Testicular ischemia/reperfusion (I/R) injury develops after torsion and following detorsion of the testis. Reactive oxygen species were produced and oxidative damage begins to occur due to I/R process. Nimesulide, which is a specific cyclooxygenase-2 inhibitor drug, have antioxidant, antiinflammatory, analgesics and antipyretic effects. We aimed to investigate biochemically and histopathologically effect of nimesulide on testis I/R injury in rats induced by the testicular torsion–detorsion.
Material and methodsIn this study, 24 albino Wistar male rats were divided into four groups (6 rats in each group): ischemia/reperfusion applied+50mg/kg nimesulide administrated (NIM-50), ischemia/reperfusion applied+100mg/kg nimesulide administrated (NIM-100), ischemia/reperfusion applied (IR) and Sham surgery (SS) groups. Nimesulide was administered to NIM-50 and NIM-100 groups at the 50mg/kg and 100mg/kg doses before 2h applied I/R procedures. The IR group were applied only I/R procedures, no drug treatment was applied. Animals were sacrificed under high dose anesthesia and left testes were extracted. Testes were examined biochemically and histopathologically.
ResultsTotal glutathione (tGSH) and cyclooxygenase-1 (COX-1) levels were increased in the NIM-50 and NIM-100 groups compared to IR group. The levels of COX-2, malondialdehyde (MDA), interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) were lower in the NIM-50 and NIM-100 groups than in the IR group. Some histopathological changes seen in IR group. This findings were decreased in NIM-50 group and prevented in NIM-100 group.
ConclusionNimesulide prevented inflammation and oxidative stress. Our results suggest that nimesulide may be have a protective effect on testicular I/R injury.
La lesión de isquemia y reperfusión testicular (I/R) se desarrolla después de la torsión y la consiguiente detorsión del testículo. Se produjeron especies reactivas de oxígeno y el daño oxidativo comienza a producirse debido al proceso de I/R. La nimesulida, que es un fármaco inhibidor específico de la ciclooxigenasa 2, tiene efectos antioxidantes, antiinflamatorios, analgésicos y antipiréticos. El objetivo fue investigar el efecto bioquímico e histopatológico de la nimesulida sobre la lesión testicular I/R en ratas inducida por la torsión-detorsión testicular.
Material y métodosEn este estudio se dividió a 24 ratas albinas Wistar macho en 4 grupos (6 ratas en cada grupo): isquemia y reperfusión aplicada+50mg/kg de nimesulida administrada (NIM-50), isquemia y reperfusión aplicada+100mg/kg de nimesulida administrada (NIM-100), isquemia y reperfusión aplicada (IR) y cirugía simulada (SS). La nimesulida se administró a los grupos NIM-50 y NIM-100 a las dosis de 50 y 100mg/kg, respectivamente, 2h antes de aplicar los procedimientos de I/R. Al grupo IR se aplicó solo procedimientos I/R, no se aplicó tratamiento farmacológico. Los animales se sacrificaron con anestesia a dosis altas y se extrajeron los testículos izquierdos. Los testículos se examinaron bioquímicamente e histopatológicamente.
ResultadosLos niveles totales de glutatión (tGSH) y ciclooxigenasa-1 (COX-1) aumentaron en los grupos NIM-50 y NIM-100 en comparación con el grupo IR. Los niveles de COX-2, malondialdehído (MDA), interleucina 1β (IL-1β) y factor de necrosis tumoral-α (TNF-α) fueron menores en los grupos NIM-50 y NIM-100 que en el grupo IR. Se vieron algunos cambios histopatológicos en el grupo IR. Estos hallazgos disminuyeron en el grupo NIM-50 y se evitaron en el grupo NIM-100.
ConclusiónLa nimesulida previno la inflamación y el estrés oxidativo. Nuestros resultados sugieren que la nimesulida puede tener un efecto protector sobre la lesión testicular I/R.
Ischemia is a phenomenon in which blood flow to the tissue is reduced or removed due to various reasons resulting in the tissue not being sufficiently oxygenated.1 Ischemia leads to a number of chemical events in the cells, from functional disorders to necrosis.2 Therefore, the first intervention to prevent the development of injury in long-term ischemic tissue is the re-establishment of blood flow to the tissue. However, the process in which increased xanthine oxidase (XO) converts hypoxanthine to xanthine using the increased amount of oxygen to the tissue through reperfusion leads to the formation of excessive free oxygen radicals (FORs). Increased FORs result in reduced antioxidant capacity,3 and by oxidizing cell membrane lipids, lead to the formation of toxic products, such as malondialdehyde (MDA).4
Active polymorphonuclear leukocytes (PNLs) have an important role in the pathogenesis of reperfusion injury. PNLs release the myeloperoxidase (MPO) enzyme with an oxidant effect.5 In the presence of Cl− ions, H2O2 is reduced to hypochlorous acid through the MPO enzyme. Hypochlorous acid is a strong oxidant that easily reacts with many biological molecules and can cause tissue damage.2,6 The cycloxygenase-2 (COX-2) enzyme produce inflammatory prostaglandins (PGs) from arachidonic acid. It has been held responsible for the development of ischemia reperfusion injury.2 Testicular ischemia/reperfusion (I/R) injury is a pathological event that results in detorsion of the torsed testis. Testicular torsion leads to ischemic injury while detorsion leads to reperfusion injury.7 Despite early surgical interventions, only 32% of testicular torsion can be recovered.8 Unilateral testicular torsion results in infertility in 25% of the cases.9 The literature suggests that I/R injury is a complex pathologic process that begins with the lack of oxygen being sent to the tissue, continues with the production of FORs, and expands in response to inflammation. Therefore, it is considered that anti-inflammatory drugs which both antioxidants and selectively inhibit the COX-2 enzyme responsible for inflammation can be beneficial in preventing I/R injury. In this study, we investigated the protective effects of nimesulide against testicular I/R injury. Nimesulide is an anti-inflammatory, analgesic and antipyretic drug that is a selective inhibitor of COX-2 enzyme.10 Nimesulide differs from other non-selective COX-2 inhibitor drugs in that the side effects resulting from COX-1 inhibition (e.g., tissue damage, hemorrhage, and stomach ulcer) are milder.10 The review provided there was no information about the protective effect of nimesulide against testis I/R injury in the literature. Therefore, in this study, we aimed to biochemically and histopathologically investigate the effect of nimesulide on oxidative testis I/R injury induced by the testicular torsion–detorsion in rats.
Material and methodsExperimental animalsThe experimental animals were obtained from Atatürk University Medical Experimental Application and Research Center. A total of 24 male albino Wistar rats weighing 230–240 grams were randomly selected for use in the experiment. To adapt to the environment, the animals were housed and fed at room temperature (22°C) for one week in the laboratory before experimentation. The ethical approval of all the steps of the study was obtained from Atatürk University Ethical Committee of Animal Experiments decision dated 30.03.2017 and numbered 3/34).
Experimental groupsThe experimental animals were equally divided into the following four groups: ischemia/reperfusion+50mg/kg nimesulide (NIM-50), ischemia/reperfusion+100mg/kg nimesulide (NIM-100), ischemia/reperfusion (IR) and sham surgery (SS). In previous study, nimesulide was found effective on I/R injury at same doses.11
Experimental procedureOne hour before anesthesia, animals in the NIM-50 and NIM-100 groups received orally a single dose of 50 and 100mg/kg nimesulide, respectively. Distilled water was applied to IR and SS groups by the same route.12 Surgical interventions were performed under sterile conditions in an appropriate laboratory environment with 50mg/kg intraperitoneally ketamine anesthesia (KETALAR® Injectable Vial 500mg, Pfizer Turkey, Turkey) and application of sevoflurane (Sevorane Liquid 100% Solution 250ml, Abbott, Turkey) at appropriate intervals. The scrotum region of the animals in the experimental groups was cleaned with a 10% povidone iodine solution. Then, the scrotal cavity was accessed by a 2 cm vertical incision on the midline of the subjects’ scrotum. The left testicles in the scrotal cavity of animals in the IR, NIM-50, NIM-100 and SS groups were removed from the gubernaculum together with tunica vaginalis and spermatic cord via blunt dissection. In the SS group animals, the scrotum was placed back in the scrotum without any further intervention. In the remaining groups, the testes were torsed at 720° for 2h and placed back in the scrotal cavity. At the end of the 2 h waiting period, the testes were detorsed and perfused for 2 h. Following the reperfusion period, the animals in the IR, NIM-50, NIM-100 and SS groups were sacrificed with high-dose anesthesia and the left testes were removed. In previous studies, ischemia/reperfusion was applied in 1–2h periods.13,14 Biochemical and histopathological examinations were performed on the extracted testicular tissue.
Biochemical analysesPreparation of the samplesA 0.2g section was cut from each of the removed testicular tissues and homogenized in an ice-cold medium with a 1.15% KCl solution for an MDA assay and complemented to 2ml in a phosphate buffer at pH 7.5 for the measurement of total glutathione (tGSH). Then, after centrifugation at +4°C at 10,000rpm for 15min, the supernatant was used as the sample for analysis.
MDA quantificationMDA quantification is based on the spectrophotometric measurement of the adsorption of the pink color complex formed by MDA with thiobarbituric acid (TBA) at a high temperature (95°C) and a wavelength of 532nm.15 The amount of MDA was spectrophotometrically measured (Beckmandu 500, Global Medical Instrumentation, USA). The MDA amount in each sample was determined using a standard curve generated using the MDA stock solution [Lipid Peroxidation (MDA) Assay Kit, Sigma–Aldrich, USA].
GSH quantificationThe spectrophotometric method was used to quantify the GSH.16 The amount of GSH in the samples was determined using the standard curve generated with the previously prepared GSH stock solution (Glutathione Assay Kit, Sigma–Aldrich, USA). The GSH and MDA levels in the testicular tissues were given as nmol/mg protein.
Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α)The concentrations of the IL-1β and TNF-α homogenates in the testicular tissues were measured using rat-specific sandwich enzyme-linked immuno-sorbent assay kits (Rat Interleukin 1β ELISA Kit and Rat Tumor Necrosis Factor α ELISA Kit, respectively, Shanghai LZ, China). The analyses were performed according to the manufacturer's instructions. Monoclonal antibodies specific for rat IL-1β and TNF-α were placed in the microplate wells. Tissue homogenization, standards, biotinylated specific monoclonal antibodies and streptavidin-HRP (HRP-Conjugated Streptavidin, Thermo Fisher Scientific, USA) were pipetted into these wells and incubated at 37°C for 60min. After washing, to form a color, the chromogenic reagents A and B that act through the linked enzyme, were added. Then, a stop solution was added to terminate the reaction. The density of the colored product is directly proportional to the concentration of IL-1β and TNF-α in the original sample. On completion of the procedure, the adsorbance values of the well plates were obtained at 450nm using a microplate reader (PowerWave HT Microplate Spectrophotometer, Bio-Tek, USA). The adsorbance of the samples was calculated using formulas including standard graphics. The results were given as pg/g protein.
Measurement of COX activityThe measurement of COX activity in testicular tissues was performed using a COX activity assay kit (Cayman, Ann Arbor, MI, USA). The principle of this method is based on the 590nm colorimetric measurement of the oxidized N,N,N,N′- tetramethyl-p-phenylenediamine (Sigma–Aldrich, ABD) formed as a result of the reaction.
Histopathological analysesThe testicular tissues obtained from the rats were fixed in a 10% formalin solution. As a routine processing procedure, the tissues were embedded in paraffin. Then, sections of 4μm thickness were cut from the paraffin blocks and stained with hematoxylin and eosin (H & E). All sections were coded and examined under a light microscope (Olympus BX 52, Tokyo, Japan) by a pathologist blind to the treatment protocol. Testicular injury and spermatogenesis were graded as described by Johnsen.17 Each slide was scored on a scale of 1–10 based on the level of spermatogenesis.
Statistical analysesThe results obtained from the experiments were expressed as ‘mean value±standard error of the mean’ (x±SEM). Differences between the groups was determined using a one-way ANOVA test, followed by a Tukey test for biochemical analyses. Testicular biopsy scoring data were evaluated by Kruskal–Wallis and Mann–Whitney U test, Bonferroni correction was used in pairwise comparisons. All statistical procedures were performed in SPSS for Windows, v. 22.0 statistical software, and p<0.05 was considered significant.
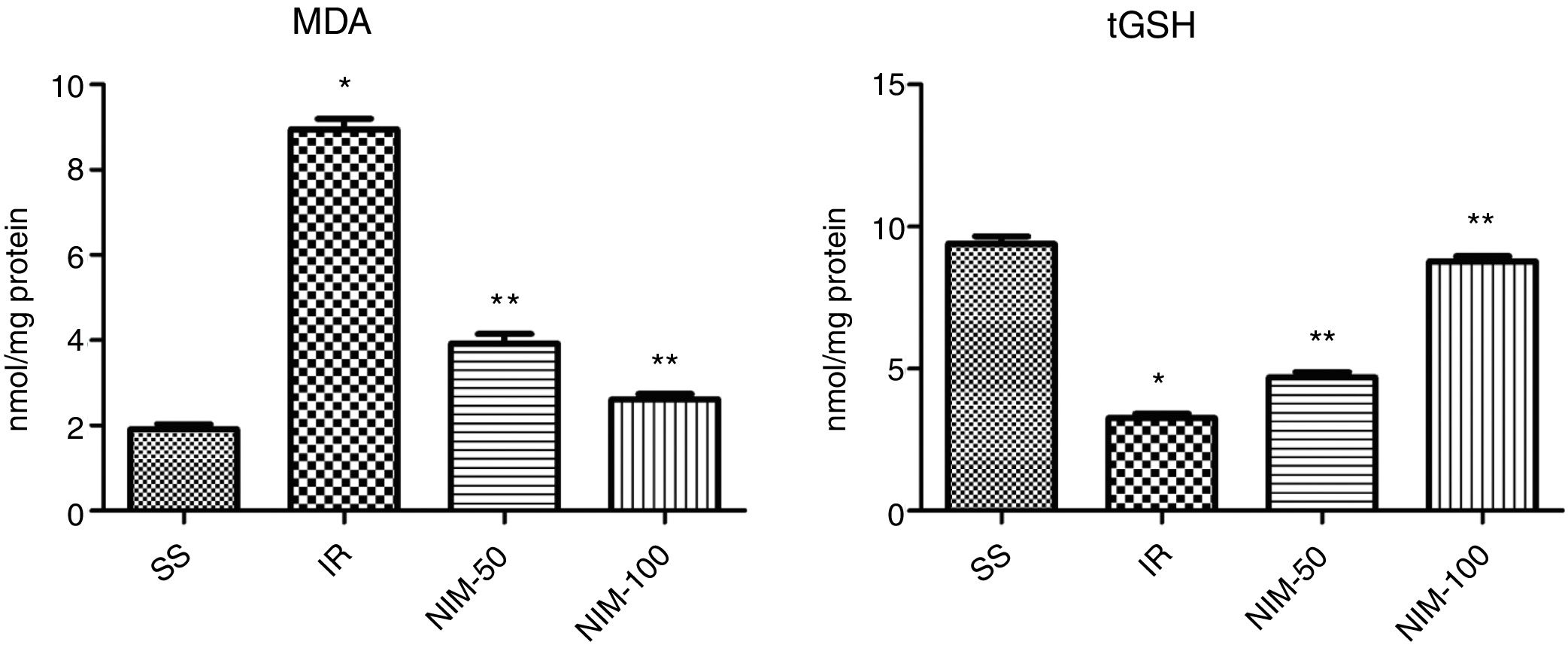
ResultsBiochemical findingsResults of the MDA analysisAccording to the results obtained from analysis performed on the tissues, the MDA values were determined as 1.92±0.11, 8.95±0.2, 3.92±0.23 and 2.62±0.12 for the SS, IR, NIM-50 and NIM-100 groups, respectively. The MDA values of the IR group were significantly higher according to the SS group (p<0.001). In addition, the MDA values were found to be significantly higher in the IR group (p<0.001) according to the NIM-50 and NIM-100 groups (Fig. 1).
Results of the tGSH analysisAccording to the results obtained from the tGSH analysis, the tGSH values were found to be 9.4±0.26, 3.25±0.15, 4.70±0.18 and 8.77±0.19 in the SS, IR, NIM-50 and NIM-100 groups, respectively. The tGSH values decreased in the IR group according to the SS group (p<0.001). Furthermore, the tGSH values were significantly higher in the NIM-50 and NIM-100 groups than in the IR group (p<0.001) (Fig. 1).
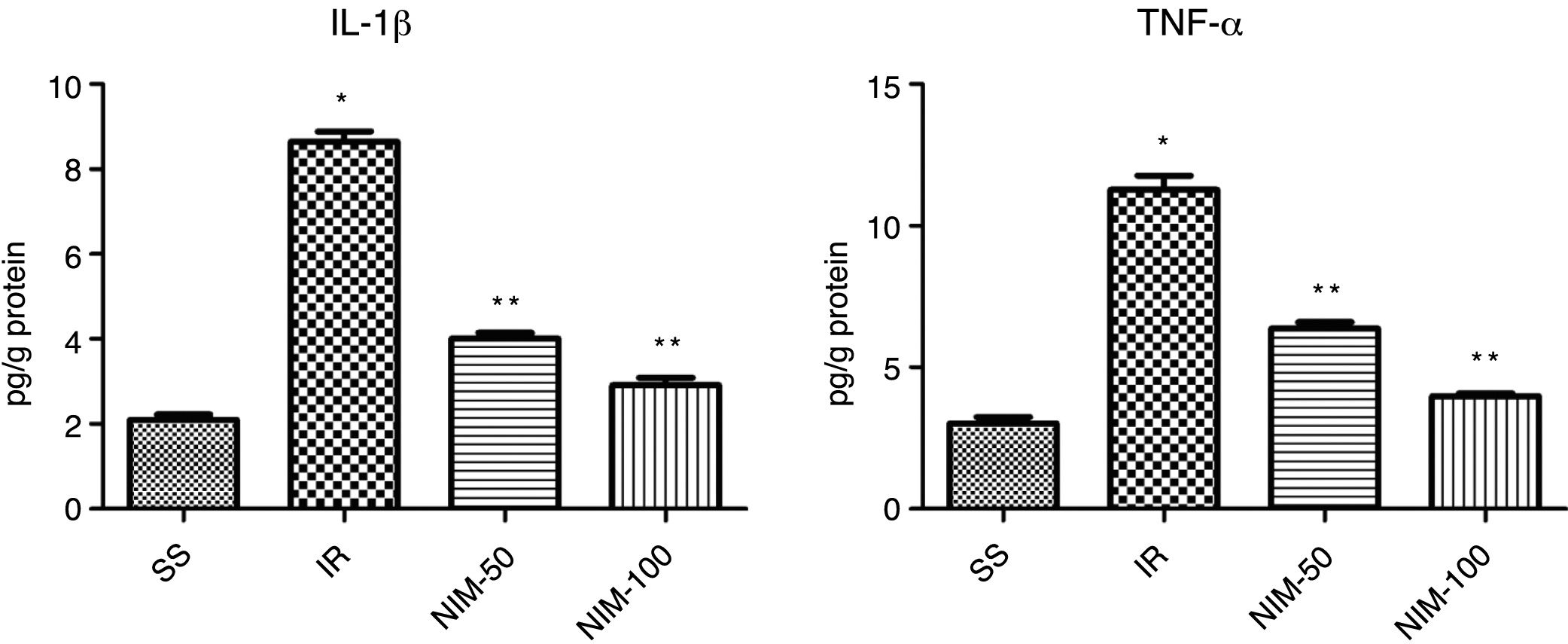
Results of the IL-1β analysisThe IL-1β values of the tissues were determined as 2.1±0.12, 8.65±0.23, 4.02±0.13 and 2.92±0.17 in the SS, IR, NIM-50 and NIM-100 groups, respectively. The IL-1β levels in the IR group were statistically significantly higher compared to the SS group (p<0.001) and the NIM-50 and NIM-100 groups (p<0.001) (Fig. 2).
Results of the TNF-α analysisThe TNF-α values were found to be 3.02±0.23 in the SS group, 11.27±0.51 in the IR group, 6.37±0.23 in the NIM-50 group and 3.98±0.10 in the NIM-100 group. The findings revealed that the TNF-α levels were significantly higher in the IR group according to the SS group (p<0.001) and the NIM-50 and NIM-100 groups (p<0.001) (Fig. 2).
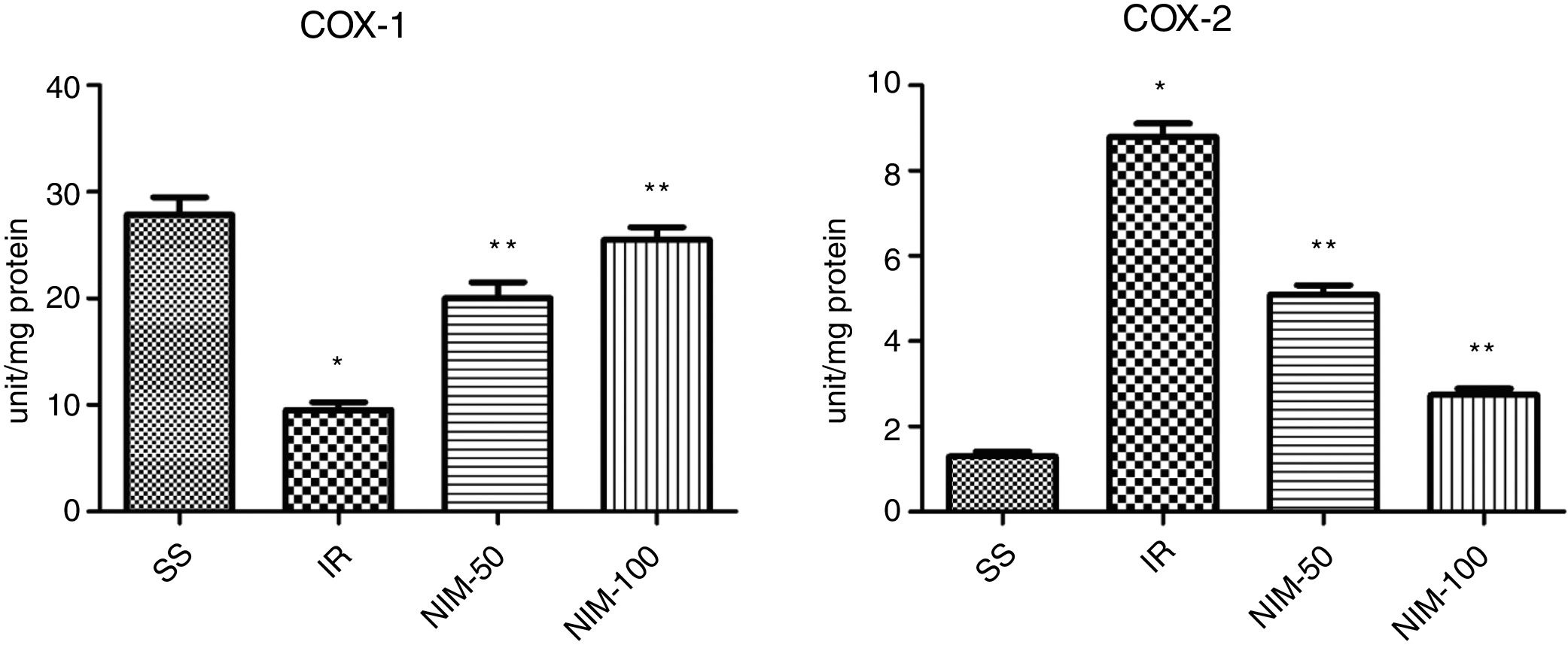
Results of the COX-1 analysisThe COX-1 levels of the testicular tissues of the subjects were determined as 27.83±1.64, 9.50±0.76, 20.00±1.53 and 25.50±1.18 in the SS, IR, NIM-50 and NIM-100 groups, respectively. The COX-1 levels in the SS group were found to be approximately three times higher than the IR group (p<0.001). In addition, the COX-1 values were lower in the IR group (p<0.001) according to the NIM-50 and NIM-100 groups (Fig. 3).
Results of the COX-2 analysisThe COX-2 values were measured as 1.30±0.12, 8.78±0.32, 5.10±0.21 and 2.73±0.15 in the SS, IR, NIM-50 and NIM-100 groups, respectively. The COX-2 values were found significantly higher in the IR group according to the SS group (p<0.001). Furthermore, the COX-2 values in the NIM-50 and NIM-100 groups were lower than in the IR group (p<0.001) (Fig. 3).
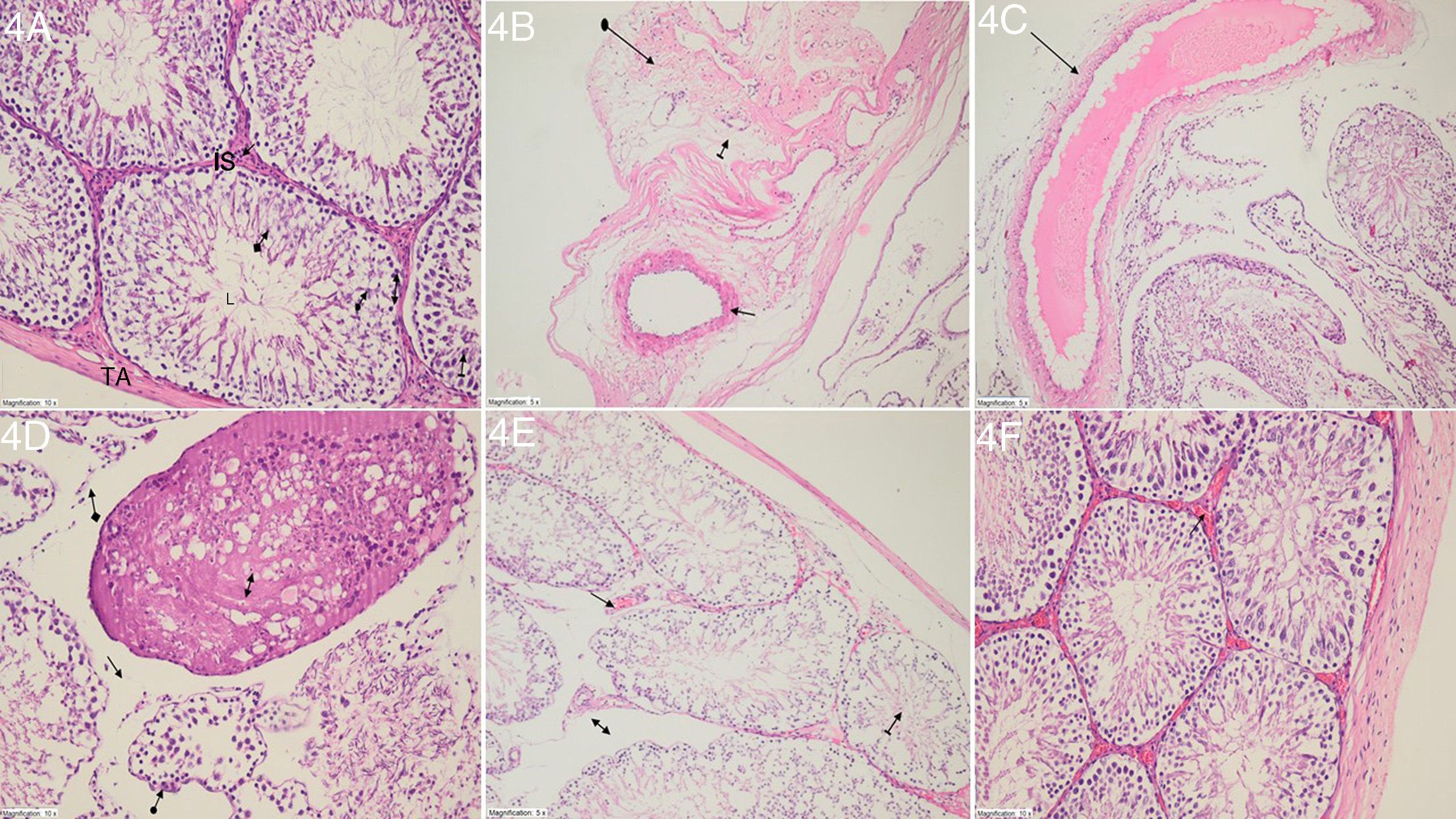
Histopathological findingsThe histopathological examination revealed the normal structure of tunica albuginea, the interstitial space of Leydig cells, lumen, Sertoli cells, spermatogonium, primary spermatocytes and spermatids in the testicular tissue of the rats in the SS group (Fig. 4A). In the IR group in which ischemia/reperfusion had been undertaken, degeneration of tunica albuginea, extensive interstitial damage including edema and dilated and congested blood vessels were observed (Fig. 4B). In this group, dilated and congested blood vessels were also seen in the stroma (Fig. 4C). The testicular tissue of this group also had necrotic seminiferous tubules, disintegration, edema and atrophic seminiferous tubules (Fig. 4D). Fig. 4E presents the dilated and congested blood vessels, edema and more organized seminiferous tubule structures observed in the NIM-50 group that had been administered nimesulide at a 50mg/kg dose. Nimesulide protected the testicular tissue from I/R injury at a dose of 100mg/kg. In the NIM-100 group, dilated and congested vessels had an almost normal appearance (Fig. 4F).
(A) Histopathological appearance of the testicular tissue in the SS group: TA (tunica albuginea), Leydig cells (straight arrow), IS (interstitial space), L (lumen), Sertoli cells (arrow with a circle), spermatogonium (double-headed arrow), primary spermatocytes (arrow with a line) and spermatids (arrow with a square) (H&E; ×200). (B) Histopathological appearance of the testicular tissue in the IR group: Degenerated tunica albuginea (arrow with a circle), edema (arrow with a line) and partially congested dilated vascular structure (straight arrow) (H&E; ×200). (C) Histopathological appearance of the testicular tissue in the IR group: dilated congested blood vessel in the stroma (straight arrow) (H&E; ×200). (D) Histopathological appearance of the testicular tissue in the IR group: necrotic seminiferous tubules (double-headed arrow), disintegrated interstitial tissue (arrow with a square), edema (straight arrow) and atrophic seminiferous tubule (arrow with a circle) (H&E; ×200). (E) Histopathological appearance of the testicular tissue in the NIM-50 group: dilated and congested blood vessels (straight arrow), edema (double-headed arrow) and more organized structure of the seminiferous tubule (arrow with a line) (H&E; ×200). (F) Histopathological appearance of the testicular tissue in the NIM-100 group: an almost normal appearance except for the dilated and congested blood vessels (straight arrow) (H&E; ×200).
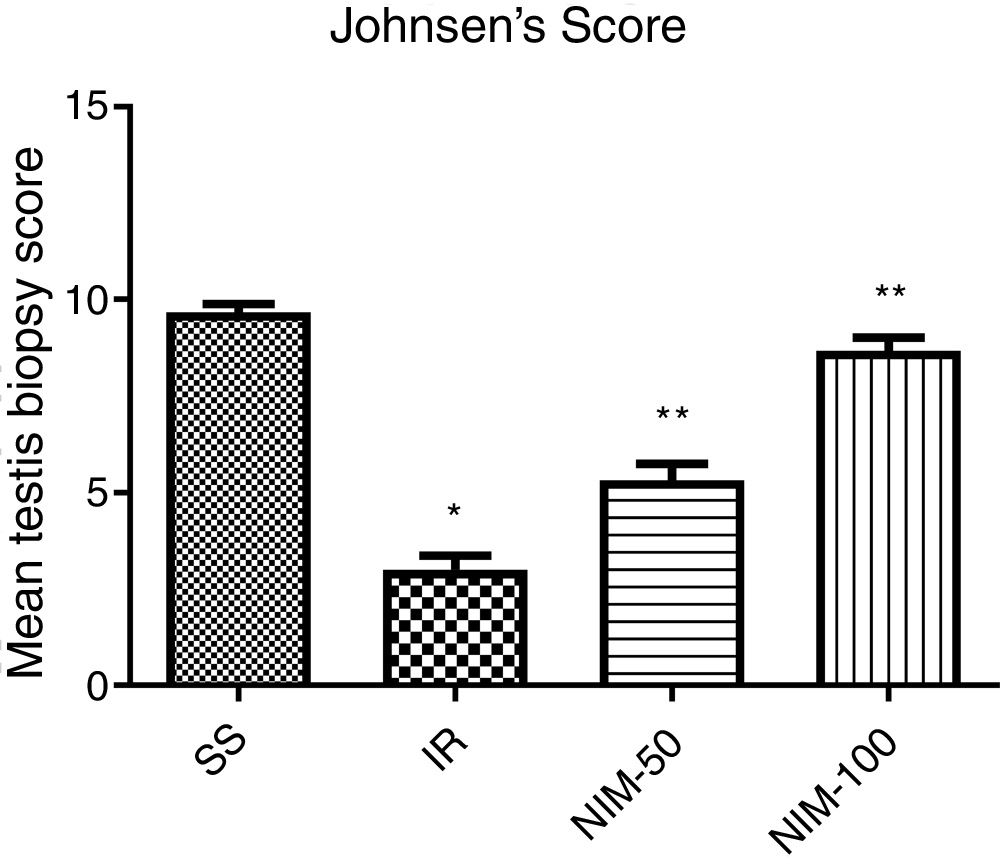
Mean testis biopsy score in the IR group was lower according to the SS group (p<0.005). Mean testis biopsy score in the NIM-50 and NIM-100 groups was higher than in the IR group (p<0.01) (Fig. 5).
DiscussionIn clinical practice, the surgical method used for the treatment of torsed testes is the detorsion and reperfusion of testicular tissues in a short time following the injury. However, excessive reperfusion of the testis results in the formation of FORs, which reduces the antioxidant capacity of the tissue.18 As indicated by our results, the torsion/detorsion procedure performed on the testicles increased the amount of MDA in the testicular tissue. As the end product of lipid peroxidation, increased MDA results in the cross-linking of cell membrane compounds and impaired ion permeability and enzyme activity, eventually leading to cell death.19 Previous studies have also reported increased MDA levels in testicular I/R injury.20–22 Similarly, in the current study, the amount of tGSH significantly decreased in the testicular tissue that had a high level of MDA. Endogenous tGSH has been shown to have the ability to eliminate free radicals formed due to oxidative damage.23 A number of experimental studies have reported that the amount of tGSH is reduced in testicular I/R injury.21,24 In the literature, the state in which oxidant parameters are increased and antioxidants are decreased is called oxidative stress.25
In this study, the testicular tissue of the IR group were found to have significantly higher MDA values and lower tGSH values compared to the NIM-50, NIM-100 and SS groups. Depending on the dose, when used against testicular I/R injury nimesulide inhibits the increase in MDA. The inhibitor effect of nimesulide on MDA was more pronounced, especially at the dose of 100mg/kg. To the best of our knowledge, in the literature, there is no information on the protective effect of nimesulide on testicular I/R injury. However, nimesulide has been reported to inhibit I/R-induced oxidative liver damage at a significantly higher rate at a dose of 100mg/kg compared to 50mg/kg.11 It has also been reported that by preventing the increase of MDA and reduction of tGSH, nimesulide protects kidney tissue from I/R.26 In another studies, glibenclamide that a nonselective KATP blocker, edaravone and short-interval postconditioning have been shown to be effective in reducing MDA levels in testicular I/R injury.27–29 The inhibition of MDA production and tGSH consumption in the testicular tissue of nimesulide-treated groups is in agreement with the data reported in the literature.
In the current study, it was also noted that the proinflammatory parameters, IL-1β and TNF-α, increased in the testicular tissue of the IR group. IL-1β and TNF-α are secreted from PNLs activated during I/R.6 Turner et al. showed that the levels of TNF-α and IL-1β increased in the testicular tissue following the reperfusion process.30 Many experimental studies have reported that the IL-1β and TNF-α levels are elevated in the ischemia/reperfusion applied testis.20,31,32 However, in the current study, it was found that the amounts of IL-1β and TNF-α were lower in the NIM-50 and NIM-100 groups receiving nimesulide compared to the IR group. In addition, nimesulide better inhibited IL-1β and TNF-α production at the dose of 100mg/kg. To date, no study has demonstrated that nimesulide prevents the increase of IL-1β and TNF-α in the testicular tissue due to I/R injury. However, nimesulide has been shown to create an anti-neuroinflammatory effect by inhibiting antioxidants and IL-1β gene expression in brain cells.33 Niranjan et al. also argued that the anti-inflammatory activity of nimesulide results from its inhibitor effect on IL-1β and TNF-α.34
Our experimental results show that in the testicular tissue of the IR group rats with increased oxidant and pro-inflammatory parameters, COX-2 activity increased and COX-1 activity decreased. COX-2 is an enzyme that can be induced by inflammatory mediators and is involved in the synthesis of pro-inflammatory PGs from arachidonic acid.35 COX-1 is known as a structural enzyme involved in the synthesis of cytoprotective PGs in cells.36 The inhibition of the COX-1 enzyme causes damage to tissues.37 Nimesulide appears to significantly inhibit the increase in COX-2 activity and the decrease in the COX-1 activity in the testicular tissue due to I/R.
In this study, nimesulide inhibited COX-2 activity and prevented the reduction of COX-1 activity at a significantly higher rate at the dose of 100mg/kg compared to the 50mg/kg dose. Some experimental studies have reported that nimesulide inhibits COX-2 enzyme, resulting in the suppression of inflammation and toxic radical formation in tissues.10 In the literature, studies on I/R injury in organs, such as the ovaries, liver and kidney found the COX-1 values to be higher in the IR injury groups than in the healthy groups.11,12,26
The histopathological examination revealed irregularities in the tunica albuginea, typical interstitial space injury including edema, dilated and congested blood vessels, necrotic seminiferous tubules, disintegrated interstitial tissue, edema and atrophic seminiferous tubules in the testicular tissue of the IR group rats. Degeneration in the tunica albuginea has previously been shown to be one of the pathological manifestations of I/R in testicular tissue.38 Interstitial spaces with edema observed in the testicular tissue are another pathological marker of I/R injury.39 In addition, Chi et al. reported that I/R can cause the necrosis of seminiferous tubules in the testicular tissue.40 Other studies on testicular I/R injury additionally revealed congestive vascular structures in the tissue and edema and structural abnormalities in the seminiferous tubules.28,41
In the current study, nimesulide was shown to prevent histopathological changes. In addition, it was clearly demonstrated that tissue injury resulting from I/R was partially prevented histopathologically in the group treated with a 50mg/kg dose of nimesulide. However, the 100mg/kg nimesulide was more successful in improving the histopathological markers of I/R injury compared to the 50mg/kg dose. Based on the results the following conclusions can be made: The torsion–detorsion procedure leads to oxidative stress in the testicular tissue. There was a significant increase in the MDA, IL-1β, TNF-α and COX-2 levels, and a decrease in the tGSH and COX-1 levels in the testicular tissues subjected to torsion–detorsion. Nimesulide 100mg/kg better inhibited the increase in MDA, IL-1β, TNF-α and COX-2, and the decrease in tGSH and COX-1 due to torsion–detorsion compared to the 50mg/kg dose. The histopathological examination also provided evidence that nimesulide protects the testicular tissue from I/R injury. It is considered that due to its ability to suppress the testicular I/R injury through its antioxidant and selective anti-inflammatory properties, nimesulide will have fewer side effects and it may be prevent the testicular I/R injury.
Authorship- 1.
The conception and design of the study, or acquisition of data, or analysis and interpretation of data: DA, ECP, ASB, MG, FKC.
- 2.
Drafting the article or revising it critically for important intellectual content: DA, ECP.
- 3.
Final approval of the version to be submitted: DA, ECP, ASB, MG, FKC.
The authors declare no conflict of interests.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.