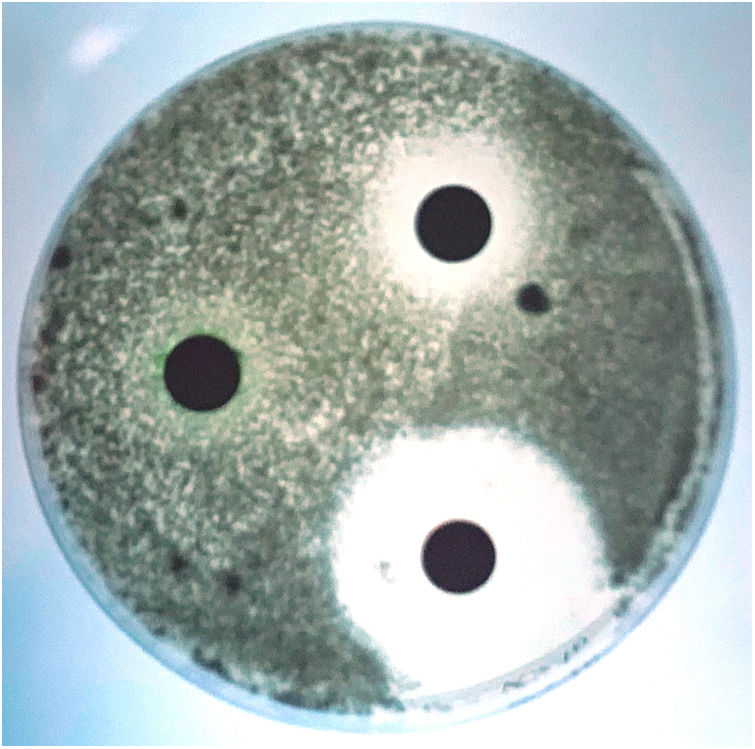
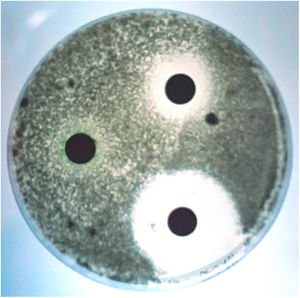
Although scedosporiosis in dogs also includes disseminated infections, localised infections, mainly affecting the nasal cavity, abdominal cavity and cornea, are more frequent. Many years ago, we had the opportunity to describe in our laboratory, in collaboration with several specialists from the Veterinary School of the Autonomous University of Barcelona, the first case of a fungal granuloma in the nasal cavity caused by S. apiospermum.2 The aetiological agents commonly isolated in mycoses with this clinical presentation are usually Aspergillus species, mainly Aspergillus fumigatus.1 Apart from isolating the aetiological agent from the samples, its role in the pathological process was evidenced by radiological and histological techniques. Rhinoscopy revealed the destruction of the vomer bone and the presence of a large mass that completely occluded the nasal cavity (Fig. 3). Although the techniques for testing the susceptibility of moulds to antifungal agents were not standardised at that time (e.g. CLSI, EUCAST), an assessment of some antifungal agents that could be of interest for the treatment of the case was also performed using a diffusion technique. The isolate was sensitive to ketoconazole, had intermediate sensitivity to clotrimazole and was resistant to amphotericin B, 5-fluorocytosine, fluconazole and itraconazole (Fig. 4). Although in vivo results cannot always be extrapolated from the in vitro results obtained in such tests, a general improvement of the lesions was observed during ketoconazole treatment. One month after the last check-up, nasal discharge had decreased and sneezing had disappeared. Unfortunately, a few months later the dog was run over and killed. These accidents remain being a major cause of mortality in dogs.
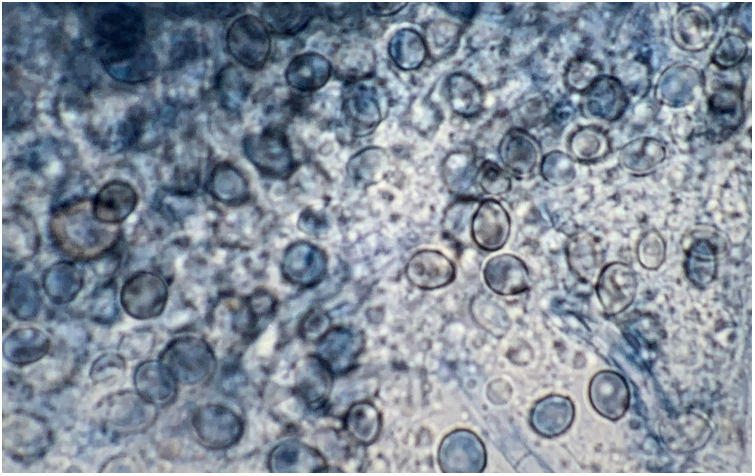
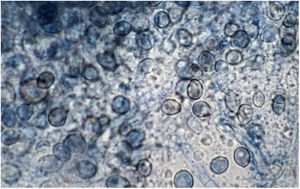
Lactophenol cotton blue stain of a smear from the nasal biopsy material from a dog with scedosporiosis2 showing numerous obovoidal conidia and hyphae.
Antifungal susceptibility testing of the Scedosporium apiospermum strain isolated from the dog with scedosporiosis,2 using a diffusion technique. The largest zone of inhibition was obtained with ketoconazole and the smallest with clotrimazole. Itraconazole showed no inhibition zone.
Author has no conflict of interest.
Financial support came from Servei Veterinari de Bacteriologia i Micologia of the Universitat Autònoma de Barcelona.
This Mycologic Forum article can be consulted in Spanish on the Animal Mycology section on the website of the Spanish Mycology Association (https://aemicol.com/micologia-animal/).