Throughout the coronavirus disease 2019 (COVID-19) pandemic, a greater severity and lethality of the disease has been highlighted in male patients, so we set out to evaluate the prognostic role of serum testosterone levels in the clinical results of this population.
MethodsIn this single-center and cross-sectional design, we included male patients admitted to our hospital with COVID-19 confirmed diagnosis. The biochemical analysis included lymphocytes, lactate dehydrogenase (LDH), total testosterone (TT), dehydroepiandrosterone, follicle-stimulating hormone, and luteinizing hormone. Receiver operating characteristic curves, univariate and bivariate analysis, and binary logistic regression for multivariate analysis were performed. A p value<0.05 was consider significant.
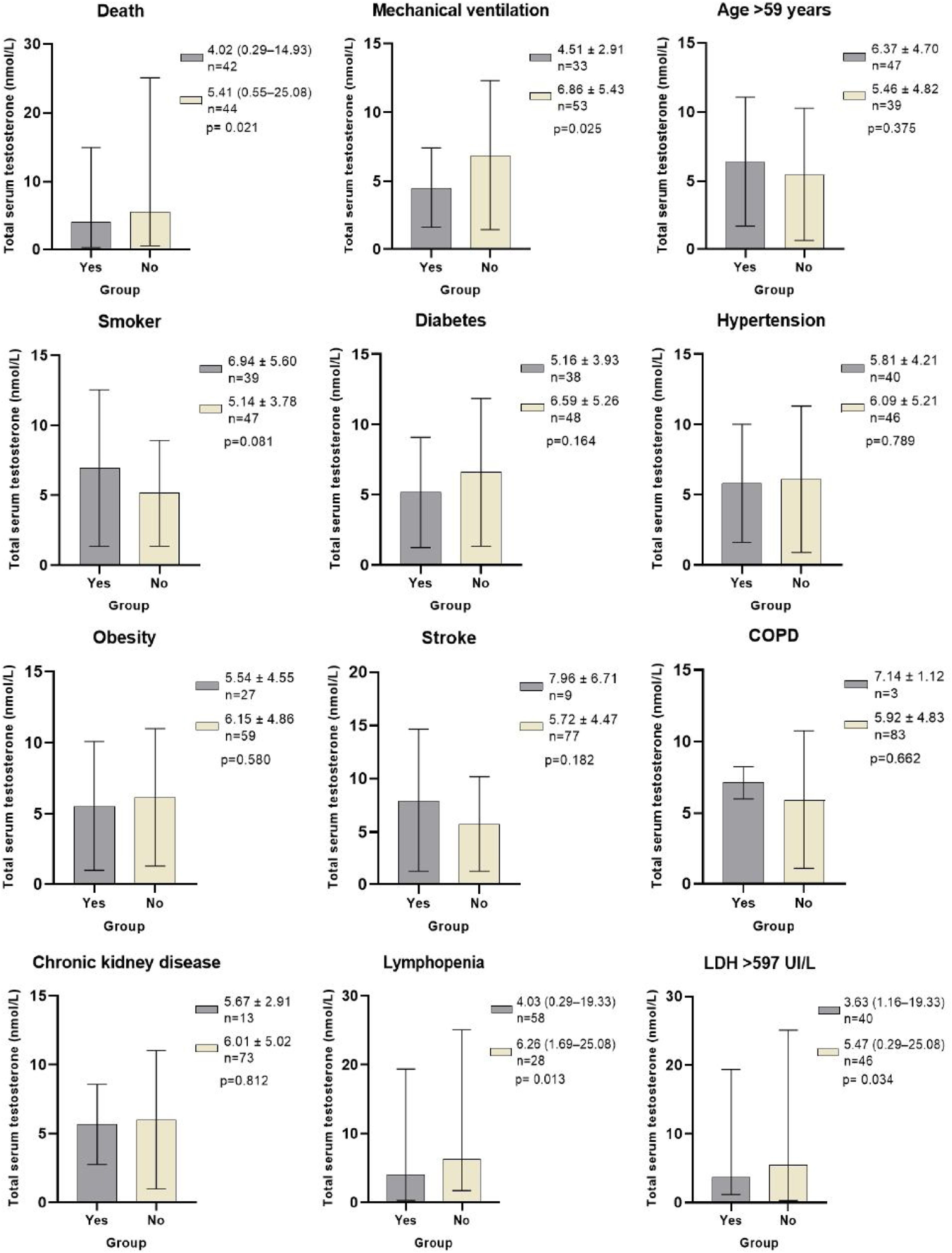
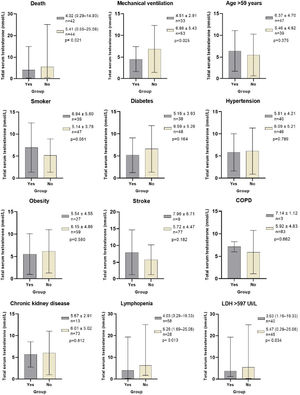
ResultsFrom 86 men included, 48.8% died. TT levels were lower in non-survivor patients than in survivor patients (4.01nmol/L [0.29–14.93] vs 5.41 (0.55–25.08) nmol/L, p=0.021). The independent risk factors that increased the relative risk (RR) of dying from COVID-19 were: age>59 years (RR 3.5 [95% IC 1.0–11.6], p=0.045), TT levels<4.89nmol/L (RR 4.0 [95% IC 1.2–13.5], p=0.027) and LDH levels>597IU/L (RR 3.9 [95% IC 1.2–13.1], p=0.024). Patients who required mechanical ventilation (p=0.025), had lymphopenia (p=0.013) and LDH levels>597IU/L (p=0.034), had significantly lower TT levels compared to those who did not present these conditions. There were no differences in TT levels between patients who had or did not have comorbidities.
ConclusionsA TT level<4.89nmol/L increase four times the RR of death from COVID-19 in men, regardless of age or presence of comorbidities.
A lo largo de la pandemia de la enfermedad por coronavirus de 2019 (COVID-19), se ha destacado una mayor gravedad y letalidad de la enfermedad en pacientes del sexo masculino, por lo que nos propusimos evaluar el papel pronóstico de los niveles séricos de testosterona en los resultados clínicos de esta población.
MétodosEn este diseño transversal de un único centro, incluimos a pacientes masculinos ingresados en nuestro hospital con diagnóstico confirmado de COVID-19. El análisis bioquímico incluyó linfocitos, lactato deshidrogenasa (LDH), testosterona total (TT), dehidroepiandrosterona, hormona estimulante del folículo y hormona luteinizante. Se elaboraron curvas «característica operativa del receptor», análisis univariado y bivariado y regresión logística binaria para análisis multivariado. Se consideró significativo un valor de p<0,05.
ResultadosDe los 86 hombres incluidos, el 48,8% falleció. El nivel de TT fue más bajo en los pacientes no supervivientes que en los supervivientes (4,01 [0,29-14,93] nmol/L vs. 5,41 [0,55-25,08] nmol/L; p=0,021). Los factores de riesgo independientes que aumentaron el riesgo relativo (RR) de muerte por COVID-19 fueron: edad>59 años (RR 3,5: IC 95% 1,0-11,6; p=0,045), cifra de TT<4,89 nmol/L (RR 4,0; IC 95%: 1,2-13,5; p=0,027) y de LDH>597 UI/L (RR 3,9; IC 95%: 1,2-13,1; p=0,024). Los pacientes que requirieron ventilación mecánica (p=0,025), tenían linfopenia (p=0,013), un nivel de LDH>597 UI/L (p=0,034) y de TT significativamente más bajos que aquellos que no presentaban estas condiciones. No hubo diferencias en los niveles de TT entre los pacientes que tenían o no comorbilidades.
ConclusionesUn nivel de TT<4,89 nmol/L aumenta 4 veces el RR de muerte por COVID-19 en hombres, independientemente de la edad o la presencia de comorbilidades.
Just over a year after coronavirus disease 2019 (COVID-19) was declared a pandemic, various studies continue to emerge with the aim of improving understanding of the disease and devising treatment strategies. In COVID-19, it stands out that the greater fatality that occurs in men, which is why an attempt has been made to identify some genetic, immunological and hormonal factor that causes men to have this disparity in contrast to women.1
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) enters the host cell through the angiotensin-converting enzyme 2 (ACE2), which is normally expressed at the level of the lung, cardiovascular system, hypothalamus, pituitary, adrenal glands, immune cells, testis, etc.2–4 Like other viruses, such as Zika virus, Ebola virus, Marburg virus, coxsackievirus, among others5; SARS-CoV-2 can reach the testes because ACE2 is highly expressed in spermatogonia, Leydig cells, and Sertoli cells,2 and the blood-testicular barrier does not completely prevent the passage of viruses.6 This has been demonstrated by the histopathological changes that occur in orchitis caused by other viruses that cause severe acute respiratory syndrome (SARS).7
There is a significant hormonal imbalance around the “stress response” in serious diseases such as COVID-19, in favor of a pro-inflammatory-catabolic profile with deterioration of anti-inflammatory-anabolic hormones and therefore low levels of hormone of the human growth and testosterone. Low testosterone levels lead to increased protein breakdown and complications including sarcopenia, loss of lean mass, anemia, impaired healing process, and weakness.8
Currently, some studies report a deleterious prognosis in men with COVID-19 and low testosterone levels. To understand these findings, we conducted a biochemical study that evaluated the prognostic role of total testosterone (TT) levels in the evolution of men with COVID-19 admitted to our hospital.
Materials and methodsPatientsIn a cross-sectional design, we included 86 consecutive men over 18 years old, on admission to hospitalization, with confirmed COVID-19 diagnosis by reverse transcription-polymerase chain reaction (RT-PCR) assays in nasopharyngeal swab samples, admitted to the hospitalization area of the “Hospital de Especialidades del Centro Médico Nacional La Raza”, Instituto Mexicano del Seguro Social in Mexico City, from May 1st to July 31st, 2020. Patients who, prior to admission, were under treatment with steroid (n=5) or testosterone (n=0), patients with cancer (n=11, due to lymphocyte or lactate dehydrogenase levels secondary to oncological disease or chemotherapy effect), human immunodeficiency virus infection (n=2), thyroid disease (n=6), and with hypothalamic-pituitary-gonadal axis disease (n=0) were not included. The protocol was approved by the Research Committee and the Research Ethics Committee of the Hospital with registration number R-2020-3501-143. Informed consent was obtained from all participants.
Upon admission, the severity of the disease was determined according to the “COVID-19 treatment guidelines” published by the National Institutes of Health.9 A blood sample was taken from the patients by peripheral puncture the first day after their hospital admission between 06:00 and 08:00a.m. The biochemical analysis included hematic biometry, glucose, lactate dehydrogenase (LDH), and hormones such as TT, dehydroepiandrosterone sulfate (DHEAS), follicle-stimulating hormone (FSH), and luteinizing hormone (LH). The concentrations of TT, DHEAS, FSH, and LH were determined by chemiluminescence. Demographic characteristics, comorbidities, and outcomes were obtained from the medical record.
Statistical analysisDescriptive statistics were performed for all data, categorical variables were expressed in number and percentage and quantitative variables as mean or median and standard deviation or range, depending on their distribution. To compare categorical variables, we used X2. Kolmogorov–Smirnov test was used to know the distribution of the variables. Student's T-test and U of Mann–Whitney were used depending on variables distribution. The receiver operating characteristic (ROC) curve was used to obtain the mortality cut-off points of variables of interest such as age, LDH, TT and DHEAS, according to the higher sensitivity (Sn%) and specificity (Sp%) shown in the analysis, calculating the positive predictive value (PPV), and negative predictive value (NPV). Area under the curve (AUC) was reported. In the multivariate analysis we used binary logistic regression analysis, selecting the variables that in the bivariate analysis had a p<0.05 and adjusted with variables considered of interest. The statistical package IBM SPSS statics version 25 and Prisma V6 were used.
ResultsEighty-six male patients were included, 38.4% (n=33) had a critical illness and the rest had severe illness. For their analysis, the patients were divided into survivors (51.16%) and non-survivors (48.8%). The median age was 60.5 (17–84) years; non-survivors (NSv) were older than survivors (Sv). There were no differences in body mass index (Table 1).
Univariate analysis of demographic, hormonal, and biochemical characteristics of surviving and non-surviving male patients due to COVID-19.
| All patientsn=86 (100%) | No survivorsn=42 (48.8%) | Survivorsn=44 (51.16%) | P | |
|---|---|---|---|---|
| Age, years | 60.5 (17–84) | 65.5 (39–84) | 50.5 (17–79) | <0.001 |
| BMIa | 28.33±4.06 | 28.87±3.89 | 27.81±4.19 | 0.226 |
| TTb, nmol/Lg | 4.89 (0.29–25.08) | 4.01 (0.29–14.93) | 5.41 (0.55–25.08) | 0.021 |
| DHEASc, μg/dLg | 47.54 (2.8–367.4) | 40.78 (2.8–367.4) | 67.66 (8.2–210.4) | 0.044 |
| LHd, mIU/mLg | 6.1±4.8 | 6.7±4.8 | 5.5±4.9 | 0.269 |
| FSHe, mIU/mLg | 5.5±6.8 | 5.7±5.6 | 5.2±7.9 | 0.723 |
| Lymphocytes, cells×109/Lg | 0.78 (0.18–6.83) | 0.74 (0.28–2.03) | 0.93 (0.18–6.83) | 0.039 |
| LDHf, IU/Lg | 582 (203–17,057) | 717 (203–17,057) | 499 (250–1258) | 0.010 |
TT levels were lower in NSv than in Sv (4.01 [0.29–14.93] vs 5.41 (0.55–25.08) nmol/L, p=0.021), as were DHEAS (40.78 [2.8–367.4] vs 67.66 [8.2–210.4] μg/dL, p=0.044) and lymphocytes (0.74 [0.28–2.03] vs 0.93 [0.18–6.83]×109/L, p=0.039). NSv had higher LDH levels compared to SV (717 [203–17,057] vs 499 [250–1258] IU/L, p=0.010). LH and FSH levels were not different between the groups (Table 1 and Fig. 1).
Total testosterone levels according to classification variables in male patients with COVID-19. * Laboratory reference parameters: TT in men 20–49 years 5.03–55.58nmol/L, TT in men>50 years 6.28–26.77nmol/L. Ω ROC cutoff points: Age>59 (AUC 0.735, Sn 71%, Sp 61%, PPV 64%, NPV 69%), LDH>597IU/L (AUC 0.661, Sn 64%, Sp 70%, PPV 68%, NPV 67%). COPD: Chronic obstructive pulmonary disease. Lymphopenia: Lymphocytes<1.0×109/L. LDH: Lactate dehydrogenase. Statistical analysis: Student's t test, Mann–Whitney U test, and ROC curves.
The best cut-off points for mortality of variables of interest were: Age>59 (AUC 0.735, Sn 71%, Sp 61%, PPV 64%, NPV 69%), TT levels<4.89nmol/L (AUC 0.645, Sn 64%, Sp 64%, PPV 63%, NPV 65%), DHEAS levels<47.2μg/dL (AUC 0.627, Sn 59%, Sp 61%, PPV 59%, NVP 61%), LDH levels>597IU/L (AUC 0.661, Sn 64%, Sp 70%, PPV 68%, NPV 67%).
Age>59 years (p=0.002), diabetes (p=0.001), TT levels<4.89nmol/L (p=0.010), lymphopenia (p=0.002) and LDH levels>597IU/L (p=0.001) were the variables associated with mortality (Table 2). In the multivariate analysis, the independent risk factors that increased the relative risk (RR) of dying from COVID-19 were: age>59 years (RR 3.5 [95% IC 1.0–11.6], p=0.045), TT levels<4.89nmol/L (RR 4.0 [95% IC 1.2–13.5], p=0.027) and LDH levels>597IU/L (RR 3.9 [95% IC 1.2–13.1], p=0.024) (Table 3).
Bivariate analysis of demographic and laboratory characteristics of surviving and non-surviving male COVID-19 patients.
| All patientsn=86 (100%) | No survivorsn=42 (48.8%) | Survivorsn=44 (51.16%) | p | |
|---|---|---|---|---|
| Age>59 yearsa | 47 (54.7%) | 30 (71.4%) | 17 (38.6%) | 0.002 |
| TTb<4.89nmol/La | 43 (50%) | 27 (64.3%) | 15 (35.7%) | 0.010 |
| DHEASc<47.2μg/dLa | 41 (47.7%) | 24 (58.5%) | 17 (38.6%) | 0.067 |
| Lymphopeniae | 58 (67.44%) | 35 (83.3%) | 23 (52.3%) | 0.002 |
| LDHf>597IU/La | 40 (46.5%) | 27 (64.3%) | 13 (29.5%) | 0.001 |
| Diabetes | 38 (44.2%) | 26 (61.9%) | 12 (27.3%) | 0.001 |
| Hypertension | 40 (46.5%) | 24 (57.1%) | 16 (36.4%) | 0.053 |
| Obesity | 27 (31.4%) | 15 (35.7%) | 12 (27.3%) | 0.399 |
| Stroke | 9 (10.5%) | 5 (11.9%) | 4 (9.1%) | 0.670 |
| Chronic kidney disease | 13 (15.1%) | 6 (14.3%) | 7 (15.9%) | 0.834 |
| COPDd | 3 (3.5%) | 2 (4.8%) | 1 (2.3%) | 0.529 |
| Smoking | 39 (45.3%) | 20 (47.6%) | 19 (43.2%) | 0.679 |
Multivariate analysis for mortality from COVID-19 in male patients.
| RRa (CI 95%) | p | |
|---|---|---|
| Age>59 yearsb | 3.5 (1.0–11.6) | 0.045 |
| TTc<4.89nmol/Lb | 4.0 (1.2–13.5) | 0.027 |
| DHEASd<47.2μg/dLb | 2.8 (0.9–8.9) | 0.088 |
| Lymphopeniad | 3.4 (1–12.4) | 0.059 |
| LDHe>597IU/Lb | 3.9 (1.2–13.1) | 0.024 |
| Diabetes | 2.8 (0.9–9.2) | 0.095 |
| Hypertension | 2.5 (0.7–8.7) | 0.156 |
| Obesity | 1.1 (0.3–4.0) | 0.906 |
In addition to the patients who died, the patients who required mechanical ventilation (MV) (p=0.025), had lymphopenia (p=0.013) and LDH levels>597IU/L (p=0.034), had significantly lower TT levels compared to those who did not present these conditions. There were no differences in TT levels between patients who had or did not have comorbidities, smoking, or age older or younger than 59 years (Fig. 1).
DiscussionAge, comorbidities, and mortality in male patients with COVID-19We report a cohort of 86 consecutive male patients who required hospitalization for COVID-19. The age of our patients was like that reported in two Italian studies,10,11 but higher than that reported in Chinese, North American, and Turkish populations.5,12–14 According to literature, hypertension, diabetes, and obesity were the most frequent comorbidities, but only diabetes was associated with death; patients who died were older and had higher LDH levels.13,15,16
The 48.8% of our patients died, a percentage much higher than that reported by Rastrelli G et al. (6%) and Salonia A et al. (8%). The difference in the mortality percentage with Rastrelli et al. lies in the fact that their cohort had only 31 patients with severe COVID-19, of which four evolved to critical illness (12.9%); however, at the time of its analysis, 19.4% had not yet defined its outcome (recovery or death). Moreover, our percentage of critically ill patients since their hospital admission was higher. Compared with Salonia et al., their cohort size (n=286) explains their lower percentage of mortality. It is noteworthy that they managed to withdraw MV in 17.8% of their critical patients; but on the other hand, 9.4% of their patients with severe disease were discharged from the emergency service to their home, it is not specified if they did not return to the hospital for evaluation in the event of a possible progression of the disease.10,11
Testosterone, lymphocytes, and mortality in male patients with COVID-19Because not only the presence of comorbidities explains the greater severity and fatality of COVID-19 in men, attention has been paid to hormonal factors; especially testosterone, since it not only has metabolic and cardiovascular functions, but also immune functions.1,17
The presence of significantly lower TT levels in male patients with COVID-19 has been demonstrated by Salonia et al., Ma et al. and Kadihasanoglu M et al., when comparing male patients with COVID-19 with healthy controls and patients with lower respiratory tract infection not due to COVID-19.5,11,12
Rastrelli et al. reported that TT levels<5nmol/L increased the risk of ICU admission and/or mortality by 10 to 15 times. They also observed that patients with SARS had significantly lower TT levels compared to those without SARS. Compared to the authors, our cut-off point for mortality of TT level was very similar to theirs, although our RR for mortality was markedly lower. We also observed that our patients with MV, indicated by SARS, had lower TT levels than those without MV. Our lymphocytes count's median was like that of the authors (0.7 [0.5–0.9]×109/L) and, like them, this figure was lower in the NSv. Rastrelli et al. observed that TT levels had a positive correlation with lymphocyte count (r=0.493, p=0.007); in our case, we did not observe this correlation, but we did observe that patients with lymphopenia had lower TT levels and that lymphopenia was significantly associated with mortality and had a tendency to increase this risk in men with COVID-19.10
The median TT levels (2.5 [1.0–4.7] nmol/L) reported by Salonia A et al. was lower than ours and, on the contrary, their lymphocytes count's median (1.0 [0.7–1.4]×109/L) was higher, probably due to their cohort size. These authors reported that patients who required MV (1.0 [0.5–1.7] nmol/L) or who died (0.7 [0.3–2.3] nmol/L) had significantly lower TT levels compared to those that did not, the same was observed in our cohort but the TT values for each of these conditions were higher.11
Since Ma L et al. and Kadihasanoglu M et al. included patients with mild, moderate, and severe disease, their TT levels and lymphocytes count were higher than ours: Ma et al. (13.76 [10.82–19.69] nmol/L and lymphocytes count 1.88 [0.22–3.77]×109/L) and Kadihasanoglu M et al. (6.43 [IQR 6.21] nmol/L and lymphocytes count 1.4 [IQR 0.83]×109/L). Ma L et al. negatively associated TT levels with disease severity indirectly through the T:LH ratio.11 Kadihasanoglu M et al. reported a significant negative correlation between TT levels and hospitalization time and a significant positive correlation with blood oxygen saturation, both situations related to disease progression to more severe forms of COVID-19. As well as us, Kadihasanoglu M et al. did not observe a correlation between TT levels and lymphocyte count.5,12
In male patients with COVID-19 in ICU, Schroeder M et al. found that 68.6% (24/35) had low TT levels. They found that TT levels were negatively correlated with interleukin (IL)-2 and interferon (IFN)-γ, which reflected a loss of the immunosuppressive effect of testosterone against infections.18
In outpatients, Okcelik S (n=44) reported that patients who developed COVID-19 pneumonia had significantly lower TT levels than those who did not. The authors’ TT average (9.04±5.37mmol/L) is higher than ours due to the lower severity of his patients.14
Like Rastrelli et al., we did not find that TT levels were associated with age or obesity.10 Additionally, we observed that they were not associated with comorbidities or smoking. Therefore, we think that the low TT levels are due to acute testicular damage produced by SARS-CoV-2, specifically Leydig cells, which has also been proposed by other authors.12,14
Unlike the previously cited studies, and except for Rastrelli et al., we established a cut-off point for TT levels below which the RR of mortality from COVID-19 increases by four times significantly and independently.
DHEAS, LH, and FSH in male patients with COVID-19Schroeder M et al. found that 48.6% of their male COVID-19 patients had low dihydrotestosterone levels (<10ng/dL).18 In our case, we measured DHEAS, and a similar percentage had it low. The decrease in DHEAS could influence TT decreases, especially in NSv; however, its impact could be minimal considering that most testosterone is produced at the gonadal level.19 The low values of both androgens reflect an adrenal cortex dysfunction during COVID-19.4
Regarding LH and FSH, in our cohort they were within the laboratory reference parameters (LRP) and there were no differences between Sv and NSv. Due to the heterogeneity of the patients in each cohort, our LH values were higher than those reported by Salonia et (4.7 [3.0–6.7] mIU/mL), Ma L et al. (5.93 [4.31–8.25] mIU/mL), Kadihasanoglu M et al. (5.67 [4.52] mIU/mL) and Okcelik S (5.5 [2–12] mIU/mL), and lower than reported by Rastrelli et al. (16.3 [7.9–20.3] mIU/mL).5,11,12,14
Only in Rastrelli et al.’s cohort a tendency for hypergonadotropic hypogonadism was observed based on the report of their LH levels10; in our case, we could only observe this compensation in 17 of 69 patients. This could be due to the time of the COVID-19 evolution in which LH was measured; but on the other hand, it may also be due this reflects an inadequate response of the hypothalamic-pituitary-gonad axis in COVID-19. It is not clear if this inadequate response is due to a direct effect of the virus on the hypothalamus or pituitary, or if it is secondary to a humoral response and release of inhibitory cytokines.4
Ma L et al. found no differences in FSH levels between their COVID-19 patients and healthy controls (4.13 [3.02–6.31] vs 4.51 [3.39–5.97] mIU/mL, p=0.578), this because FSH is not altered since the Sertoli cells are less disturbed than Leydig cells in COVID-19, which explains why our FSH levels are in the LRP range.12
Testosterone in comorbidities, immunity, and COVID-19Testosterone is the main androgen, 95% is produced at the gonadal level, by Leydig cells stimulated by LH, and to a lesser extent from dehydroepiandrosterone, it is last produced in adrenal glands from cholesterol by various enzymatic pathways.19 Normally, testosterone decreases by 0.4–2% per year after the age of 30, leading to a state known as late-onset hypogonadism, usually evident after the age of 60. In late-onset hypogonadism, decreased testosterone is not only associated with loss of libido, erectile dysfunction, and loss of muscle and bone mass, but also with an increased likelihood of metabolic syndrome, obesity, diabetes, obstructive sleep apnea, and cardiovascular disease,20,21 all of them with high prevalence in patients with COVID-19.15,22
There are two possible mechanisms by which androgens can influence COVID-19 clinical outcomes. The first mechanism is related to the expression of the transmembrane protease serine 2 (TMPRSS2) and the second mechanism to androgen-driven immune modulation.23
SARS-CoV-2 requires two host cellular proteins: ACE2 and TMPRSS2.24 SARS-CoV-2 enters the host cell through the binding of its peak protein to ACE2, which is expressed at extremely high levels in Leydig cells, Sertoli cells, seminiferous duct tissues, and spermatogonia, being the male reproductive system a gate for the systemic spread of SARS-CoV-2.2,3,22,24–26 TMPRSS2 cleaves the SARS-CoV-2 peak antigen at the S1/S2 and S2 sites and this is crucial to allow viral fusion with the host cell membrane. The expression of the TMPRSS2 gene is reinforced by androgens, which favors a greater viral entry that correlates with the severity of the disease in men.25
In humans and animals, testosterone is an immunomodulator capable of altering the number, function, and differentiation of most immune cell populations.27 Through the androgen receptor (AR), expressed in immune cells such as macrophages, neutrophils, and lymphocytes T (LT), testosterone promotes the expression of anti-inflammatory mediators and thus protects against a variety of inflammation-mediated diseases.27–29 Testosterone also exerts its immunosuppressive effect by inhibiting the pro-inflammatory pathway of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), thereby reducing IL-6 and tumoral necrosis factor (TNF)-α levels.29 JAK (Janus kinase)-STAT (signal transducers and activators of transcription) are critical for signaling the modulatory mechanism by which cytokines contribute to the progression of inflammatory diseases, including COVID-19.30 Previous studies have shown that testosterone suppresses the lipopolysaccharide-induced JAK-STAT1 pathway and thereby inhibits the expression of pro-inflammatory cytokines such as IL-1β, IL-6, and IL-8.31
By decreasing testosterone, its immunosuppressive effect is lost due to the immune cells differentiation is affected with a decrease in the number of LT regulatory, a decrease in LT CD8+ with a reduction in the expression of IFN-γ (which in the initial stages of infection is essential to decrease viral replication) and suppression of NK cell proliferation; there are a greater formation of inflammatory cytokines, mainly IL-6, and loss of inhibition of the JAK-STAT pathways.27,30–32 All these events favoring an intense inflammatory state and a deficient immune response to infection by SARS-CoV-2, which not only causes damage to the lungs but to other systems. Furthermore, the apoptosis of lymphocytes directly induced by SARS-CoV-2 also favors the decrease of these lymphocytes’ subpopulations.25
Pozzilli and Lenzi have suggested that low testosterone levels, in the COVID-19 clinical course, can cause a reduction in muscle strength, including respiratory muscles, while normal testosterone levels can have a protective effect on severe respiratory events.20 The testosterone beneficial effects on respiratory muscles have also been suggested by other authors.33,34
The role of testosterone as an anti-inflammatory hormone is reflected in the study by Rambhatla A et al. The authors compared two groups of male patients with COVID-19, one of the groups had testosterone replacement therapy (TRT); they found that the rates of thromboembolism and death were similar in both groups. There were lower rates of ICU admission and MV needing in the TRT group, despite having a higher rate of baseline comorbidities, but without statistical significance.13
In animals, Vom Steeg et al. administered testosterone to elderly male mice infected with influenza A virus, and they observed improvement in survival after infection; while in young male mice, it reduced the synthesis of inflammatory cytokines (IFN-γ and TNF-α), increased anti-inflammatory cytokines (IL-10) and decreased Th1 lymphocyte activity, for which they hypothesized that testosterone mitigates the inflammation and improves outcomes from influenza infection.35 In another study, Ho CH et al. administered testosterone in prostate cell cultures infected by uropathogenic escherichia coli and observed a reduction in inflammation by inhibiting the JAK-STAT1 and JAK-STAT3 signaling pathways, thus attenuating the severity of prostatitis.31
Testosterone can be considered a double-edged sword since, on the one hand, its high (normal) levels are related to a greater expression of TMPRSS2 (favoring a greater entry of SARS-CoV-2) and, on the other, its low levels they are related to a poor immunomodulatory and anti-inflammatory response.36
Our study has some limitations such as the cross-sectional design, the size of the sample and that we do not have TT levels after recovery from COVID-19. Other limitation was that the sex hormone-binding globulin (SHBG) levels were not measured.37 About 60% of circulating testosterone is tightly bound to SHBG, rendering this fraction of testosterone biologically inactive.37 SHBG levels have generally been reported decreased in obesity, diabetes mellitus, hypothyroidism, acromegaly, nephrotic syndrome, and patients with steroid, progesterone, or insulin use; and increased in aging, cirrhosis, hyperthyroidism, human immunodeficiency virus, use of androgens, and anticonvulsants; however, in our population this reference could not be absolute, since the NSv were older and diabetic, and on the other hand there was no difference in BMI between Sv and NSv.37
The strength of this study is that it reaffirms the prognostic value of TT in COVID-19. Furthermore, it is the first study in Latin America, to our knowledge, to establish a cut-off point for below which the RR of death from COVID-19 increases. Our data indicate that the low levels of TT are due to an acute damage induced by the virus at the testicular level rather than to a preexisting permissive state, which affects the anti-inflammatory and immunomodulatory response of testosterone. The knowledge of the effects of testosterone on the immune system gives the guideline to try TRT in men with severe or critical COVID-19 and low TT levels to reduce the lethality in this group of patients. However, in prostate cancer patients, androgen deprivation therapies decrease TMPRSS2 levels, which may partially protect them from severe SARS-CoV-2 infections.38
ConclusionTT levels<4.89nmol/L increase significantly four times the RR of death from COVID-19 in Mexican men, regardless of age or presence of comorbidities. Based on this finding, the possibility open ups of considering testosterone as an adjunctive treatment in selected patients with severe and critical COVID-19. Further studies are required to corroborate our results.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Sources of fundingOwn resources of Hospital de Especialidades Centro Médico Nacional “La Raza”, Instituto Mexicano del Seguro Social.
Conflict of interestNone.
To all our hospital workers, especially Internal Medicine service.