The aim of the study is to investigate the protective effect of taxifolin (3,5,7,3,4-pentahydroxy flavanone), a strong antioxidant, against testicular I/R injury in rats biochemically and histopathologically.
Materials and methods50mg/kg taxifolin was administered to taxifolin+testicular torsion–detorsion (TTTD, n-10) group of Albino Wistar male rats by oral gavage. Distilled water .5ml as a solvent was administered to testicular torsion–detorsion (TTD, n-10) and Healthy Control (SG, n-10) groups using the same method. An hour after the administration of taxifolin and distilled water, anaesthesia (ketamine 60mg/kg) was administered to all animal groups. TTD and TTTD group animals were subjected to testicular torsion at 720 degrees for four hours during anaesthesia. At the end of this period, testicular detorsion was applied and perfusion was allowed for four hours. Sham operation was applied to SG group.
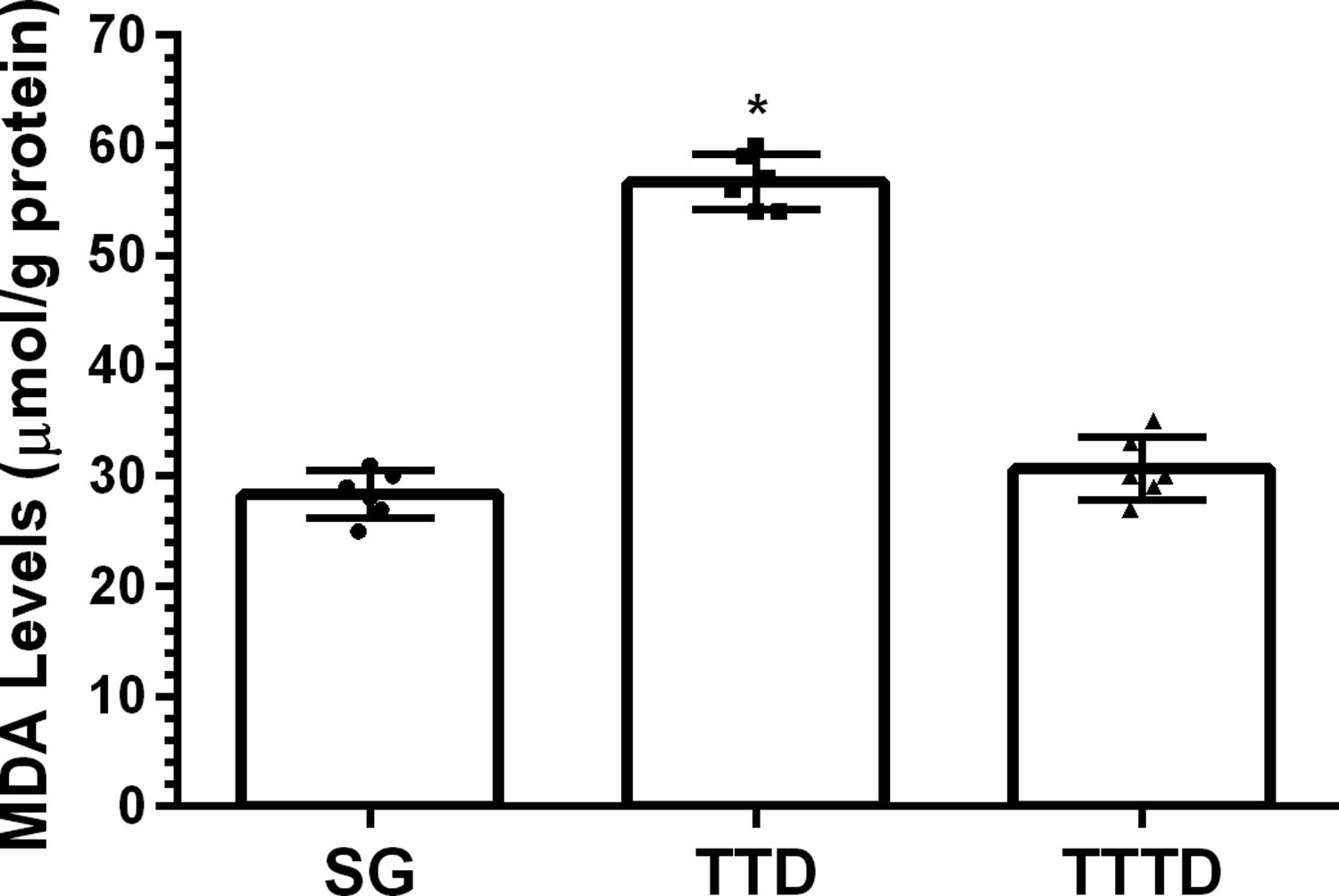
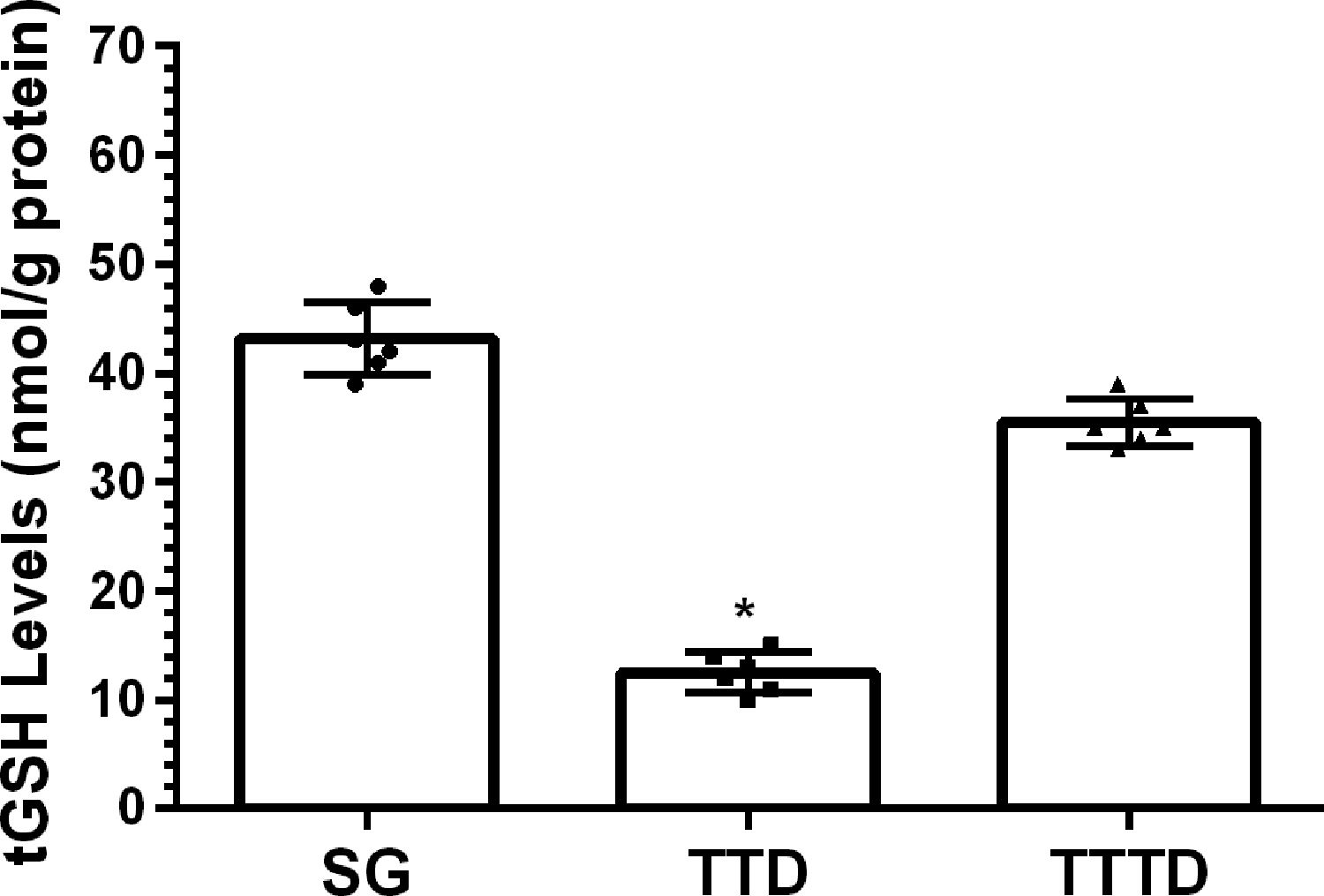
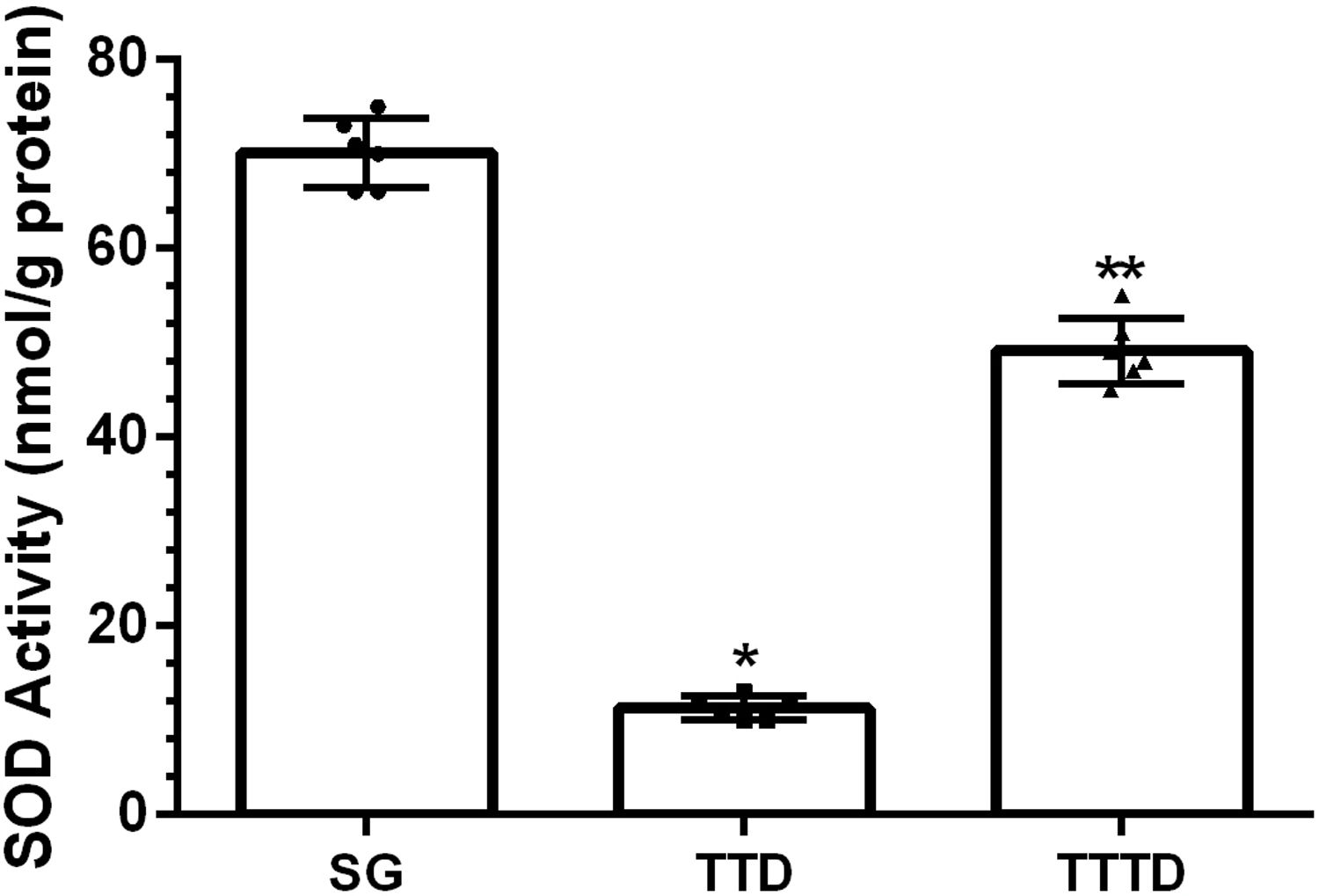
ResultsOur biochemical experiment results showed that the amount of malondialdehyde (MDA) in testicular tissue of TTD group presented a significant increase compared to SG and TTTD groups whereas total glutathione (tGSH) and superoxide dismutase (SOD) levels decreased. In addition, while TTD group presented severe histopathological damage in germinal epithelium cell and seminiferous tubule, mild damage was observed in TTTD group.
ConclusionsThe results of our experiment indicate that taxifolin could be useful in the treatment of testicular I/R damage.
El objetivo del estudio fue analizar el efecto protector de la taxifolina (3,5,7,3,4-pentahidroxi flavanona), un fuerte antioxidante, en la lesión por reperfusión-isquemia (R/I) en ratas, a nivel bioquímico e histopatológico.
Materiales y métodosSe administraron 50 mg/kg de taxifolina a un grupo de ratas macho Albino Wistar con torsión-destorsión y taxifolina+testicular (TTTD, n-10) mediante una sonda oral, y una solución de 0,5 mL de agua destilada a un grupo con torsión-destorsión testicular (TTD, n-10) y a controles sanos (SG, n-10), utilizando el mismo método. Una hora después de la administración de taxifolina y agua destilada, se aplicó anestesia (ketamina 60 mg/kg) a todos los grupos de animales. Los grupos TTD y TTTD fueron sometidos a una torsión testicular a 720 grados por cuatro horas durante la anestesia. Al finalizar este período, se aplicó destorsión testicular, permitiéndose la perfusión durante cuatro horas. Se aplicó un placebo al grupo SG.
ResultadosLos resultados de nuestro experimento bioquímico reflejaron que el incremento de malondialdehído (MDA) en el tejido testicular del grupo TTD presentó un aumento significativo en comparación con los grupos SG y TTTD, mientras que disminuyeron los niveles de glutatión (tGSH) y superóxido dismutasa (SOD). Además, mientras que el grupo TTD presentó daño histopatológico severo en las células del epitelio germinal y el tubo seminífero, se observó un daño leve en el grupo TTTD.
ConclusionesLos resultados de nuestro experimento indican que la taxifolina podría ser de utilidad para el tratamiento de la lesión testicular por R/I.
Ischemic injury is tissue or organ deoxygenation due to the reducing or cutting off blood flow to tissues for various reasons.1 In persistent ischemia, an irreversible tissue and cellular damage may occur.2 Therefore, the first intervention on the ischemic tissue is to obtain reperfusion. However, it induces overproduction of reactive oxygen species (ROS) like molecular oxygen (O2), superoxide anion (**O−2), hydroxyl radicals (**OH) and hydrogen peroxide (H2O2) that are abundantly introduced to the ischemic tissue through blood in reperfusion.3 As is known, ROS are important radicals initiating lipid peroxidation (LPO).4 LPO terminates with the formation of toxic malondialdehyde (MDA), reaction of further destruction.5 Thus, MDA is widely used as the indicator of the oxidative state.6 ROS-associated damage is also argued to be the result of the overuse of the natural antioxidant defense system in tissues and cells.7 Testicular ischemia perfusion (I/R) injury occurs with the surgical detorsion of testicular torsion in clinical environment.8 However, only 32% of the testis could be saved despite the early surgical interventions.9 These literature data suggest that it could be helpful to use antioxidant medications at the pre- and post-surgical intervention to testicular torsion. Taxifolin (3,5,7,3,4-pentahydroxy flavanone or dihydroquercetin) to be analyzed in this study with respect to its protective effect against testicular I/R injury is a powerful antioxidant flavonoid being abundant in citrus fruits and onion.9 Flavonoids are reported to be inhibiting lipid peroxidation and enzymatic reactions that are responsible for the formation of ROS in the literature.10 Taxifolin has been reported to be protecting tissues from oxidative damage by inhibiting the reduction of an endogenous antioxidant, glutathione, and the increase of MDA in various organ tissues.11 The use of taxifolin is also recommended to minimize or eliminate lipid oxidation in foods and pharmaceuticals and to retard the generation of toxic oxidation products.12 These data show that ROS are the major components in the pathogenesis of testicular I/R injury. They also suggest that taxifolin could be helpful in the treatment of I/R-induced oxidative testicular injury. Literature review revealed no data on the protective effect of taxifolin against oxidative testicular I/R injury. Therefore, the purpose of our research is to investigate the effect of taxifolin on experimentally induced testicular I/R injury in Albino Wistar male rats biochemically and histopathologically.
Material and methodsAnimalsAlbino Wistar male rats to be used in our study were supplied from Ataturk University Medical Experimental Research and Application Centre. Albino Wistar male rats with a weight range of 255–270g were preferred for the experiment. The animals were housed and fed at normal room temperature (22°C) prior to the experiment.
Chemical substancesOne of the chemical substances used for the experiments, ketamine was supplied from Pfizer Ltd. Sti. (Turkey) and taxifolin was supplied from Evalar (Russia).
Animal groupsThe experiment animals were divided into three groups as; (TTD) group to be subjected to testicular torsion–detorsion, (TTTD) group to be subjected to taxifolin+testicular torsion–detorsion and the Healthy Control (HG) group to be subjected to sham operation.
Experimental procedurePharmacological procedure50mg/kg (n-10) taxifolin was administered to TTTD group of animals by oral gavage. 0.5ml distilled water, as a solvent, was administered to TTD (n-10) and SG (n-10) groups using the same method. An hour after the administration of taxifolin and distilled water, anesthesia was administered to all animal groups.
Anesthesia procedurePrior to the surgical operation, all animal groups were anaesthetized by administering 60mg/kg ketamine intraperitoneally (i.p.) and xylazine at suitable intervals. After ketamine injection, the rats were kept being observed for the suitable time period for surgical operation. The time when the animals remain motionless lying on their back is accepted to be a favorable anesthesia period.13
Surgical procedureSurgical procedures were performed in laboratory environment under sterile conditions. Scrotum area of TTD, TTTD and HG groups of animals were disinfected with 10% povidone iodine solution. The skin and under-skin scrotum midline of the experimental animals were incised at a length of 2cm. Right testis tunica vaginalis and the spermatic cord at the scrotal space were subjected to blunt dissection and removed. Testis of HG group animals were placed back to the scrotum without being subject to any operation. Testis of TTD and TTTD groups of animals were subjected to torsion at 720° for 4h. Testicular detorsion was applied at the end of this period and perfusion was allowed for four hours. After each operation, the incision area was covered by a sterile sponge wetted with physiological solution. Then all animals were killed by a high dose anesthesia and their testicles were removed. Biochemical and histopathological examinations were performed on the removed testicular tissues. The results of TTD and TTTD groups were evaluated by comparing with SOC.
Biochemical analysesHomogenates from tissues were prepared for the biochemical analyses of testicular tissues. Total glutathione (tGSH) and malondialdehyde (MDA) levels in the supernatants obtained from these homogenates were determined using suitable literature-based methods.
Sample preparation0.2g was weighted from each dissected tissue at this stage of the study. These were completed to 2ml in 1.15% potassium chloride solution for evaluating MDA and in pH=7.5 phosphate buffer for measuring tGSH and they were homogenized in an iced environment. Then, they were centrifuged at +4°C and 10,000rpm for 15min. The supernatant was used as an analysis sample.
MDA evaluationIt is based on the spectrophotometric measurement at 532nm wavelength of the absorbance of the pink-colored complex produced by thiobarbituric acid (TBA) and MDA at a high temperature (95°C).14 Homogenates were centrifuged for 20minutes at 5000g and these homogenates were used in the evaluation of MDA amount. 250μl homogenate, 100μl 8% sodium dodecyl sulphate (SDS), 750μl 20% acetate acid, 750μl 0.08%TBA and 150μl distilled water were pipetted into capped experimental test tubes and vortexed. The mixture was incubated at 100°C for 60min; 2.5ml n-butanol was added into the mixture and spectrophotometric measurement was performed. The amount of pink color was measured using 3ml cuvette at 532nm. MDA amounts of the samples were then evaluated with the help of the standard graph produced by using the prearranged MDA stock solution considering the dilution coefficient.
tGSH evaluationDTNM [5,5′-Ditiyobis (2-nitrobenzoic acid)] available in the measurement environment is a disulphide chromogen and is easily reduced by sulfhydryl group compounds. The yellow color was subjected to spectrophotometric measurement at 412nm.15 Homogenates were centrifuged at 12,000×g for 10min and the supernatants were used in the assessment of GSH amount. 1500μl measurement buffer (0.2mM EDTA containing 200mM Tris-HCl, pH=8.2), 500μl supernatant, 100μl 5.5′-Dithio-bis (2-nitrobenzoic acid) (DTNB) and 7900μl methanol were pipetted into capped test tubes and vortexed. The mixture was incubated at 37°C for 30min, then measurements were performed using a spectrophotometer. The amount of yellow color was measured using 3ml cuvette at 412nm. GSH amounts of the samples were then assessed with the help of the standard graph produced by using the prearranged MDA stock solution considering the dilution coefficient.
Superoxide dismutase (SOD) analysisThe measurements were performed according to the method of Sun et al.16 When xanthine is converted into uric acid by xanthine oxidase, SOD forms. If nitro blue tetrazolium (NBT) is added to this reaction, SOD reacts with NBT and a purple-colored formazan dye occurs. The sample was weighed and homogenized in 2ml of 20mmol/L phosphate buffer containing 10mmol/L EDTA at pH 7.8. The sample was centrifuged at 6000rpm for 10min and then the brilliant supernatant was used as an assay sample. The measurement mixture containing 2450μL (0.3mmol/L xanthine, 0.6mmol/L EDTA, 150μmol/L NBT, 0.4mol/L Na2CO3, 1g/l bovine serum albumin), 500μL supernatant and 50μL xanthine oxidase (167U/l) was vortexed. Then it was incubated for 10min. At the end of the reaction, formazan occurred. The absorbance of the purple-colored formazan was measured at 560nm. As more of the enzyme exists, the least O2− radical that reacts with NBT occurs.
Histopathological examinationAll of the tissue samples were first identified in a 10% formaldehyde solution for light microscope assessment. Following the identification process, tissue samples were washed under tap water in cassettes for 24h. Samples were then treated with conventional grade of alcohol (70%, 80%, 90%, and 100%) to remove the water within tissues. Tissues were then passed through xylol and embedded in paraffin. Four-to-five micron sections were cut from the paraffin blocks and hematoxylin–eosin staining was administered. Their photos were taken following the Olympus DP2-SAL firmware program (Olympus® Inc. Tokyo, Japan) assessment. Histopathological assessment was carried out by the pathologist blind for the study groups. Ischemia and reperfusion resulted in tissue damage with varying degrees of severity observed in different areas of the rat testis. Consequently, the grading system proposed by Cosentino et al.17 was used to evaluate any histological changes in the testes that were caused by ischemia and reperfusion. Therefore, a different score was assigned to each testicular area, and the final Cosentino's grade for each testis was calculated by multiplying the grade of each area by the percentage of the total surface it occupied.
Statistical analysesResults of the experiment were expressed as “mean value±standard deviation” (x±SEM). The degree of significance of intergroup differences was determined by one-way ANOVA test. Fisher's post-hoc LSD (least significance differences) were carried out in the follow up process. All statistical operations were performed on “SPSS for Windows, 21.0” statistics program and p<0.05 was considered to be significant.
ResultsBiochemical findingsAs can be seen from Fig. 1, the amount of MDA showed a significant (p<0.001) increase in testicular tissue of TTD group animals subjected to torsion and detorsion compared to SOC group subjected to sham operation and TTTD group treated with taxifolin. The difference of the amounts of MDA in HG and TTTD groups were statistically insignificant (p>0.05). The amount of tGSH in testicular tissue of the group subjected to torsion and detorsion operation was significantly lower (p<0.0001) compared to both groups subjected to sham operation and taxifolin. The difference between the amounts of tGSH in sham group and taxifolin group was also statistically insignificant (p>0.05) (Fig. 2). In Fig. 3, torsion and detorsion operations significantly (p<0.0001) decreased SOD activity in testicular tissue compared to sham group and taxifolin group. In addition, the difference between sham group and taxifolin group in terms of SOD activity was measured to be significant (p<0.05).
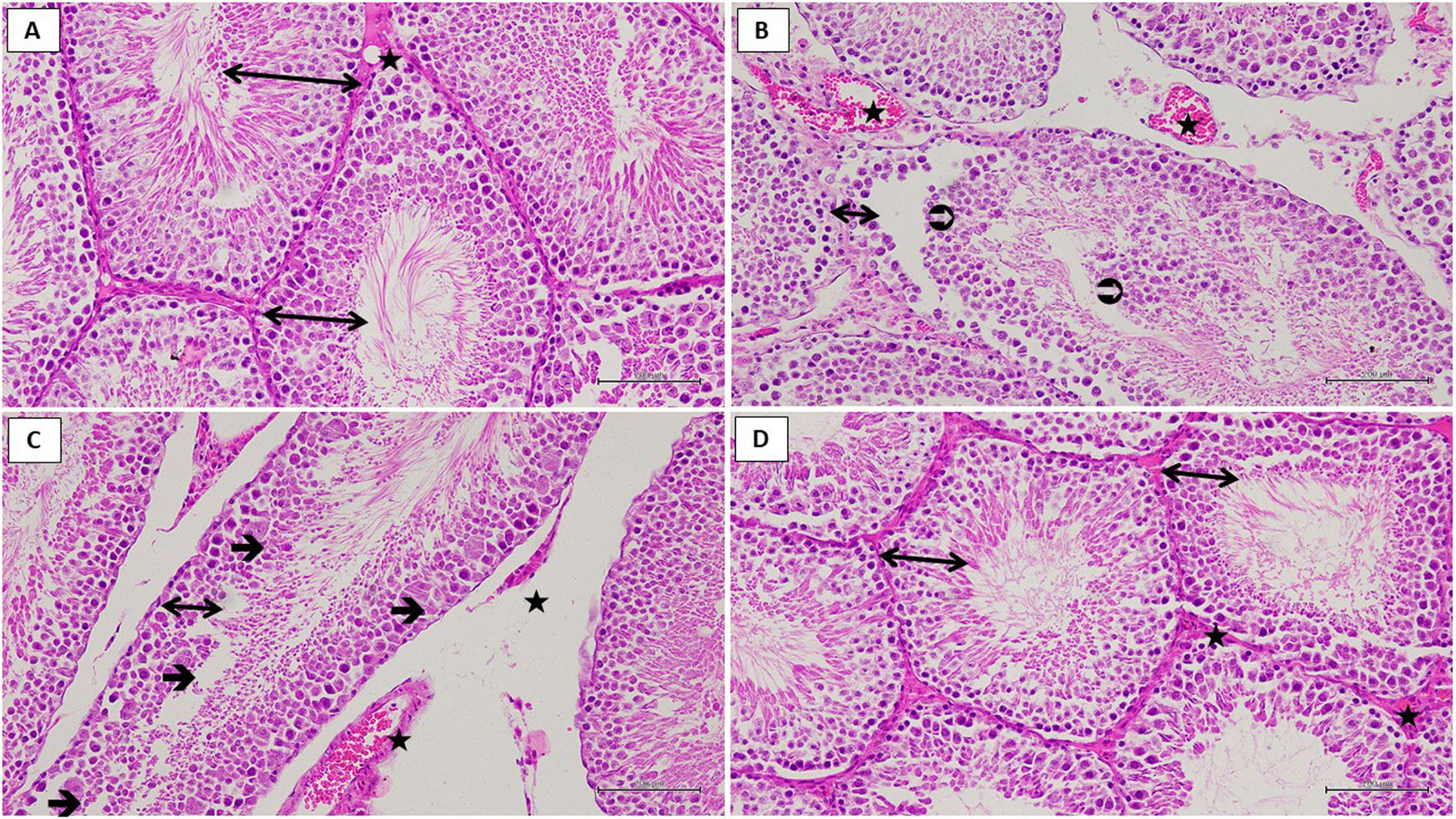
As a result of histological examination in testis tissue; germinal epithelium containing developing spermatogenic cell lines, multiple rounded seminiferous tubules with regular outlines and vascular formation showed normal histological structure in the sham operation healthy control (HG) group (Fig. 4A). (TTD) group to be subjected to testicular torsion–detorsion, decreased the thickness of the germinal epithelium, irregular seminiferous tubule borders, detached germinal cell lines, necrotic cell debris spilled into the lumen of the seminiferous tubule attracted the attention (Fig. 4B). However, multinucleated giant cells were observed in the degenerated germinal epithelium (Fig. 4C). (TTTD) group to be subjected to taxifolin+testicular torsion–detorsion sections showed increased germinal epithelium thickness, developing spermatogenic cell lines, normal seminiferous tubule shape. Also there was no dilatation and congestion in this group (Fig. 4D).
Hematoxylin–eosin staining in testis tissue in the SG group; ↔. germinal epithelium,
: blood vessel, ×200 (A). Hematoxylin–eosin staining in testis tissue in the TTD group; ↔. thin germinal epithelium, : necrotic cell debris, : dilated and congested blood vessel, ×200 (B). Hematoxylin–eosin staining in testis tissue in the TTD group; ↔. thin germinal epithelium, →: multinucleated giant cell formation, : dilated and congested blood vessel, ×200 (C). Hematoxylin–eosin staining in testis tissue in the TTTD group; ↔. germinal epithelium, : blood vessel, ×200 (D).Biochemical and histopathological examinations were performed in order to examine the effect of taxifolin on experimental testicular I/R injury through torsion and detorsion in rats. As is known, testicular torsion is a pathological event that requires urgent surgical intervention. If testicular torsion lasts for more than a few hours, an irreversible injury may occur in testis. On the other hand, only 32% of testis can be saved despite the early surgical interventions to testicular torsion in clinic.9 Besides, approximately 25% of men with torsion history are estimated to develop infertility in adulthood.18 In literature, the reason for this is suggested to be the overproduction of free radicals like ROS and pro-inflammatory cytokines.19 ROS induce LPO reaction which leads to further destruction.4 MDA, the final product of LPO reaction, is a more cytotoxic endogenous oxidant molecule.5 As can be understood from our experimental findings, torsion and detorsion have induced MDA production in testicular tissue of animals. MDA amount has increased in testicular tissue subjected to torsion and detorsion, which is compatible with the previous researches.19,20
It was observed that testicular tissue subjected to torsion and detorsion displayed a reduction in tGSH amount in parallel with the increase in MDA amount, suggesting that the oxidant-antioxidant balance in testicular tissue changed in favor of oxidants. As is known, ROS that are overproduced to sustain the integrity and functions of cells and tissues at normal levels under physiological conditions, are neutralized by endogenous glutathione, glutathione peroxidase, glutathione reductase, glutathione-s-transferase, superoxide dismutase, catalase and other antioxidant (vitamins A,C,E) defense systems.21 If antioxidants remain insufficient in neutralizing oxidants, the balance between oxidants and antioxidants changes in favor of the oxidants.22 GSH is a tripeptide consisting of l-glutamate, l-cysteine and glycine that are available in several cells. GSH displays an antioxidant effect by reacting with H2O2 and organic peroxides under the catalytic effect of GPO, an enzyme that contains selenium in its active region, and it eliminates H2O2 from cells. GSH chemically detoxifies hydrogen peroxide or organic oxides and protects cells from SOR damage.23
Torsion and detorsion operations have led to reduction in SOD activity in testicular tissue, which explains that the enzymatic antioxidant defense system gets reduced in the I/R injury of the testicular tissue. SOD is known to be protecting cells and tissues from ROS.24 SOD particularly catalyzes transformation of superoxide into hydrogen peroxide and molecular oxygen and dismutation reaction of superoxide.25 It has been shown by Dejban P et al. that SOD activity gets reduced in testicular I/R injury and SOD activity increases with antioxidant treatment.26
Taxifolin has inhibited elevation of MDA generation in testicular tissue of the animals subjected to torsion and detorsion, suggesting that it has an antioxidant activity. As stated above, flavonoids create an antioxidant effect by inhibiting lipid peroxidation and enzymatic reactions responsible for ROS formation.10 Koc et al. also reported that flavonoids suppress the increase of I/R-induced LPO in kidney tissue.27 Taxifolin has been reported to be inhibiting oxidative enzymes and overproduction of ROS.28 Taxifolin has also been reported to be suppressing the depletion of non-enzymatic and enzymatic antioxidants like GSH, SOD, glutathione-S-transferase and glutathione peroxidase as well as ROS.29 Duman A et al. also that flavonoids protect testicular tissue from I/R oxidative damage by suppressing not only the increase in MDA production but also its depletion in GSH.30 In several researches performed at different intervals, taxifolin has been reported to be protecting the cardiac and brain tissue from ischemia reperfusion injury due to its antioxidant and anti-inflammatory effects.31–33 Our experimental findings are compatible with previous reports stating that ROS cleaners suppress oxidative stress by inhibiting lipid peroxidation and preserving the levels of antioxidant mechanisms.34
In the group subjected to torsion and detorsion, which had a high level of MDA and low levels of tGSH and SOD, a reduction in germinal epithelium thickness, irregularity in seminiferous tubular boundaries, separated germinal cell lines, necrotic cellular eruption pouring out to the seminiferous tubular lumen and multinucleate giant cells in degenerated germinal epithelium, were observed. However, taxifolin significantly reduced these histopathological symptoms induced by torsion and detorsion operations. In our study, the finding of reduction in germinal epithelium thickness after torsion and detorsion operations was compatible with previous studies.20 Yapanoglu T et al. showed that seminiferous tubular destruction, necrosis, interstitial tissue damage and generalized germ cell damage seen after testicular torsion and detorsion operations were associated with oxidative stress.35 Hirik E et al. reported an evident injury in germinal epithelium cell and seminiferous tubule in I/R exposed testicular tissue where the balance oxidant-antioxidant balance changed in favor of the oxidants.36 Polat EC et al. reported that I/R was associated with dilated congested blood vessel in testis and seminiferous tubular and interstitial tissue damage were associated with oxidative stress.37 Yousefi-Manesh H et al. reported that experimental torsion and detorsion operations on testicular tissue caused degeneration and disorganization in germinal epithelium cells.11
ConclusionsIt has been shown by biochemical and histopathological findings that torsion and detorsion applied on testicular tissue cause oxidative I/R injury in tissue. Through biochemical and histopathological findings, taxifolin has been determined to be significantly reducing I/R-related oxidative damage in testicular tissue. The results of our experiment suggest that taxifolin could be helpful in the treatment of I/R injury that may come out following detorsion of testis subjected to torsion in clinic.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis research did not receive any specific grant from public, commercial and non-profit funding agencies.
Conflict of interestThe authors declare that they have no conflict of interest.
We would like to thank Ataturk University Medical Experimental Application and Research Center.