Erectile dysfunction (ED) is one of the main threats in diabetic patients. This research aimed to assess the relationship between glycated hemoglobin (HbA1c) level and pharmacopenile duplex ultrasonography (PPDU) indices in diabetic patients with ED.
Materials and methodsA total of 130 males with ED were recruited (100 had diabetes mellitus (DM) and 30 did not as control). The International Index of Erectile Function (IIEF) was used to evaluate patients for ED. Measurement of HbA1c, lipid profile and assessment of erectile function using PPDU were performed. All participants were assessed to take the medical history.
ResultsThe mean age±SD was 53.8±8.9 and 53.6±2.8 years for patients and controls, respectively. Patients had variable grades of ED: mild in 20%, mild to moderate in 32.3%, moderate in 35.3%, and severe in 12.3%. A significant association was found between the existence of DM and a deprived response to intracorporeal injection (ICI), rising end-diastolic velocity (EDV), and reducing resistance index (RI) values. Comparing all diabetic groups according to HbA1c with controls, a significant relationship was found in; severity of IIEF-5 score, poor response to ICI, decreased peak systolic velocity (PSV) at 10min, increased EDV at 10, 20min and decreased RI at 10, 20min. A significant relationship was found between smoking, dyslipidaemia, and decreased PSV at 10, 20min and decreased increment ratio. However, a non-significant relationship was observed between age, type of DM and PPDU parameters.
ConclusionPoor glycaemic control of DM is associated with an increase in EDV and decrease in RI, and PSV of PPDU.
La disfunción eréctil (DE) es una de las principales amenazas en los pacientes diabéticos. El objetivo de este estudio fue evaluar la relación entre el nivel de hemoglobina glicosilada (HbA1c) y los índices de la ecografía dúplex fármaco-penile (PPDU) en los pacientes diabéticos con DE.
Materiales y métodosSe reunió a un total de 130 varones con DE (100 con diabetes mellitus [DM] y 30 no diabéticos como control). Se utilizó el Índice Internacional de Función Eréctil (IIEF) para evaluar la DE en los pacientes. Se midieron los valores de HbA1c, perfil lipídico y evaluación de la función eréctil utilizando PPDU. Se evaluó a todos los participantes para realizar la historia médica.
ResultadosLa edad±DE fue de 53,8±8,9 y 53,6±2,8 años para los pacientes y controles, respectivamente. Los pacientes tenían grados variables de DE: leve en el 20% de los casos, de leve a moderado en el 32,3%, moderado en el 35,3% y grave en el 12,3%. Se encontró una asociación significativa entre la existencia de DM y la ausencia de respuesta a la inyección intracorpórea (ICI), incremento de la velocidad diastólica final (VDF) y reducción de los valores del índice de resistencia (IR). Con arreglo a la HbA1c, la comparación entre todos los grupos diabéticos y los controles arrojó una relación significativa en cuanto a: gravedad de la puntuación IIEF-5, mala respuesta a la ICI, reducción de la velocidad sistólica pico (VSP) a los 10min, incremento de VDF a los 10 y 20min y reducción de IR a los 10 y 20min. Se encontró una relación significativa entre tabaquismo, dislipidemia y reducción de VSP a los 10 y 20min y reducción del ratio de incremento. Sin embargo, se observó una relación no significativa entre la edad, tipo de DM y parámetros PPDU.
ConclusiónUn mal control glucémico en la DM está asociado al incremento de VDF y a la reducción del IR y de la VSP de PPDU.
Erectile dysfunction (ED) is an incapacity to gain and/or sustain an erection enough to allow satisfactory sexual relationships.1 The population affected is anticipated to amplify from 152 million in 1995 to 322 million in 2025 worldwide.2 Unfortunately, there are no concrete statistics on the proper occurrence of ED in Arab countries, however, anecdotal reviews indicate an excessive incidence among diverse age groups and in patients with various comorbidities.3 In another Upper Egyptian study, it was found that of 658 people with ED, 40.1% were smokers, 17.3% had hypertension, and 21.4% had diabetes mellitus (DM). The incidence of these risk elements among controls was 28.7%, 2.8%, and 3.7% respectively, and they stated that hypertension, DM and smoking were major danger elements for ED.4 The incidence levels of ED in diabetic population cross-sectional studies vary from 20% to 71%. The greatest of these researches did not manage disease severity, disease duration, or hyperglycemia control.5
DM is one of ED's most frequent comorbidities, the incidence of ED among diabetic males differs from 20 to 85% (ranging from mild to entire ED) which shows up at sooner age than non-diabetic males.6,7
Six Middle East and North African countries are among the top 10 for DM prevalence in the world, and a similar state relates to the prevalence of impaired glucose tolerance. Such nations are the United Arab Emirates, Bahrain, Egypt, Kuwait, Oman, and Saudi Arabia. Population aging, along with changes in socio-economic and lifestyle, has contributed to a drastic rise in DM occurrence.8
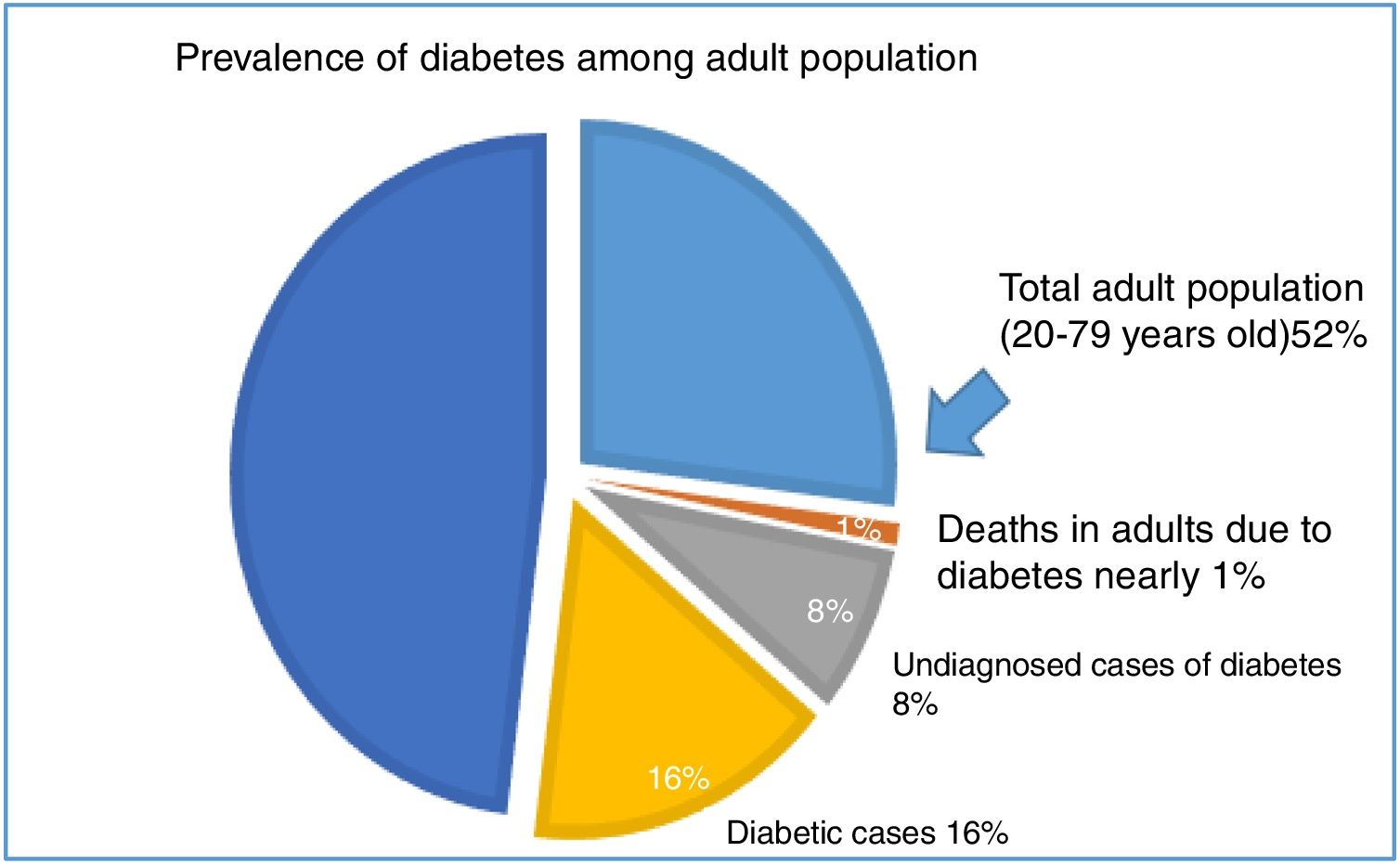
In Egypt, there were 48 million (52%) total adult population (20–79 years old), 7.5 million (16%) of them have diabetes. Additionally, the estimated undiagnosed cases of diabetes in adults were 3.8 million (8%) and the overall mortality in adults due to diabetes estimated 86 thousand (nearly 1%) (Fig. 1). Also, the incidence of type 1 diabetes in population aged 0–14 years was 8 per 100.000.9,10
Pie chart show the prevalence of diabetes among adult population aged 20–79 years old (48 million, 52%). The prevalence of diabetic cases was 16%, undiagnosed cases of diabetes in adults was 8%, and the overall mortality in adults due to diabetes was 86 thousand (nearly 1%).9,10
The level of HbA1c is broadly used in patients with type I and type II diabetes for regular evaluation of the long-term glycemic conditions. HbA1c is the guide of suggesting glycemia and therefore records the degree of glycemic control, treatment response and the hazard of diabetes problems emerging or worsening.11
The current study was conducted to establish the relation between HbA1c and variations in the pharmacopenile Doppler ultrasonography (PPDU) in ED patients. We also analyzed the impact of DM, length of DM, smoking, and metabolic regulation of DM on indices of PPDU.
Material and methodsThis was a cross-sectional descriptive study in which 130 male patients with ED clinical diagnosis were recruited (100 had diabetes mellitus (DM) and 30 did not as control). The study was achieved at the Department of Dermatology and Andrology and Department of Diagnostic Radiology at the period from March 2018 to October 2019. Patients were evaluated for ED by the International Index of Erectile Function (IIEF-5). Medical history including history of DM, period of DM, and diabetes-associated complications were taken at the same visit that they were evaluated for ED. The data provided by the patients were verified with the clinical records, particularly for the existence of chronic problems like heart disease, nephropathy, retinopathy, neuropathy, and diabetic foot. All participants were subjected to laboratory analyses for diabetic patients as HbA1c and lipid profile. The PPDU was done in an atmosphere of privacy for all patients by only an andrologist and a radiologist after written informed consent. All patients were assessed by a linear probe using (GE® RT 3200 series instrument) with 7.5-MHz frequency, by intracorporeal injection (ICI) of 10 mcg prostaglandin E1 (alprostadil) at one of the corpora cavernosa laterally in the distal portion of the penis as it is easily handled and with a lower incidence of prick bleeding with a 30-gauge needle.12–14 Patients were permitted of manual self-stimulation for 5min. Color Doppler imaging of the cavernous arteries was conducted together with an arterial segment corresponds to a Doppler position of 60° for better illumination of duplex waves and easy measurement of duplex parameters in this angle and a specimen size of 2mm in all participants to acquire comparable information.15,16 The Doppler variables (peak systolic velocity (PSV), end-diastolic velocity (EDV), and resistive index (RI)) were reported 5, 10, and 20min after ICI.17
The study protocol was approved by the Medical School Ethical Review Board (Ethical Committee N: IRB17100826). All participants signed an informed consent prior to inclusion in the study.
Disease grades of ED on IIEF-5 scores were classified into: severe ED (IIEF-5 score: 1–7), moderate ED (8–11), mild to moderate ED (12–16), mild ED (17–21), and no ED (22–25).17,18 The visual grading for the erectile response was classified into E1: elongation of shaft only; E2: moderate tumescence, no rigidity; E3: full tumescence, no rigidity, easily bendable; E4: full erection, partial rigidity; E5: full rigidity for at least 20min.19,20 The response to ICI was categorized as normal (E4+E5) response and ED (E1+2+3). The PSV was categorized as below 25cm/s, 25–35cm/s, and above 35cm/s. The EDV was ordered as 5cm/s or fewer and more than 5cm/s.21–23 The RI was categorized as 0.9 or higher and less than 0.9.14 Glycemic control was detected according to HbA1c analysis dating not more than 1 month prior to the interview. It was graded as follows: good, 6.2–6.8%; fair, 6.9–8.3%; and poor, greater than 8.4%.24
Blood samples and biochemical assays of HbA1c and lipid profileA total of 5mL of fasting blood samples were obtained from an antecubital vein and divided into two parts. Two milliliters of blood were collected in EDTA tubes and used for HbA1c assays. The remaining 3mL was withdrawn in a serum separator gel tube and allowed to clot for 30min at 37°C before centrifugation and the sera separated by centrifugation. In the plasma samples of the studied groups, HbA1c levels were measured using an ELISA kit (Cloud Clone Corporation, USA, Catalog No: CEA190Hu) according to the manufacturer's instructions. The serum levels of blood glucose, total cholesterol, low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL) and triglyceride were determined by the Biodiagnostic Assay Kits, Dokki, Giza, Egypt (catalog no. CH 12 20, CH 12 31, CH 12 30, TR 20 30) according to the manufacturer's guidelines. Dyslipidemia is identified as an elevation of the total cholesterol, LDL, and triglyceride concentrations, and a fall in HDL concentrations in the blood.25
Statistical analysisThe data were analyzed using the Statistical Package of Social Science (SPSS.11.0, SPSS, Inc., Chicago, IL) software program. Data were statistically in terms of range, mean, standard deviation (SD), median, frequencies (number of cases) and relative frequencies (percentages) when appropriate. A comparison of quantitative data between different groups in the present study was done using a Student t-test and ANOVA for the independent sample when normally distributed and Mann–Whitney U test for an independent sample when not normally distributed. Comparisons of the categorical variables were made by the chi-squared test (X2). A probability value (p value) less than 0.05 was considered statistically significant.
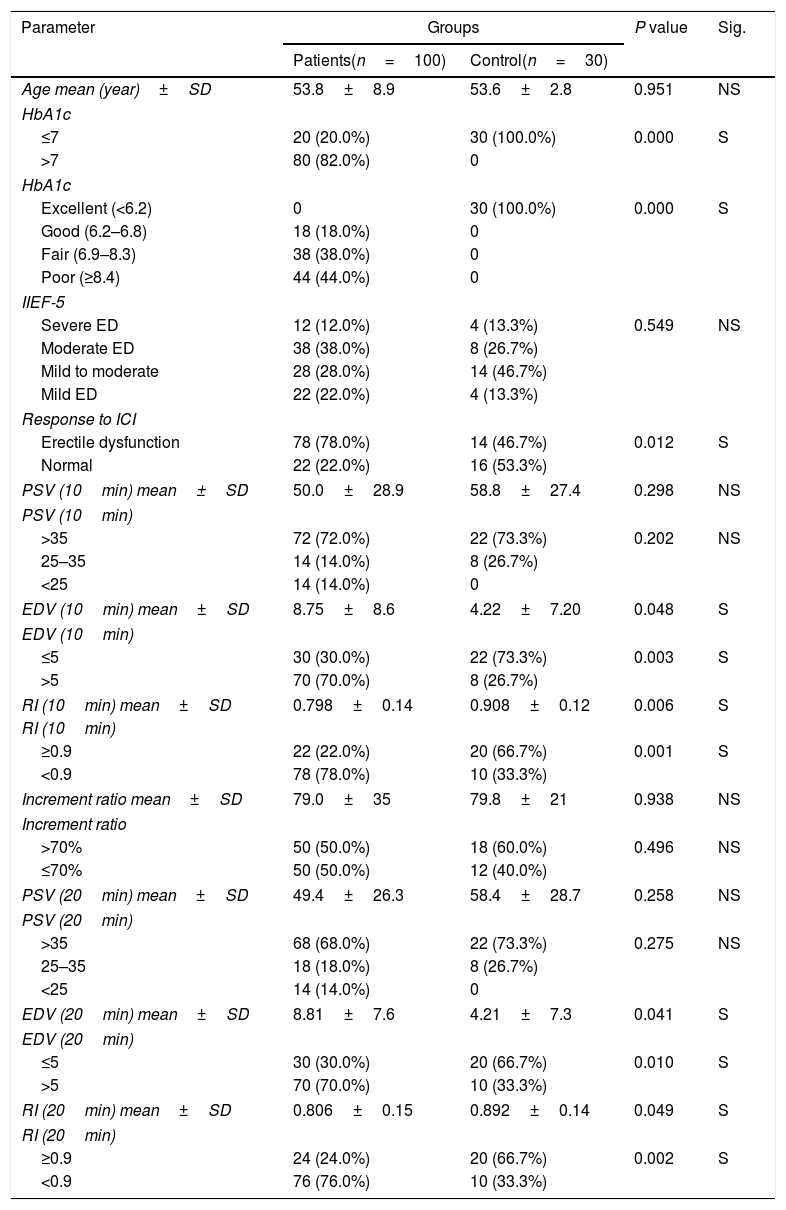
ResultsA statistically significant association between the existence of diabetes and a deprived response to ICI, rising EDV levels and reducing RI levels was found. However, no statistically significant association was found between the existence of diabetes and decreasing PSV levels or increase increment ratio (Table 1).
Descriptive of demographic data of patients and control.
| Parameter | Groups | P value | Sig. | |
|---|---|---|---|---|
| Patients(n=100) | Control(n=30) | |||
| Age mean (year)±SD | 53.8±8.9 | 53.6±2.8 | 0.951 | NS |
| HbA1c | ||||
| ≤7 | 20 (20.0%) | 30 (100.0%) | 0.000 | S |
| >7 | 80 (82.0%) | 0 | ||
| HbA1c | ||||
| Excellent (<6.2) | 0 | 30 (100.0%) | 0.000 | S |
| Good (6.2–6.8) | 18 (18.0%) | 0 | ||
| Fair (6.9–8.3) | 38 (38.0%) | 0 | ||
| Poor (≥8.4) | 44 (44.0%) | 0 | ||
| IIEF-5 | ||||
| Severe ED | 12 (12.0%) | 4 (13.3%) | 0.549 | NS |
| Moderate ED | 38 (38.0%) | 8 (26.7%) | ||
| Mild to moderate | 28 (28.0%) | 14 (46.7%) | ||
| Mild ED | 22 (22.0%) | 4 (13.3%) | ||
| Response to ICI | ||||
| Erectile dysfunction | 78 (78.0%) | 14 (46.7%) | 0.012 | S |
| Normal | 22 (22.0%) | 16 (53.3%) | ||
| PSV (10min) mean±SD | 50.0±28.9 | 58.8±27.4 | 0.298 | NS |
| PSV (10min) | ||||
| >35 | 72 (72.0%) | 22 (73.3%) | 0.202 | NS |
| 25–35 | 14 (14.0%) | 8 (26.7%) | ||
| <25 | 14 (14.0%) | 0 | ||
| EDV (10min) mean±SD | 8.75±8.6 | 4.22±7.20 | 0.048 | S |
| EDV (10min) | ||||
| ≤5 | 30 (30.0%) | 22 (73.3%) | 0.003 | S |
| >5 | 70 (70.0%) | 8 (26.7%) | ||
| RI (10min) mean±SD | 0.798±0.14 | 0.908±0.12 | 0.006 | S |
| RI (10min) | ||||
| ≥0.9 | 22 (22.0%) | 20 (66.7%) | 0.001 | S |
| <0.9 | 78 (78.0%) | 10 (33.3%) | ||
| Increment ratio mean±SD | 79.0±35 | 79.8±21 | 0.938 | NS |
| Increment ratio | ||||
| >70% | 50 (50.0%) | 18 (60.0%) | 0.496 | NS |
| ≤70% | 50 (50.0%) | 12 (40.0%) | ||
| PSV (20min) mean±SD | 49.4±26.3 | 58.4±28.7 | 0.258 | NS |
| PSV (20min) | ||||
| >35 | 68 (68.0%) | 22 (73.3%) | 0.275 | NS |
| 25–35 | 18 (18.0%) | 8 (26.7%) | ||
| <25 | 14 (14.0%) | 0 | ||
| EDV (20min) mean±SD | 8.81±7.6 | 4.21±7.3 | 0.041 | S |
| EDV (20min) | ||||
| ≤5 | 30 (30.0%) | 20 (66.7%) | 0.010 | S |
| >5 | 70 (70.0%) | 10 (33.3%) | ||
| RI (20min) mean±SD | 0.806±0.15 | 0.892±0.14 | 0.049 | S |
| RI (20min) | ||||
| ≥0.9 | 24 (24.0%) | 20 (66.7%) | 0.002 | S |
| <0.9 | 76 (76.0%) | 10 (33.3%) | ||
Categorical data are presented in the form of frequency and percent. Quantitative data are presented in the form of mean±SD. NS: Not significant; S: Significant (p<0.05).
IIEF-5, International Index of Erectile Function scores; ICI, intracavernous injection; RI, resistive index; EDV, end diastolic velocity; PSV, peak systolic velocity.
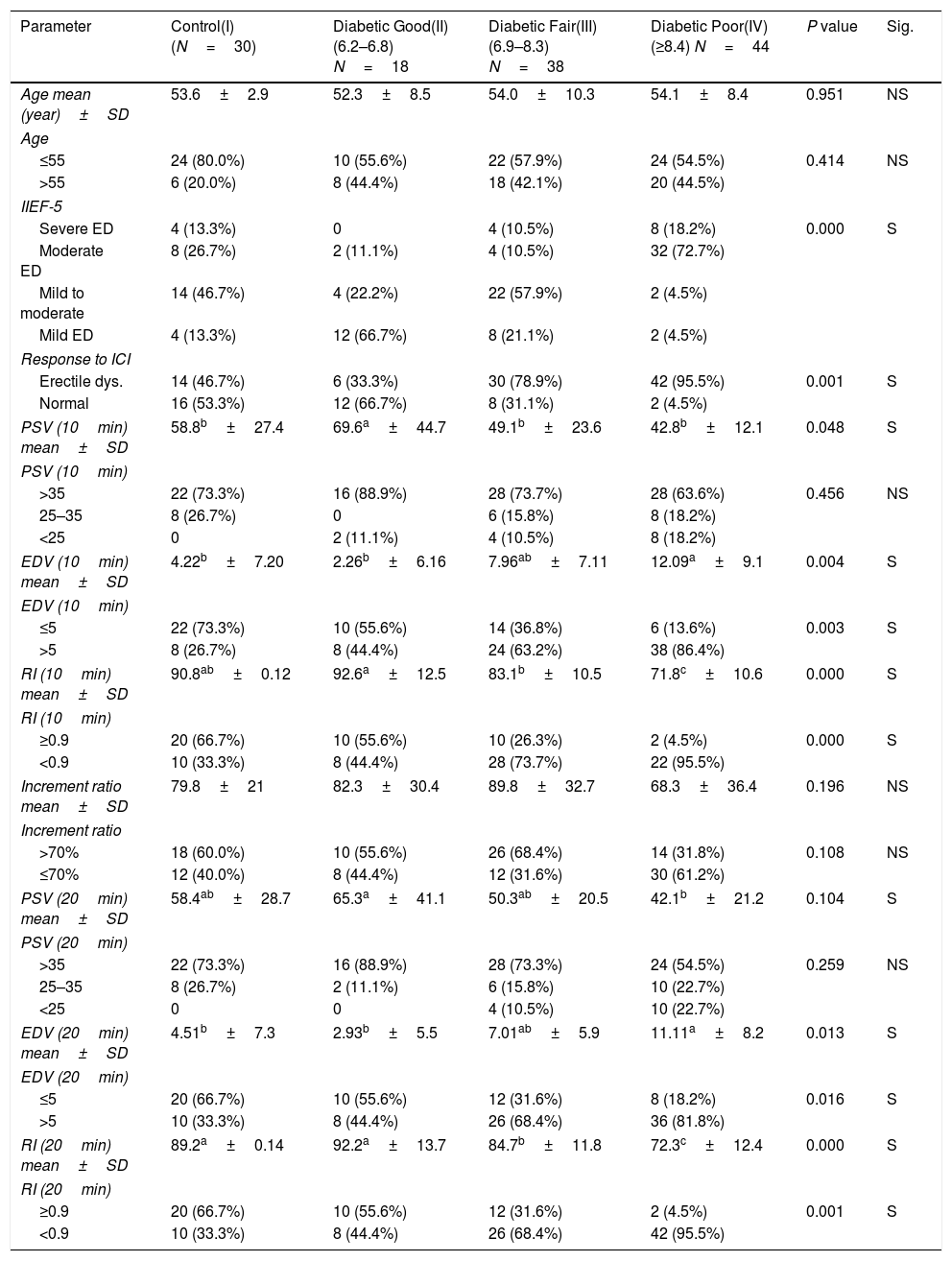
When compared all diabetic groups according to HbA1c with control group, we found a significant relation in the following; severity of IIEF-5 score, poor response to ICI, decrease PSV at 10min, increase EDV at 10, 20min and decrease RI at 10, 20min (Table 2).
Comparison among control and all HbA1c categories of diabetics.
| Parameter | Control(I)(N=30) | Diabetic Good(II)(6.2–6.8) N=18 | Diabetic Fair(III) (6.9–8.3) N=38 | Diabetic Poor(IV)(≥8.4) N=44 | P value | Sig. |
|---|---|---|---|---|---|---|
| Age mean (year)±SD | 53.6±2.9 | 52.3±8.5 | 54.0±10.3 | 54.1±8.4 | 0.951 | NS |
| Age | ||||||
| ≤55 | 24 (80.0%) | 10 (55.6%) | 22 (57.9%) | 24 (54.5%) | 0.414 | NS |
| >55 | 6 (20.0%) | 8 (44.4%) | 18 (42.1%) | 20 (44.5%) | ||
| IIEF-5 | ||||||
| Severe ED | 4 (13.3%) | 0 | 4 (10.5%) | 8 (18.2%) | 0.000 | S |
| Moderate ED | 8 (26.7%) | 2 (11.1%) | 4 (10.5%) | 32 (72.7%) | ||
| Mild to moderate | 14 (46.7%) | 4 (22.2%) | 22 (57.9%) | 2 (4.5%) | ||
| Mild ED | 4 (13.3%) | 12 (66.7%) | 8 (21.1%) | 2 (4.5%) | ||
| Response to ICI | ||||||
| Erectile dys. | 14 (46.7%) | 6 (33.3%) | 30 (78.9%) | 42 (95.5%) | 0.001 | S |
| Normal | 16 (53.3%) | 12 (66.7%) | 8 (31.1%) | 2 (4.5%) | ||
| PSV (10min) mean±SD | 58.8b±27.4 | 69.6a±44.7 | 49.1b±23.6 | 42.8b±12.1 | 0.048 | S |
| PSV (10min) | ||||||
| >35 | 22 (73.3%) | 16 (88.9%) | 28 (73.7%) | 28 (63.6%) | 0.456 | NS |
| 25–35 | 8 (26.7%) | 0 | 6 (15.8%) | 8 (18.2%) | ||
| <25 | 0 | 2 (11.1%) | 4 (10.5%) | 8 (18.2%) | ||
| EDV (10min) mean±SD | 4.22b±7.20 | 2.26b±6.16 | 7.96ab±7.11 | 12.09a±9.1 | 0.004 | S |
| EDV (10min) | ||||||
| ≤5 | 22 (73.3%) | 10 (55.6%) | 14 (36.8%) | 6 (13.6%) | 0.003 | S |
| >5 | 8 (26.7%) | 8 (44.4%) | 24 (63.2%) | 38 (86.4%) | ||
| RI (10min) mean±SD | 90.8ab±0.12 | 92.6a±12.5 | 83.1b±10.5 | 71.8c±10.6 | 0.000 | S |
| RI (10min) | ||||||
| ≥0.9 | 20 (66.7%) | 10 (55.6%) | 10 (26.3%) | 2 (4.5%) | 0.000 | S |
| <0.9 | 10 (33.3%) | 8 (44.4%) | 28 (73.7%) | 22 (95.5%) | ||
| Increment ratio mean±SD | 79.8±21 | 82.3±30.4 | 89.8±32.7 | 68.3±36.4 | 0.196 | NS |
| Increment ratio | ||||||
| >70% | 18 (60.0%) | 10 (55.6%) | 26 (68.4%) | 14 (31.8%) | 0.108 | NS |
| ≤70% | 12 (40.0%) | 8 (44.4%) | 12 (31.6%) | 30 (61.2%) | ||
| PSV (20min) mean±SD | 58.4ab±28.7 | 65.3a±41.1 | 50.3ab±20.5 | 42.1b±21.2 | 0.104 | S |
| PSV (20min) | ||||||
| >35 | 22 (73.3%) | 16 (88.9%) | 28 (73.3%) | 24 (54.5%) | 0.259 | NS |
| 25–35 | 8 (26.7%) | 2 (11.1%) | 6 (15.8%) | 10 (22.7%) | ||
| <25 | 0 | 0 | 4 (10.5%) | 10 (22.7%) | ||
| EDV (20min) mean±SD | 4.51b±7.3 | 2.93b±5.5 | 7.01ab±5.9 | 11.11a±8.2 | 0.013 | S |
| EDV (20min) | ||||||
| ≤5 | 20 (66.7%) | 10 (55.6%) | 12 (31.6%) | 8 (18.2%) | 0.016 | S |
| >5 | 10 (33.3%) | 8 (44.4%) | 26 (68.4%) | 36 (81.8%) | ||
| RI (20min) mean±SD | 89.2a±0.14 | 92.2a±13.7 | 84.7b±11.8 | 72.3c±12.4 | 0.000 | S |
| RI (20min) | ||||||
| ≥0.9 | 20 (66.7%) | 10 (55.6%) | 12 (31.6%) | 2 (4.5%) | 0.001 | S |
| <0.9 | 10 (33.3%) | 8 (44.4%) | 26 (68.4%) | 42 (95.5%) | ||
a,b,c Means in the same row with different superscripts are significantly different.
NS: Not significant; S: Significant (p<0.05).
IIEF-5, International Index of Erectile Function scores; ICI, intracavernous injection; RI, resistive index; EDV, end diastolic velocity; PSV, peak systolic velocity.
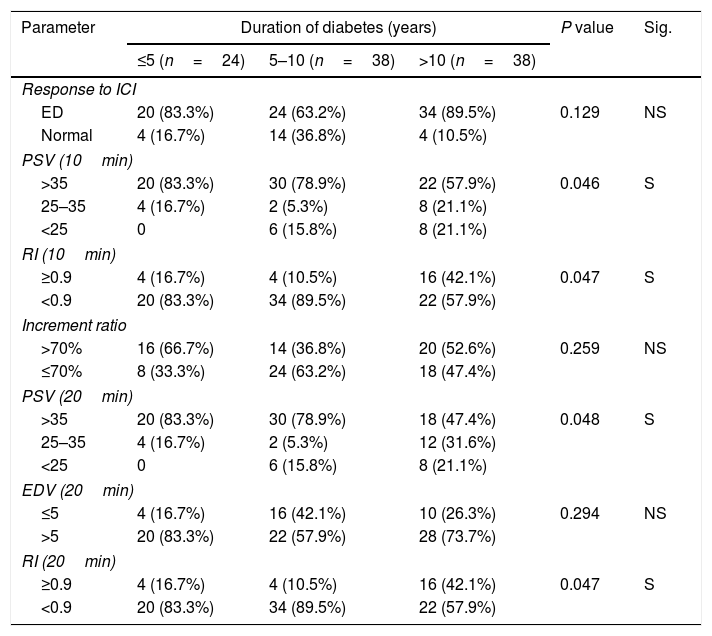
A statistically significant association was found between the duration of DM and decrease PSV 10, 20min and RI at 10, 20min (Table 3).
Duration of diabetes & PPDU parameters.
| Parameter | Duration of diabetes (years) | P value | Sig. | ||
|---|---|---|---|---|---|
| ≤5 (n=24) | 5–10 (n=38) | >10 (n=38) | |||
| Response to ICI | |||||
| ED | 20 (83.3%) | 24 (63.2%) | 34 (89.5%) | 0.129 | NS |
| Normal | 4 (16.7%) | 14 (36.8%) | 4 (10.5%) | ||
| PSV (10min) | |||||
| >35 | 20 (83.3%) | 30 (78.9%) | 22 (57.9%) | 0.046 | S |
| 25–35 | 4 (16.7%) | 2 (5.3%) | 8 (21.1%) | ||
| <25 | 0 | 6 (15.8%) | 8 (21.1%) | ||
| RI (10min) | |||||
| ≥0.9 | 4 (16.7%) | 4 (10.5%) | 16 (42.1%) | 0.047 | S |
| <0.9 | 20 (83.3%) | 34 (89.5%) | 22 (57.9%) | ||
| Increment ratio | |||||
| >70% | 16 (66.7%) | 14 (36.8%) | 20 (52.6%) | 0.259 | NS |
| ≤70% | 8 (33.3%) | 24 (63.2%) | 18 (47.4%) | ||
| PSV (20min) | |||||
| >35 | 20 (83.3%) | 30 (78.9%) | 18 (47.4%) | 0.048 | S |
| 25–35 | 4 (16.7%) | 2 (5.3%) | 12 (31.6%) | ||
| <25 | 0 | 6 (15.8%) | 8 (21.1%) | ||
| EDV (20min) | |||||
| ≤5 | 4 (16.7%) | 16 (42.1%) | 10 (26.3%) | 0.294 | NS |
| >5 | 20 (83.3%) | 22 (57.9%) | 28 (73.7%) | ||
| RI (20min) | |||||
| ≥0.9 | 4 (16.7%) | 4 (10.5%) | 16 (42.1%) | 0.047 | S |
| <0.9 | 20 (83.3%) | 34 (89.5%) | 22 (57.9%) | ||
a,b,c Means in the same row with different superscripts are significantly different.
NS: Not significant; S: Significant (p<0.05).
IIEF-5, International Index of Erectile Function scores; ICI, intracavernous injection; RI, resistive index; EDV, end diastolic velocity; PSV, peak systolic velocity.
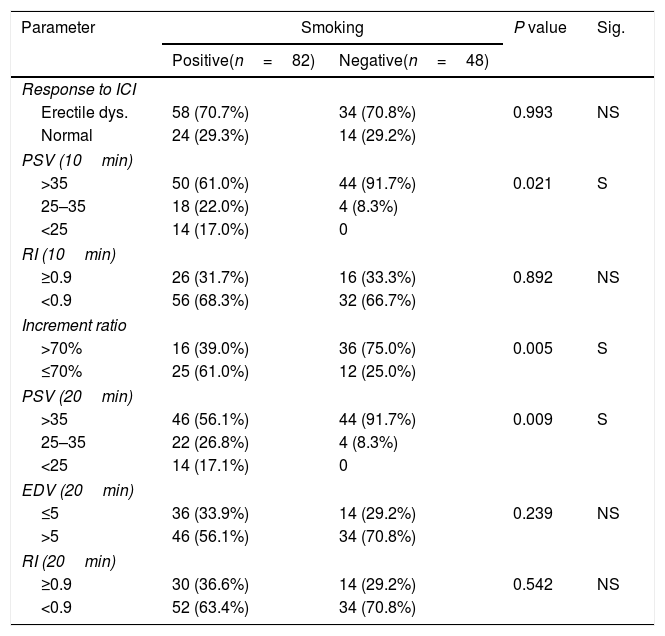
In relation to smoking, it was found a significant relation with decrease PSV at 10, 20min and decrease increment ratio and non-significant relation with the response, EDV, and RI at 10, 20min (Table 4).
Smoking and PPDU parameters.
| Parameter | Smoking | P value | Sig. | |
|---|---|---|---|---|
| Positive(n=82) | Negative(n=48) | |||
| Response to ICI | ||||
| Erectile dys. | 58 (70.7%) | 34 (70.8%) | 0.993 | NS |
| Normal | 24 (29.3%) | 14 (29.2%) | ||
| PSV (10min) | ||||
| >35 | 50 (61.0%) | 44 (91.7%) | 0.021 | S |
| 25–35 | 18 (22.0%) | 4 (8.3%) | ||
| <25 | 14 (17.0%) | 0 | ||
| RI (10min) | ||||
| ≥0.9 | 26 (31.7%) | 16 (33.3%) | 0.892 | NS |
| <0.9 | 56 (68.3%) | 32 (66.7%) | ||
| Increment ratio | ||||
| >70% | 16 (39.0%) | 36 (75.0%) | 0.005 | S |
| ≤70% | 25 (61.0%) | 12 (25.0%) | ||
| PSV (20min) | ||||
| >35 | 46 (56.1%) | 44 (91.7%) | 0.009 | S |
| 25–35 | 22 (26.8%) | 4 (8.3%) | ||
| <25 | 14 (17.1%) | 0 | ||
| EDV (20min) | ||||
| ≤5 | 36 (33.9%) | 14 (29.2%) | 0.239 | NS |
| >5 | 46 (56.1%) | 34 (70.8%) | ||
| RI (20min) | ||||
| ≥0.9 | 30 (36.6%) | 14 (29.2%) | 0.542 | NS |
| <0.9 | 52 (63.4%) | 34 (70.8%) | ||
NS: Not significant; S: Significant (p<0.05).
PPDU, pharmacopenile Doppler ultrasonography; IIEF-5, International Index of Erectile Function scores; ICI, intracavernous injection; RI, resistive index; EDV, end diastolic velocity; PSV, peak systolic velocity.
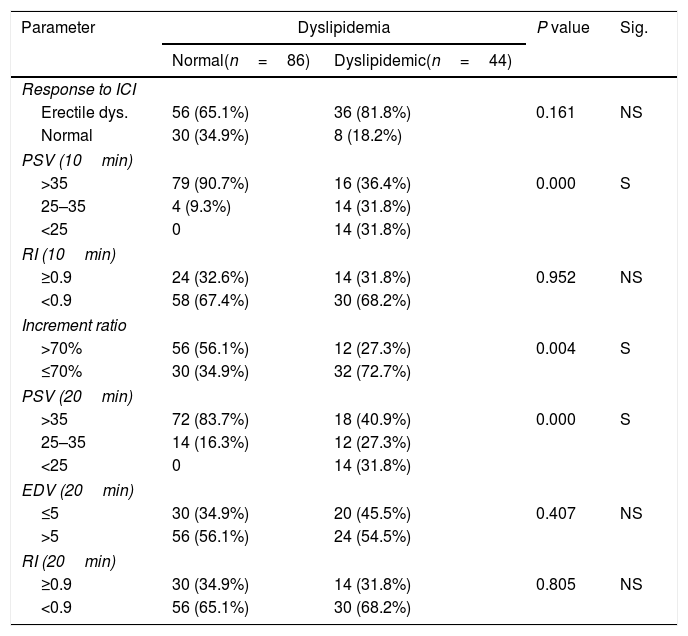
Concerning dyslipidemia, it was a significant relation with decrease PSV at 10, 20min and increment ratio and non-significant relation with the response, EDV, and RI at 10, 20min (Table 5).
Dyslipidemia & PPDU parameters.
| Parameter | Dyslipidemia | P value | Sig. | |
|---|---|---|---|---|
| Normal(n=86) | Dyslipidemic(n=44) | |||
| Response to ICI | ||||
| Erectile dys. | 56 (65.1%) | 36 (81.8%) | 0.161 | NS |
| Normal | 30 (34.9%) | 8 (18.2%) | ||
| PSV (10min) | ||||
| >35 | 79 (90.7%) | 16 (36.4%) | 0.000 | S |
| 25–35 | 4 (9.3%) | 14 (31.8%) | ||
| <25 | 0 | 14 (31.8%) | ||
| RI (10min) | ||||
| ≥0.9 | 24 (32.6%) | 14 (31.8%) | 0.952 | NS |
| <0.9 | 58 (67.4%) | 30 (68.2%) | ||
| Increment ratio | ||||
| >70% | 56 (56.1%) | 12 (27.3%) | 0.004 | S |
| ≤70% | 30 (34.9%) | 32 (72.7%) | ||
| PSV (20min) | ||||
| >35 | 72 (83.7%) | 18 (40.9%) | 0.000 | S |
| 25–35 | 14 (16.3%) | 12 (27.3%) | ||
| <25 | 0 | 14 (31.8%) | ||
| EDV (20min) | ||||
| ≤5 | 30 (34.9%) | 20 (45.5%) | 0.407 | NS |
| >5 | 56 (56.1%) | 24 (54.5%) | ||
| RI (20min) | ||||
| ≥0.9 | 30 (34.9%) | 14 (31.8%) | 0.805 | NS |
| <0.9 | 56 (65.1%) | 30 (68.2%) | ||
NS: Not significant; S: Significant (p<0.05).
PPDU, pharmacopenile Doppler ultrasonography; IIEF-5, International Index of Erectile Function scores; ICI, intracavernous injection; RI, resistive index; EDV, end diastolic velocity; PSV, peak systolic velocity.
The occurrence of ED in males with DM is a complicated and multifactorial mechanism. The process tends to be impaired by neurogenic and vascular factors, endothelial malfunction, oxidative damages and variations in the nitric-oxide pathway.26
The ED etiopathogenesis in DM is multifaceted, as it based on both psychological and organic elements (which play important tasks in ED), in addition to psychological and relationship concerns, which frequently exist. The suggested pathways of ED in diabetic subjects are reflected by neuropathy, vasculopathy, insulin resistance, visceral adiposity, and hypogonadism.27
In DM, production of glycation end products, tissue oxidative damages and endothelial dysfunction are frequently occurred and leading to microvascular problems but interrelationships with glycation appearing greatest direct pathway.28
PPDU is a first-line diagnostic tool of ED and is frequently used in clinical practice as it is safe, free from radiation, enables continuous and regular monitoring of hemodynamic changes, and can assess the parameters of the cavernous arteries and veins after the ICI procedure compared with the conventional cavernosography, thus offering the best clinical therapy.29,30
In our study when compared the diabetic patients and controls, we found significant association between the existence of diabetes and a deprived response to ICI and increasing EDV levels and diminishing RI levels. However, no statistically significant association was found between the existence of diabetes and diminishing PSV values or upsurge increment ratio that may be due to related autonomic diabetic neuropathy.
These results are in concordance with one study that was conducted on 34 patients with diabetic impotence. This study reported that venous leakage was found alone or with arterial insufficiency in 23 diabetic patients (67%). Of these 23 patients, there was a total loss of intermittent bursts of spontaneous cavernosal activity (SCA) in 19 (83%). Also, This study determined that venous leakage in diabetic impotence was linked to autonomic neuropathy.31 Another study was done on 265 patients aged 17–75 years whereas a larger rise in EDV and a decline in RI happened in groups comprising DM.32
In a different research by Kadioglu et al.33 sexually active diabetic men had a significantly reduced cavernous PSV and a higher mean EDV relative to non-diabetics. In another study, patients with diabetes as a common vascular risk aspect suffered comparable declines in PSV and rises in EDV relative to those without vascular risk features.34
In contrast, additional studies found significant relations between the incidence of diabetes and a reduced response to ICI including declined PSV values.35,36
When compared all diabetic groups with control group showed significant relation in the following; severity of IIEF-5 score, poor response to ICI, decrease PSV at 10min, increase EDV at 10, 20min and decrease RI at 10, 20min, however non-significant relation with age, type of DM, treatment of DM, duration of DM, smoking or dyslipidemia that increase significant value of the result table.2
These results match with the theory that says diabetic men with highest values of HbA1c had a greater incidence of severe ED, while those with minor levels of HbA1c inclined to have lower rates of ED. El-Sakka reported on 481 diabetic patients with ED, a significant relationship was detected between a deprived control of DM (according to HbA1c>7) and the following: a reduced response to ICI and declining PSV, RI, and axial penile rigidity levels and rising EDV values (P<0.05 for each).36
Although hyperglycemia is a contributing factor for the occurrence of ED in diabetic men; certain observational researches have shown a correlation between weak glycemic control, demonstrated by high values of HbA1c, and ED6,37,38 while other studies have not recorded any correlations.39–41
Such divergent findings can be clarified, at least partially, by the distinct methodological approaches used in the various studies. In addition, DM is usually linked with hypertension, hyperlipidemia, obesity, metabolic syndrome, cigarettes smoking, lack of exercise, and autonomic neuropathy, which are documented as risk variables for ED. In diabetic men, both microvascular and macrovascular diabetes complications also raise the risk of ED.27
When compared effect of groups of duration of the DM on PPDU parameters, a significant association was detected between increase duration of DM and decrease PSV 10, 20min and RI at 10, 20min.
The incidence of cavernous arterial insufficiency was greater in patients with a longer period of diabetes.35 A longer duration of DM and the following: weak response to ICI and reducing PSV, RI, and axial penile stiffness values and excessive EDV values (P<0.05 for each).36 Certain research assessing the length of DM and ED indicates that; a longer period of DM is linked with more serious ED.6,38,41,42
Moreover, no substantial correlation between length of DM and intensity of ED has been identified.43,44
When compared the relation between type of DM and PPDU parameters, it was found non-significant relation.
In 73% of IDDM and 61% of NIDDM, cavernous arterial inflow disease was detected. This was not significantly different (P=0.11). The extent of the two groups’ arterial insufficiency was comparable and not significantly different.45 Insulin-dependent and non-dependent patients did not differ with PSV.46 Most of the research defining the incidence of ED in diabetes did not distinguish between diabetes type 1 and type 2.27
When compared the relation between the type of treatment of DM and PPDU parameters, it was found non-significant relation.
Wang et al. stated that there is no definite correlation between cavernous arterial insufficiency and the type of DM treatment.35 It was reported non-significant relationship between type of DM treatment and ED.42 Multivariate analyzes showed that insulin treatment, microangiopathy, and anorexia are all major risk factors for ED.47
There were limitations to this study. First, our research being a single-center study that may limit the generalisability of the findings. Second, the number of controls in this study was relatively small compared to the number of cases. Further works should be carried out on a larger sample size to confirm our results.
ConclusionsDiabetes mellitus is allied with a deprived response to ICI, low RI, and high EDV. Deprived glycemic control associated with an increase of EDV, and decrease of RI, PSV. No significant association with the type of DM or type of treatment of DM. The results of the current study add to the overall set of data which suggests that ED is correlated with diabetes, a longer diabetes period, and impaired metabolic regulation of diabetes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingNo particular funding for this study was provided.
Conflict of interestThe authors report no conflict of interest.