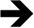This study aimed to biochemically and histopathologically investigate the effect of sunitinib on oxidative testicular damage induced by ischemia/reperfusion in rats.
Material-methodExperimental animals were divided into three groups of six rats each: testicular torsion–detorsion (TTD), sunitinib+testicular torsion–detorsion (STD), and sham control (SC). Sunitinib (25mg/kg) was administered orally to the STD group by gavage. Normal saline (0.9% NaCl) was administered orally to the TTD and control groups as the solvent. One hour after administration of sunitinib and 0.9% NaCl, all animal groups were done torsion–detorsion. Then, all the rats were killed by high-dose anesthesia, and their testicles were removed. Biochemical and histopathological examinations were performed on the removed testicular tissues.
ResultsMalondialdehyde; it was observed that the results in the STD group were close to those of the SC group and statistically significant lower compared to the TTD group (p=0.001). The glutathione values were statistically significantly higher in the STD group compared to the TTD group (p<0.001). Nuclear factor kappa B values, revealing a statistically significant difference between the TTD and STD groups (p<0.001). The TNF-α levels were measured and indicating that the results of the STD group were statistically significantly lower than those of the TTD group (p<0.001). Histopathologically, animal tissues given sunitinib were observed to resemble normal tissues.
ConclusionSunitinib was shown to prevent histopathological changes in testicular tissue against ischemia/reperfusion damage.
Este estudio tuvo como objetivo investigar bioquímica e histopatológicamente el efecto del sunitinib en el daño testicular oxidativo inducido por isquemia/reperfusión en ratas.
Material-métodoLos animales experimentales se dividieron en tres grupos de seis ratas cada uno: torsión-detorsión testicular (TTD), sunitinib + torsión-detorsión testicular (STD) y control simulado (SC). El sunitinib (25 mg/kg) se administró por vía oral al grupo STD por sonda. Se proporcionó por vía oral solución salina normal (NaCl al 0,9%) a los grupos TTD y control como disolvente. Una hora después de la administración de sunitinib y NaCI al 0,9%, se realizó una torsión-detorsión a todos los grupos de animales. A continuación, todas las ratas fueron sacrificadas con anestesia de alta dosis y se les extrajeron los testículos. Se realizaron exámenes bioquímicos e histopatológicos de los tejidos testiculares extraídos.
ResultadosMalondialdehído; se observó que los resultados en el grupo STD eran cercanos a los del grupo SC y estadísticamente significativos más bajos en comparación con el grupo TTD (p = 0,001). Los valores de glutatión fueron estadísticamente significativos más altos en el grupo STD en comparación con el de TTD (p < 0,001). Los valores del factor nuclear kappa B, revelaron una diferencia estadísticamente significativa entre los grupos TTD y STD (p < 0,001). Se midieron los niveles de TNF-α e indicaron que los resultados del grupo de ETS fueron estadísticamente significativos más bajos que los del ETV (p < 0,001). Desde el punto de vista histopatológico, se observó que los tejidos de los animales a los que se les administró sunitinib se parecían a los tejidos normales.
ConclusiónSe demostró que el sunitinib previene los cambios histopatológicos en el tejido testicular frente al daño por isquemia/reperfusión.
Testicular torsion or the torsion of the spermatic cord are among urological emergencies that mostly require surgery, especially in children and young men. Testicular torsion causes ischemia, which can lead to permanent damage to the testicles even if detorsion is successfully performed. As a result, apoptosis, testicular atrophy, and impaired spermatogenesis may cause infertility.1 Unilateral testicular torsion causes damage to the opposite testicle. Human and animal studies performed to date have shown defects in spermatogenesis after torsion, but the pathogenesis of these findings has not yet been fully elucidated.2 While ischemia results in tissue damage, the damage caused by reperfusion is greater. Due to ischemia/reperfusion (I/R), large amounts of reactive oxygen derivatives (ROS) are generated.3
The protective roles of many therapeutic agents have been reported in organ injuries due to I/R injury. Sunitinib, which was investigated in this study in terms of its protective effect against testicular I/R damage, is a tyrosine kinase inhibitor used in the treatment of metastatic renal carcinoma and gastrointestinal and pancreatic neuroendocrine tumors resistant to imatinibs.4 This group of drugs represents a new class of target-specific antineoplastics.5 Sunitinib has been reported to have the ability to inhibit the reduction in cisplatin-associated glutathione (GSH) and increase in malondialdehyde (MDA).6 It has also been documented that sunitinib inhibits the basal activity of the nuclear factor kappa B (NFκB) pathway.7 These findings from the literature suggest that sunitinib may be useful in the treatment of testicular I/R damage. However, to the best of our knowledge, there are no studies in the literature investigating the effect of sunitinib on testicular oxidative damage caused by I/R. Thus, this study aimed to investigate the effect of sunitinib on I/R-induced oxidative testicular damage in rats, using biochemical and histopathological analyses.
Material and methodEthical approval was obtained from the Animal Experiments Local Ethics Committee of Atatürk University Faculty of Medicine (dated 21.10.2019 and numbered 77040475-641.04-E.1900299746).
For the study, male Wistar albino rats were obtained from the Medical Experimental Application and Research Center of the university. A total of 18 male Wistar albino rats weighing 280–290g were used for the experiment. The animals were housed and fed at normal room temperature (22°C) prior to the experiment.
Ketamine and sunitinib used in the experiment were supplied from Pfizer Pharmaceuticals Limited Company (Turkey).
The experimental animals were divided into three groups of six rats each: testicular torsion–detorsion (TTD), sunitinib+testicular torsion–detorsion (STD), and sham control (SC).
Sunitinib (25mg/kg) was orally administered to the STD group by gavage. Normal saline (0.9% NaCl) was orally administered to the TTD and SC groups as the solvent. One hour after the administration of sunitinib and 0.9% NaCl, all the rats were done torsion–detorsion.
Before the surgical procedures, the rats were given 60mg/kg ketamine intraperitoneally and made to inhalexylazine at appropriate intervals to achieve anesthesia. When the animals are immobile in the supine position is considered to be an appropriate anesthetic period for surgical intervention.6
Surgical procedureSurgical interventions were performed under sterile conditions in a suitable laboratory environment. The scrotum of the animals in all groups was disinfected with 10% povidone iodine solution, and then a 2-cm vertical skin and subcutaneous incision was made on the scrotum midline. In the scrotal space, the right testicle was removed by blunt dissection from the gubernaculum together with the tunica vaginalis and spermatic cord. In the SC group, the testicles of the rats were replaced into the scrotum without any further treatment. For the TTD and STD groups, torsion was achieved by rotating the testes 720° for 4h. At the end of this period, detorsion was performed, and perfusion was applied for four hours. After each procedure, the incision area was closed with sterile sponge moistened with physiological solution. Then, the rats in all groups were killed by high-dose anesthesia, and their testicles were removed. Biochemical and histopathological examinations were carried out on the removed testicular tissues. The results obtained from the TTD and STD groups were evaluated by comparing them to the SC group.
Biochemical analysesFor sample preparation, from each tissue removed during the surgical procedure, all tissue were rinsed with phosphate buffered saline solution and 0.2g was weighed. Tissue homogenized in ice-cold 2ml phosphate buffers (50mM, pH 7,4) that were appropriate for the variable to be measured.8 It was then centrifuged at +4°C at 10,000rpm for 15min. The supernatant portion was used as the analysis sample for, MDA, tGSH, TNF-α, NF-κB and protein concentration. The protein concentration of the supernatant measured with the method described by Bradford.9
Malondialdehyde (MDA) detection is based on the spectrophotometric measurement, at 532-nm wavelength, of the absorbance of the pink complex formed by MDA with thiobarbituric acid (TBA) at high temperature (95°C). Into test tubes with caps were pipetted 250μl of homogenate, 100μl of 8% sodium dodecyl sulfate (SDS), 750μl of 20% acetic acid, 750μl of 0.08% TBA, and 150μl of pure water, which was then vortexed. After the mixture was incubated at 100°C for 60min, 2.5ml of n-Butanol was added, and the spectrophotometric measurement was undertaken. The amount of red color formed was read at 532nm using 3ml cuvettes, and the MDA amount of the samples was determined based on the standard graphic created using the MDA stock solution prepared by considering the dilution coefficients.10
DTNB [5,5′-dithiobis (2-nitrobenzoic acid)] in the measuring medium is a disulfide chromogen and easily reduced by compounds with the sulfhydryl group. The resulting yellow color was measured spectrophotometrically at 412nm. 1500μl measuring buffer (200mM Tris–HCl with 0.2mM EDTA, pH 8.2), 500μl supernatant, 100μl DTNB and 7,900μl methanol were pipetted into test tubes with caps and vortexed. The mixture was incubated at 37°C for 30min, and then measurements were made by a spectrophotometer. The amount of yellow color formed was read at 412nm using 3ml quartz cuvettes, and the GSH amount of the samples was determined based on the standard graphic created using the previously prepared GSH stock solution considering the dilution coefficients.11
Tissue-homogenate NF-κB and TNF-α concentrations were measured using the rat-specific sandwich enzyme-linked immunosorbent assay, rat NF-κB ELISA immunoassay kits (Cat. No:201-11-0288, SunRed), and rat TNF-α ELISA kits (Cat no: YHB1098Ra, Shanghai LZ). The analyses were performed according to the manufacturers’ instructions. Briefly, monoclonal antibody specific for rat NF-κB and TNF-α were coated onto the wells of the microplates. The tissue homogenate, standards and biotinylated monoclonal antibody-specific and streptavidin-HRP were pipetted into these wells, and then incubated at 37°C for 60min. After washing, chromogen reagent A and chromogen reagent B were added, upon which the bound enzyme acted to produce a color. The sample was incubated at 37°C for 10min; then, stop solution was added. The intensity of the colored product is directly proportional to the concentration of rat NF-κB and TNF-α present in the original specimen. At the end of the course, the well plates were read at 450nm. The absorbance of the samples was calculated with formulas based on standard graphics.
Histopathological analysisThe testicular tissues of the subjects were taken into 10% formaldehyde solution and fixed for 72h. After the fixation process, the tissues were taken into the cassette and washed in running water for 24h, and then purified by passing through increased alcohol series (70%, 80%, 90%, and 100%). The testicular tissues, which were made transparent in xylol, were embedded in paraffin blocks, and sections of 4–5μm thickness were taken. The sections were double-stained with hematoxylin–eosin and photographed in Olympus DP2-SAL firmware program (Olympus® Inc. Tokyo, Japan). The histopathological evaluation was performed by a histologist blinded to the study groups.
I/R resulted in tissue damage of varying degrees of severity in different areas of the rat testicles. The rating system recommended by Cosentino et al. was used to evaluate the histopathological changes caused by I/R injury. In this process, each region of the testicle was given a different score, and for each testicle, the final Cosentino grade was calculated by multiplying the grade of each area by the percentage of the total surface it occupied.12 Cosentino's grading system is presented in Table 1.12
Cosentino's grading system.
| Grade | Characteristics |
|---|---|
| I | Normal testicular architecture with an orderly arrangement of germinal cells |
| II | Injury showed less orderly, non-cohesive germinal cells and closely packed seminiferous tubules |
| III | Injury exhibited disordered sloughed germinal cells, with reduced size of pyknotic nuclei and less distinct seminiferous tubule borders |
| IV | Injury exhibited seminiferous tubules that were closely packed with coagulative necrosis of the germinal cells |
The data were analyzed using SPSS version 25.0 (SPSS®, IL, USA) and presented as mean±standard deviation (SD). The parametric data were evaluated by one-way ANOVA followed by the post-hoc Tukey test. The Kruskal–Wallis test was applied for the non-parametric data. A p value of <0.05 was considered statistically significant.
ResultsBiochemical resultsThe MDA levels in the SC, TTD and STD groups were measured as 1.113±0.005μmol/g protein, 5.488±0.278μmol/g protein, and 2.107±0.06μmol/g protein, respectively. It was observed that the results in the STD group were close to those of the SC group and statistically significant lower compared to the TTD group. The tGSH measurements in the SC, TTD, STD and SC groups were 7.041±0.158μmol/g protein, 3.165±0.174μmol/g protein, and 6.283±0.238μmol/g protein, respectively. The tGSH values were statistically significantly higher in the STD group compared to the TTD group. The TNF-α levels were measured as 3.433±0.504pg/g protein, 7.833±0.280pg/g protein, and 3.933±0.338pg/g protein for the SC, TTD, and STD groups, respectively, indicating that the results of the STD group were statistically significantly lower than those of the TTD group. The NFκB values were found to be 2.700±0.334pg/g protein, 6.767±0.294pg/g protein, and 3.400±0.473pg/g protein, respectively for the SC, TTD, and STD groups, respectively, revealing a statistically significant difference between the TTD and STD groups (Table 2). In the binary comparison of the groups, no statistically significant difference was detected between the SC and STD groups only in the TNF-α molecule. In all other binary comparisons, the difference between the groups was found statistically significant. The mean differences between the groups are given in Table 2.
Comparison of tissue levels of all groups in terms of the molecules and binary comparison of the molecules examined.
| MDA μmol/g proteinb | tGSH±nmol/g proteina | TNF-α pg/g proteina | NF-κB pg/g proteina | |
|---|---|---|---|---|
| SC | 1.113±0.005a | 7.041±0.158a | 3.433±0.504a | 2.700±0.334a |
| TTD | 5.488±0.278b | 3.165±0.174b | 7.833±0.280b | 6.767±0.294b |
| STD | 2.107±0.06c | 6.283±0.238c | 3.933±0.338a | 3.400±0.473c |
SC: sham control, TTD: testicular torsion–detorsion, STD: sunitinib+testicular torsion–detorsion, MDA: malondialdehyde, tGSH: total glutathione, TNF-α: tumor necrosis factor alpha, NF-κB: nuclear factor kappa B.
Data are expressed as Mean±SD. Data in each column were compared analytically. The numbers with different superscript letters significantly differ from each other (p<.05).
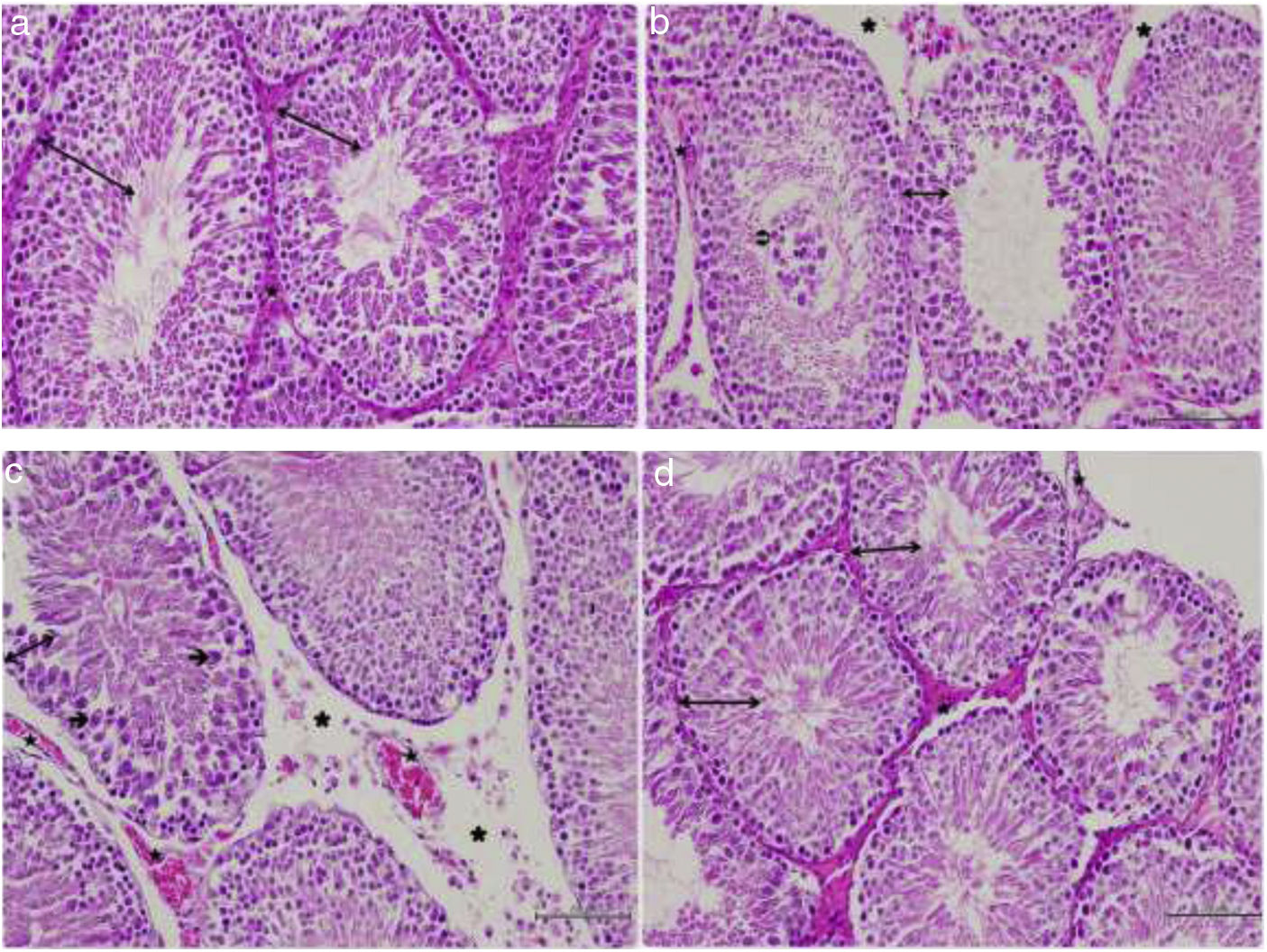
In the histopathological examination of the testicular sections of the SC group, the germinal epithelium, developing spermatogenic cell lines, the boundaries and lumen width of the seminiferous tubule, interstitial connective tissue, and blood vessel structures were normal (Fig. 1a). When the TTD, it was observed that the germinal epithelium containing spermatogenic cell series had a reduced cell density and this epithelium was thinner compared to the control group. The accumulation of necrotic cells in the lumen of the seminiferous tubule, and edema and separations in the area of the interstitial tissue between the seminiferous tubules were also noted (Fig. 1b). In the sections belonging to the same group, dilated and congested blood vessels were found in places. In addition, Sertoli cells of the germinal epithelium with a reduced number of layers were observed to be degenerated, separated from the basement membrane and released into the lumen (Fig. 1c). When the STD group was evaluated, the thickness of the germinal epithelium and the cell arrangement of the germinal epithelium were similar to the group, and the cells exhibited normal morphology. It was noted that the interstitial space between the seminiferous tubules was non-edematous and had a normal structure, and the blood vessels were also normal (Fig. 1d). The average grade of I/R injury according to the Consentino scoring system statistically significantly differed between the groups (SC<STD<TTD) (p<0.05).
Histopathological view. (a) Hematoxylin and eosin-stained testicular tissue of the control group;
: germinal epithelium, : blood vessel, ×200. (b) Hematoxylin and eosin-stained testicular tissue of the TTD; : thin germinal epithelium, : necrotic cell accumulation, : edema and separation observed in the interstitial tissue, : congested blood vessel, ×200. (c) Hematoxylin and eosin-stained testicular tissue of the TTD; : thin germinal epithelium, : degenerated Sertoli cell released into the lumen, : edema and separation observed in the interstitial tissue, : dilated and congested blood vessel, ×200. (d) Hematoxylin–eosin-stained testicular tissue of the ischemia/reperfusion group that received sunitinib; : germinal epithelium, : blood vessel, ×200.Testicular torsion creates ischemic damage and detorsion causes reperfusion damage, resulting in some structural and biochemical alterations in the tissue. Studies have shown that free oxygen radicals are responsible for tissue damage due to I/R.13 I/R caused by torsion–detorsion leads to testicular damage. During ischemia, germ cell death occurs due to low oxygen content that cannot meet metabolic needs, reduced cellular energy stores, and accumulation of toxic metabolites.14 Therefore, testicular torsion is a condition that requires urgent surgical intervention. The damage seen during ischemia increases with reperfusion. Reperfusion injury is closely related to neutrophil infiltration and increased ROS, which results in the peroxidation of lipids in the cell membrane.15 In addition to ROS generation in testicular endothelial cells, the I/R event causes the stimulation of proinflammatory cytokines, such as TNF-a and NF-κB. Furthermore, there is a decrease in reductive GSH levels and a significant increase in malondialdehyde production.16
In many studies, MDA, the end product of lipid peroxidation, has been reported to increase in I/R injury in developing tissues.17 Elevated MDA leads to the cross-linking of cell membrane compounds and impairment of ion permeability and enzyme activity, resulting in cell death. It is known that tGSH has the ability to neutralize free radicals caused by oxidative damage.18 GSH protects cells against oxidative stress damage by going into a reaction with free radicals and peroxides. It also provides the detoxification of foreign compounds and the transport of amino acids through membranes, and it is vital in protecting leukocytes and erythrocytes against oxidative stress. In the literature, GSH levels have been shown to be considerably decreased due to testicular torsion and detorsion.19 In our study, we observed that the MDA values were increased in the TTD group while in the STD group, they approached those of the SC group. Similarly, it was determined that the tGSH values were decreased in the TTD group and significantly increased in the STD group. Considering the results of both MDA and tGSH, sunitinib had an apparent protective effect of on the testicular tissue.
TNF-a is secreted from polymorphonuclear leukocytes activated during I/R.20 Lysiak et al. reported increased TNF-a levels in the testicular tissue after the reperfusion procedure.21 Many experimental studies have reported that the levels of IL-1þ and TNF-a are increased in the I/R-treated testicle.17,22 In our study, it was observed that TNF-a was significantly lower in the STD group compared to the TTD group. To date, no other study has shown that sunitinib prevents the increase in TNF-a due to I/R damage in the testicular tissue.
During the inflammation period, fibrosis occurs in the tissues via nuclear factor kappa B (NFκB).23 Sunitinib, an NFκB antagonist, is effective in reducing fibrosis and tissue damage.23 NFκB is a transcription factor that plays a critical role in many cellular processes, including embryonic and neuronal development, cell proliferation, apoptosis, and immune responses for infection and inflammation. The irregularity of NFκB signaling is associated with inflammatory diseases and some cancers.24 The NFKB pathway plays a major role in testicular reperfusion injuries; thus, it is possible to prevent such injuries using a selective NFκB inhibitor.25 Sunitinib inhibits the basal activity of the NFκB pathway.7 In our study, the NFκB values were significantly lower in the STD group compared to the TTD group. In another experimental study, Erbas et al. induced ovarian fibrosis by diabetes in rats and found that leading to the increase in the NFκB values due to fibrosis was reversed by sunitinib treatment.26
In conclusion, sunitinib prevented histopathological changes in the testicular tissue caused by I/R damage. Sunitinib was shown to protect testicular tissue against I/R injury through its antioxidant and anti-inflammatory effects. Further studies based on these findings may be useful in evaluating promising drugs for the prevention of I/R damage to testicles.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestThere is no conflict of interest of any authors in relation to the submission.