Erectile dysfunction (ED) has increased prevalence by age and significantly affects the quality of life of men and their partners. To investigate the relationship between ED and red blood cell distribution width (RDW) values.
Materials and methodBetween September 2019 and December 2019, a total of 192 individuals comprising those that were admitted to the urology outpatient clinic with ED complaints and healthy volunteers from among hospital staff were prospectively included in the study. The participants were divided into two groups according to the international erectile function index (IIEF-5) as ED group (n=148) and control group (n=44).
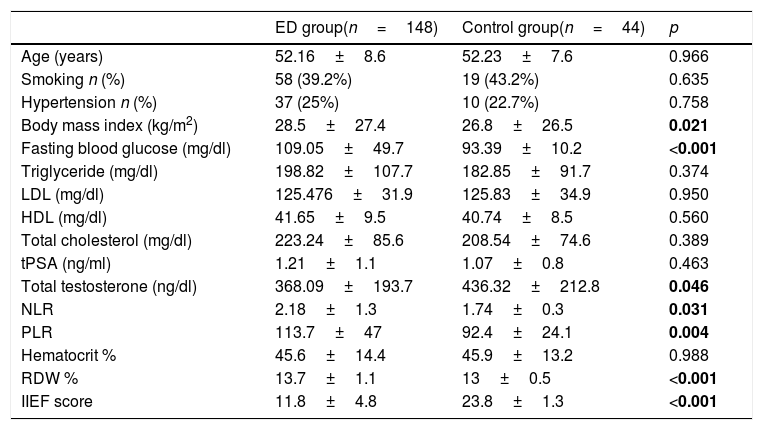
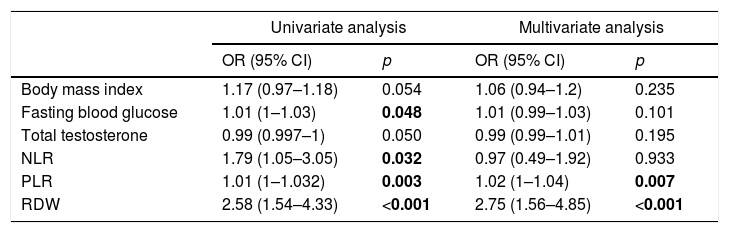
ResultsThere was no statistically significant difference between the two groups in terms of age, smoking status, presence of hypertension, triglyceride, low-density lipoprotein, high-density lipoprotein, total cholesterol, total prostate-specific antigen and haematocrit values. Body mass index, fasting blood sugar, neutrophil–lymphocyte ratio (NLR), and platelet–lymphocyte ratio (PLR) were significantly higher in the ED group (28.5±27.4kg/m2 vs 26.8±26.5kg/m2, p=.021, 109.05±49.7mg/dl vs 93.39±10.2mg/dl, p<.001, 2.18±1.3 vs 1.74±0.3, p=.031, and 113.7±47 vs 92.4±24.1, p=.004, respectively). The mean RDW values were 13.7±1.1 in the ED group and 13±0.5 in the control group (p<.001). The multivariate analysis revealed PLR [1.02 OR (1–1.04), p=.007] and RDW [2.75 OR (1.56–4.85), p<.001] as independent predictors for an ED diagnosis.
ConclusionBased on the strong relationship between RDW and ED, we consider that RDW may be a new indicator in the diagnosis of ED.
Se ha incrementado la edad de prevalencia de la disfunción eréctil (DE), afectando significativamente a la calidad de vida de los varones y sus parejas. Estudiamos las relaciones entre DE y los valores del ancho de distribución eritrocitaria (RDW).
Material y métodoEntre septiembre y diciembre de 2019 se incluyó prospectivamente en el estudio a un total de 192 individuos, que comprendía tanto a los pacientes que acudieron a la clínica ambulatoria de urología con quejas de DE como a los voluntarios sanos del personal hospitalario. Se dividió a los participantes en dos grupos, grupo DE (n=148) conforme al índice internacional de función eréctil (IIEF-5) y grupo control (n=44).
ResultadosNo se encontraron diferencias estadísticamente significativas entre los dos grupos en términos de edad, estatus de tabaquismo, presencia de hipertensión, y valores de triglicéridos, lipoproteínas de baja densidad, lipoproteínas de alta densidad, colesterol total y antígeno prostático específico total. El índice de masa corporal, glucosa en sangre en ayunas, índice neutrófilos-linfocitos (INL) e índice plaquetas-linfocitos (IPL) fueron significativamente más altos en el grupo DE (28,5±27,4kg/m2 vs. 26,8±26,5kg/m2, p=0,021, 109,05±49,7mg/dl vs. 93,39±10,2mg/dl, p<0,001, 2,18±1,3 vs. 1,74±0,3, p=0,031, y 113,7±47 vs. 92,4±24,1, p=0,004, respectivamente). Los valores eritrocitarios medios fueron 13,7±1,1 en el grupo DE y 13±0,5 en el grupo control (p<0,001). El análisis multivariante reveló que los valores de IPL [OR 1,02 (1-1,04), p=0,007] y RDW [OR 2,75 (1,56-4,85), p<0,001] se comportaron como factores predictivos independientes del diagnóstico de DE.
ConclusiónBasándonos en la fuerte relación entre RDW y DE, consideramos que RDW puede ser un nuevo indicador en el diagnóstico de DE.
Erectile dysfunction (ED) is defined as the inability to achieve or maintain an erection required for satisfactory sexual performance.1 The incidence of ED increases with age. ED is also the most common sexual dysfunction in men.2 While ED is seen in about 2% of men before the age of 40 years, this rate increases by 15% between the ages of 40 and 50 and reaches 45% prevalence by age 60 and 70% by age 70.3,4 Neuronal, hormonal and vascular mechanisms in the genital system must work in coordination for erection to occur. Disruption in any of these mechanisms may result in ED. The relationship between vasculogenic ED and coronary artery disease (CAD) has been previously shown in many studies, and it has been reported that the risk-posing factors for CAD also constitute a risk for the development of ED and both events have similar pathophysiology.5–7 ED becomes symptomatic approximately two to five years before the onset of CAD and cardiovascular events.8,9 Since the diameter of the arteries in the arterial system that provides blood to the penis is smaller than that of the coronary arteries, systemic atherosclerosis begins to affect penis vessels earlier than coronary arteries.10 Based on these data, it can be stated that ED is an independent predictor and a precursor of CAD.3,11,12
Red blood cell distribution width (RDW) is automatically calculated in the whole blood count analysis as an indicator of anisocytosis. It is calculated by measuring the heterogeneity of erythrocyte dimensions and reported as a percentage (%). Although RDW values are known to increase mainly in anemia and hematologic diseases, in recent years, it has also been suggested that RDW is increased in sepsis, malignancies, cardiovascular diseases, chronic liver diseases, and respiratory pathologies, and thus can contributes to the evaluation of disease prognosis.13,14 The strong relationship between CAD and RDW has been demonstrated by many researchers15,16; however, there are only a few studies in the literature examining the relationship between RDW and ED.17 In this study, we investigated the relationship between ED and RDW values through comparisons between patients with ED and healthy subjects.
Material and methodFollowing the approval of the ethics committee of our university hospital, patients who presented to our urology outpatient clinic with the ED complaint between September 2019 and December 2019 and healthy volunteers consisting of hospital staff were included in this prospective study. A total of 192 individuals over 40 years of age who were sexually active in the last six months and who provided written informed consent were evaluated. According to the international erectile function index (IIEF-5), the patients were classified as having mild ED (IIEF score 17–21, n=32), mild-moderate ED (IIEF score 12–16, n=36), moderate ED (IIEF score 8–11, n=34), severe ED (IIEF score 5–7, n=46) and no ED (IIEF score 22-25, n=44). Fasting blood glucose, high density lipoprotein (HDL), low-density lipoprotein (LDL), total cholesterol (TC), triglyceride, total testosterone (TT) and total prostate-specific antigen (tPSA) values were analyzed from the blood obtained at 8–10a.m. after 12h of overnight fasting. The whole blood count analysis, including the quantification of platelets, neutrophils, lymphocytes, hematocrits, and RDW was automatically performed (Sysmex Corporation, Kobe, Japan). Body mass index (BMI), smoking status, and comorbidities of all subjects were also recorded. After the physical examination, cases with genital anomalies, history of penile surgery, and Peyronie's plaques were excluded. Furthermore, patients with CAD, heart failure, renal failure, chronic vascular disease, psychiatric disease, history of hypothyroidism, and hyperthyroidism, those with anemia (Hgb<13), underwent radical prostatectomy or pelvic surgery and those receiving medical treatment for ED were not included in the study.
Statistical analysisThe data were analyzed using SPSS version 25.0 (SPSS®, IL, USA). The Shapiro–Wilk test was used to evaluate the fit of the data to the normal distribution curve. Continuous variables were compared using Student's t-test and Mann–Whitney U-test, and categorical data were compared using the chi-square test. The correlation between the data was performed by the Spearman correlation analysis. The comparison of the numerical variables between the subgroups of the ED group was undertaken using ANOVA. The receiver operating characteristic (ROC) curve was used to measure the efficacy of RDW in the diagnosis of ED. Univariate and multivariate logistic regression analyses were conducted to evaluate the independent predictors of ED. A p vale of <0.05 was considered statistically significant.
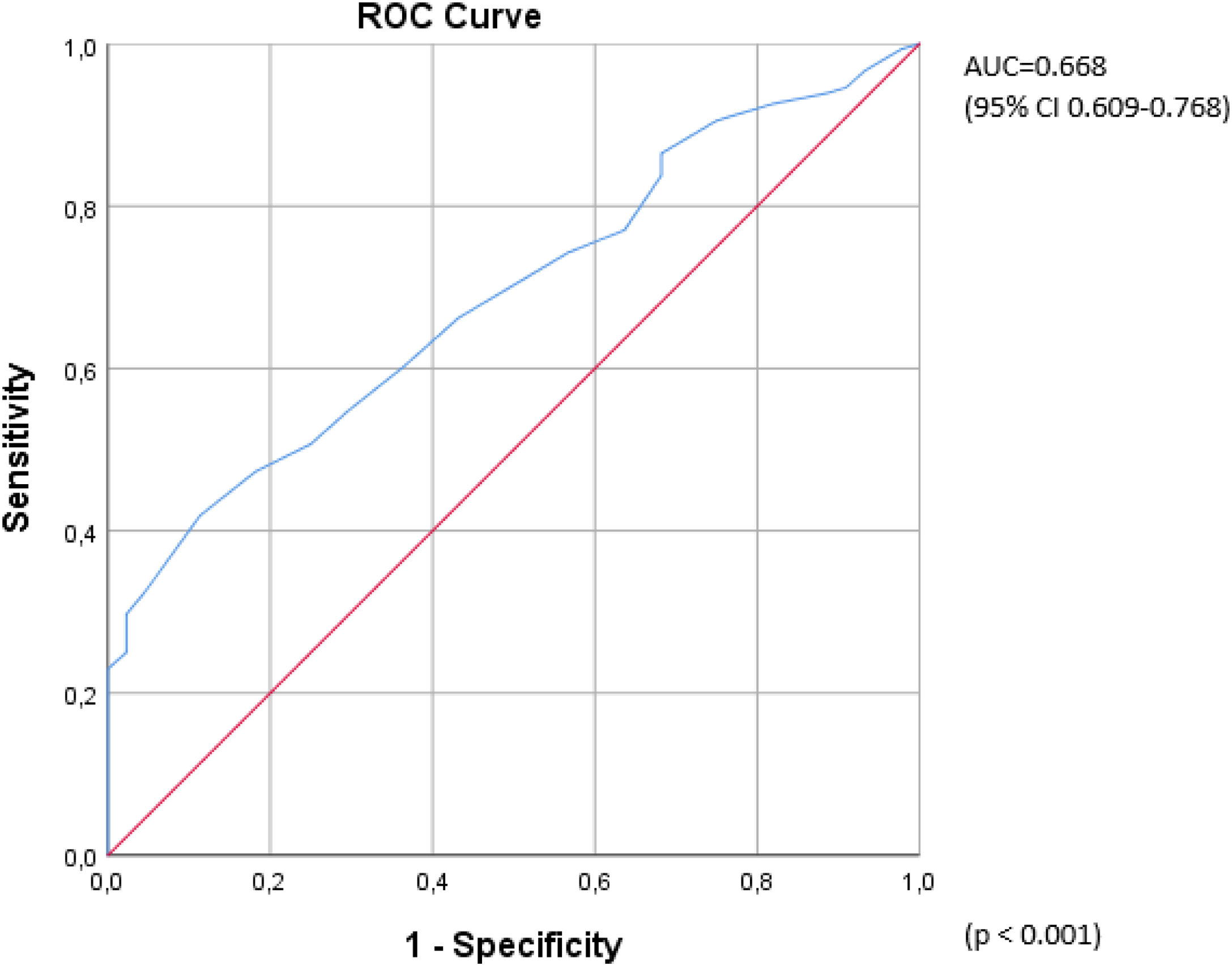
ResultsThere was no statistical difference between the ED and control groups in terms of age, smoking status, hypertension, TG, LDL, HDL, total cholesterol, tPSA and hematocrit values. BMI, fasting blood glucose, neutrophil–lymphocyte ratio (NLR) and platelet–lymphocyte ratio (PLR) were significantly higher in the ED group (28.5±27.4kg/m2 vs 26.8±26.5kg/m2, p=0.021, 109.05±49.7mg/dl vs 93.39±10.2mg/dl, p<0.001, 2.18±1.3 vs 1.74±0.3 p=0.031, and 113.7±47 vs 92.4±24.1, p=0.004, respectively). The TT values were significantly lower in the ED group than in the control group (368.09±193.7ng/dl vs 436.32±212.8ng/dl, p=0.046). The mean RDW values were calculated as 13.7±1.1 in the ED group and 13±0.5 in the control group with a statistically significant difference between the two (p<0.001) (Table 1). In order to determine the efficacy of the RDW value in ED diagnosis, a ROC analysis was undertaken, which revealed that the area under the curve (AUC) was 0.668 (95% CI 0.609–0.768, p<0.001) (Fig. 1).
Comparison of the demographic data and laboratory results between the participants with and without erectile dysfunction.
| ED group(n=148) | Control group(n=44) | p | |
|---|---|---|---|
| Age (years) | 52.16±8.6 | 52.23±7.6 | 0.966 |
| Smoking n (%) | 58 (39.2%) | 19 (43.2%) | 0.635 |
| Hypertension n (%) | 37 (25%) | 10 (22.7%) | 0.758 |
| Body mass index (kg/m2) | 28.5±27.4 | 26.8±26.5 | 0.021 |
| Fasting blood glucose (mg/dl) | 109.05±49.7 | 93.39±10.2 | <0.001 |
| Triglyceride (mg/dl) | 198.82±107.7 | 182.85±91.7 | 0.374 |
| LDL (mg/dl) | 125.476±31.9 | 125.83±34.9 | 0.950 |
| HDL (mg/dl) | 41.65±9.5 | 40.74±8.5 | 0.560 |
| Total cholesterol (mg/dl) | 223.24±85.6 | 208.54±74.6 | 0.389 |
| tPSA (ng/ml) | 1.21±1.1 | 1.07±0.8 | 0.463 |
| Total testosterone (ng/dl) | 368.09±193.7 | 436.32±212.8 | 0.046 |
| NLR | 2.18±1.3 | 1.74±0.3 | 0.031 |
| PLR | 113.7±47 | 92.4±24.1 | 0.004 |
| Hematocrit % | 45.6±14.4 | 45.9±13.2 | 0.988 |
| RDW % | 13.7±1.1 | 13±0.5 | <0.001 |
| IIEF score | 11.8±4.8 | 23.8±1.3 | <0.001 |
Abbreviations: IIEF: international erectile function index, LDL: low-density lipoprotein, HDL: high-density lipoprotein, tPSA: total prostate-specific antigen, NLR: neutrophil/lymphocyte ratio, PLR: platelet/lymphocyte ratio, RDW: red blood cell distribution width.
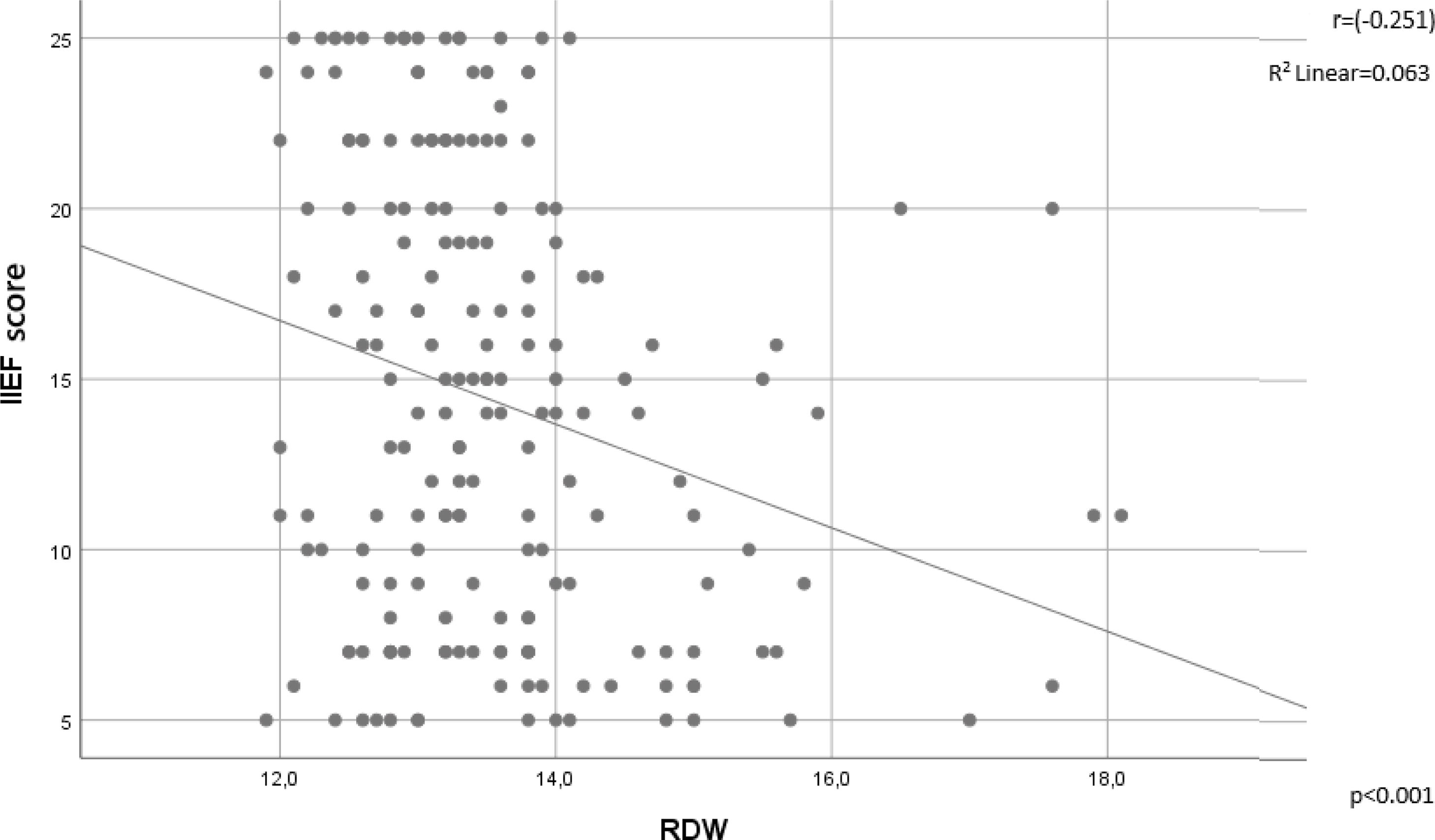
There was an inverse correlation between the IIEF and RDW values (r=−0.251, p<0.001) (Fig. 2). The RDW values statistically significantly differed between the ED group and the control group; however, there was no significant difference in the RDW values between the subgroups of ED, namely mild, mild-moderate, moderate and severe ED groups classified according to the IIEF scores (p=0.745). In the univariate logistic regression analysis, fasting blood glucose [1.01 OR (1–1.03), p=0.048), NLR [1.79 OR (1.05–3.05), p=0.032], PLR [1.01 OR (1–1.032), p=0.003], and RDW [2.58 OR (1.54–4.33), p<0.001] were associated with an ED diagnosis, whereas the multivariate analysis revealed only PLR [1.02 OR (1–1.04), p=0.007] and RDW [2.75 OR (1.56–4.85), p<0.001] as independent predictors of a diagnosis of ED (Table 2).
Results of the univariate and multivariate logistic regression analyses of the independent predictors of ED.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Body mass index | 1.17 (0.97–1.18) | 0.054 | 1.06 (0.94–1.2) | 0.235 |
| Fasting blood glucose | 1.01 (1–1.03) | 0.048 | 1.01 (0.99–1.03) | 0.101 |
| Total testosterone | 0.99 (0.997–1) | 0.050 | 0.99 (0.99–1.01) | 0.195 |
| NLR | 1.79 (1.05–3.05) | 0.032 | 0.97 (0.49–1.92) | 0.933 |
| PLR | 1.01 (1–1.032) | 0.003 | 1.02 (1–1.04) | 0.007 |
| RDW | 2.58 (1.54–4.33) | <0.001 | 2.75 (1.56–4.85) | <0.001 |
NLR: neutrophil/lymphocyte ratio, PLR: platelet/lymphocyte ratio, RDW: red blood cell distribution width.
Many conditions that cause deterioration in erectile physiology can lead to ED development. Hormonal disorders, vascular damage due to endothelial dysfunction, impairments of the nerve conduction system, and genital anomalies are the main organic causes of ED, while some psychiatric-psychological disorders can also result in ED.18 In addition, risk factors, such as diabetes mellitus, obesity, smoking, and sedentary lifestyle are effective in ED and CAD.19–21 Although reduced androgen levels contribute to the development of ED in elderly men, the development of ED as a result of vascular damage due to atherosclerosis is one of the most important organic causes.22,23 RDW, which is an indicator of anisocytosis, is known to increase in various types of anemia, except thalassemia, and furthermore, recent studies have suggested that this value is also elevated in CAD,15 heart failure,24 chronic liver disease,13 hypertension,25 stroke,26 carotid atherosclerosis,27 and malignancies.28 Although the underlying mechanism is not fully explained in many diseases, it has been reported that vascular aging, endothelial dysfunction, and chronic inflammation may cause an increase in RDW.
In a study conducted with ascending aortic aneurysm cases, Balistreri et al. simultaneously examined systemic inflammation markers, RDW values, and vascular aging markers, namely leukocyte telomere length, telomerase activity and decreased levels of endothelial progenitor cells. The authors reported that elevated values of C-reactive protein (CRP) and RDW were independent risk factors for vascular aging.29
Recent studies have found a strong association between atherosclerosis and inflammation, and shown that inflammatory markers, such as NLR and PLR were significantly higher in CAD and ED.30–32 Some studies found that in addition to RDW, inflammatory markers, including CRP were increased in CAD.15 In a meta-analysis of 10,410 patients published in 2018, Abrahan et al. reported a decrease in serum RDW values associated with a reduction in the risk of developing cardiovascular events.33 Furthermore, in their study on coronary stent re-stenosis, Qian et al. found significantly higher CAD, WBC, neutrophil, CRP, and high-sensitivity CRP (Hs-CRP) values, as well as increased RDW in the group with stent stenosis, and their univariate and multivariate logistic regression analysis revealed that RDW was correlated with stent stenosis.34
Dursun et al. first examined the RDW values in ED patients and controls and found them to be higher in the former (13.49±1.52 vs 12.91±1.13, p<0.001). Then, they conducted univariate and multivariate logistic regression analyses and demonstrated that RDW was an independent factor in the diagnosis of ED.17 In our study, the RDW values were significantly higher in the ED group (13.7±1.1 vs 13±0.5, p<0.001). In the univariate logistic regression analysis, fasting blood glucose, NLR, PLR, and RDW were found to provide significant results, but the multivariate logistic regression analysis suggested that only PLR [1.02 OR (1–1.04), p=0.007] and RDW [2.75 OR (1.56–4.85), p<0.001] were independent predictors of ED. Unlike the study of Dursun et al., our study reveals that besides RDW, PLR is also significant in univariate and multivariate logistic regression analysis. Although it is not clear whether anisocytosis detected in ED patients was due to vascular aging or chronic inflammation, the presence of high proinflammatory markers in the same patient group reinforces the idea that chronic inflammation plays a role. In addition, it has been reported that inflammation can cause erythrocyte membrane damage, thus disrupting erythrocyte maturation and leading to an increase in RDW.35 When we examined the relationship between inflammation markers and RDW values in all participants, we found no correlation between NLR and RDW (r=0.122, p=0.91), whereas there was a weak correlation between PLR and RDW (r=0.142, p=0.49).
Although a significant negative correlation was found between the IIEF scores and RDW values in our study (r=−0.251, p<0.001) (Fig. 2), no significant differences in RDW values were obtained when comparing different degrees of ED severity.
Limitations: In our study, rather than the formation of ED and changes in RDW over time, we performed the instantaneous RDW examination of patients diagnosed with ED. Although we tried to form a homogenous vascular ED group by excluding ED cases due to causes related to neurology, trauma and genital anomalies, we did not confirm this by penile Doppler ultrasonography. Lastly, our study only included Turkish men; therefore, ethnic differences could not be revealed.
ConclusionAccording to our results, there is a strong and significant correlation between the RDW value and ED disease, which can be easily demonstrated through a complete blood count analysis without any additional cost. The RDW value may be a new indicator in ED diagnosis. In order to support our findings, larger case series and prospective studies are needed to investigate the relationship between the ED development process and the changes in RDW values.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis research received no specific Grant from any funding agency in the public, commercial, or not-for-profi sectors
Conflict of interestThere is no conflict of interest of any authors in relation to the submission.