Human sperm quality is decreasing progressively. One of the foremost reasons for infertility is the failure in sperm capacitation. We examined the influence of a cAMP (cyclic-adenosine mono phosphate analog)+IBMX (3-isobutyl-1-methylxanthine) on the motility and capacitation rate of human sperm over time.
Material and methodsSamples were gotten from 20 asthenozoospermic infertile patients referring to the Academic Center for Education, Culture and Research unit of the infertility research center, Qom, Iran. Samples were processed with a Density Gradient Centrifuging. Spermatozoa were divided into 4 groups: control, experimental 1, 2 and 3 (E1, E2, E3) based on the dose/time schedules (cAMP 5mmol+IBMX 0.2mmol/2, 4, and 6h, respectively). The computer-assisted sperm analysis and chlortetracycline assays were used to measure sperm motility and capacitation.
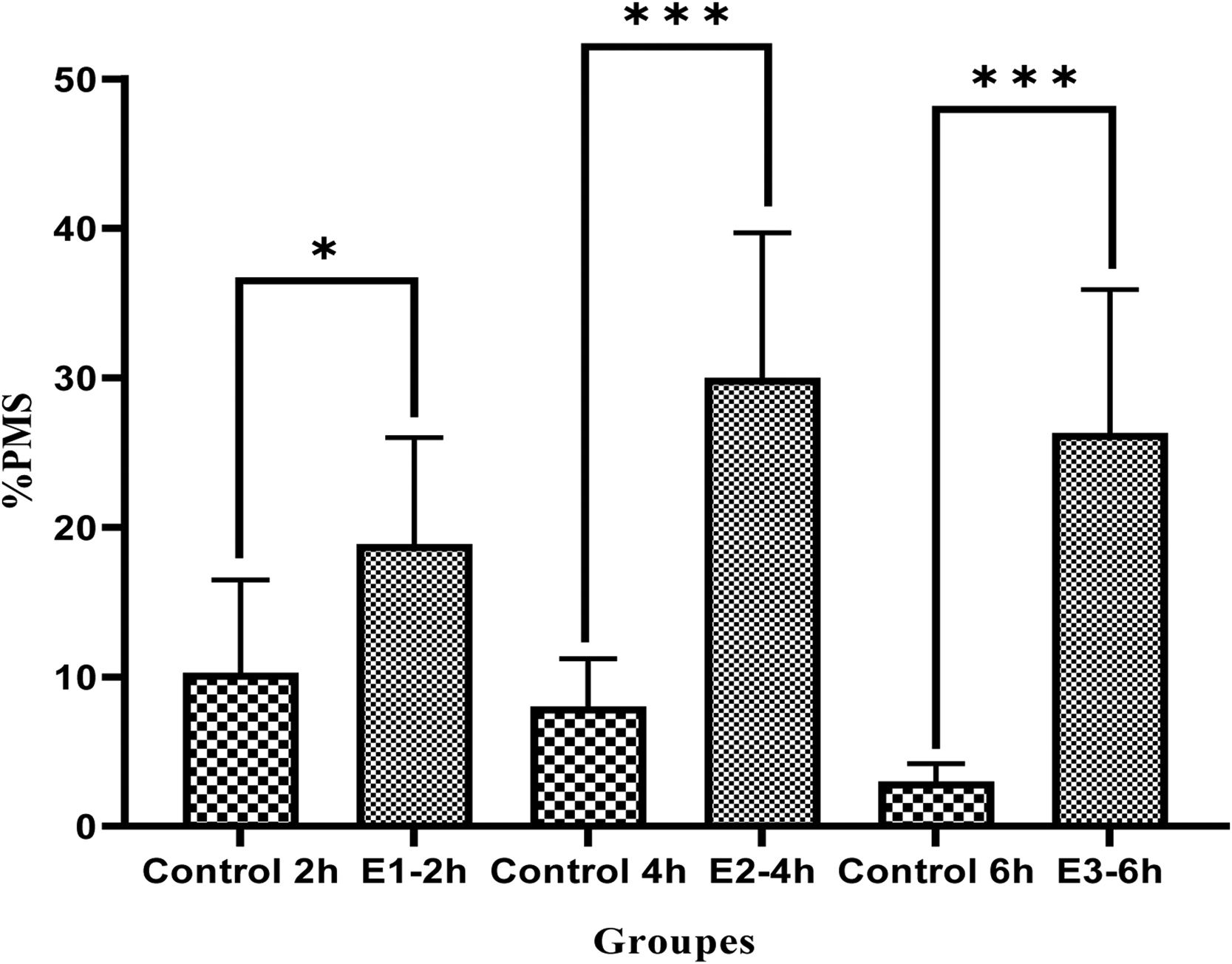
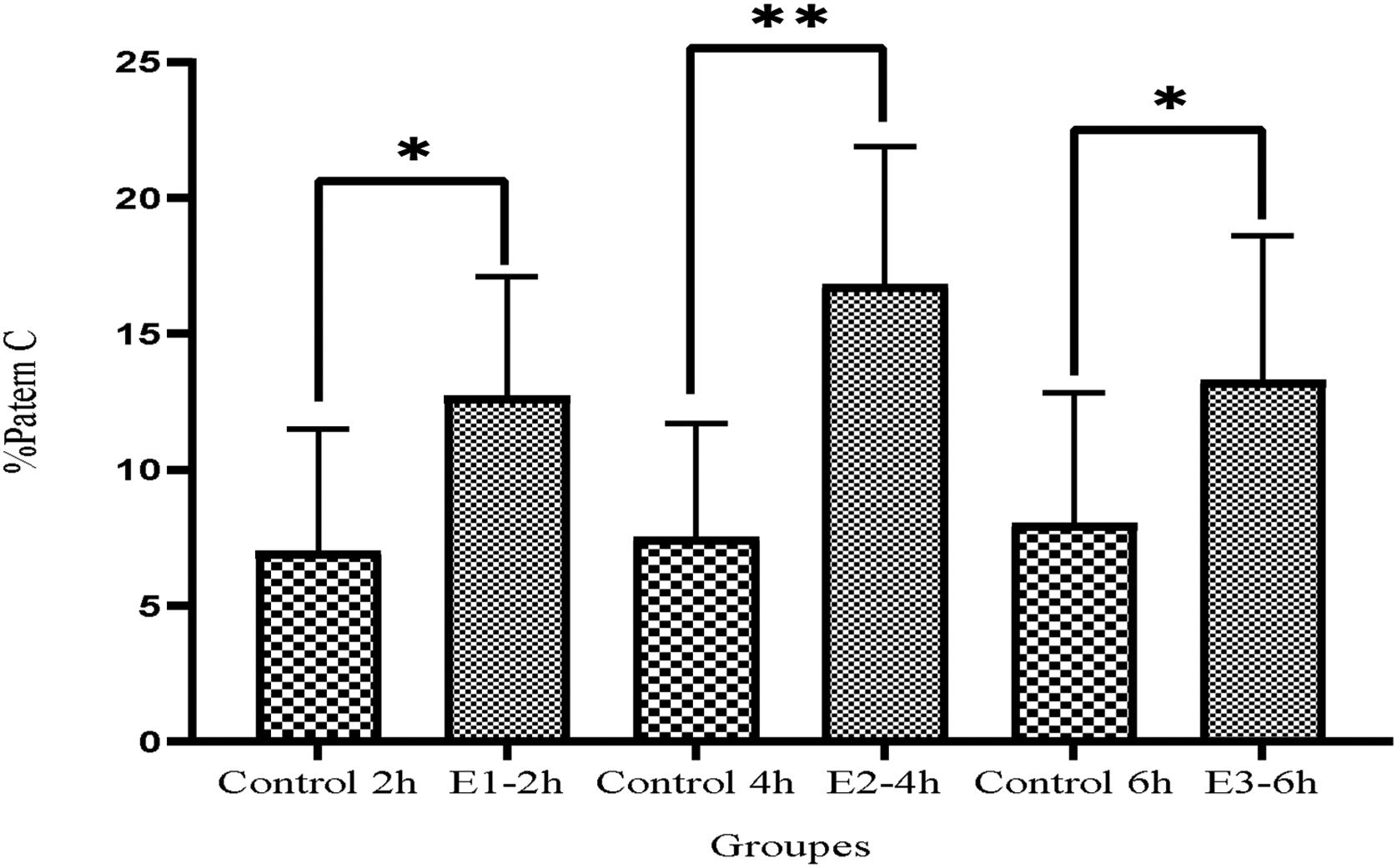
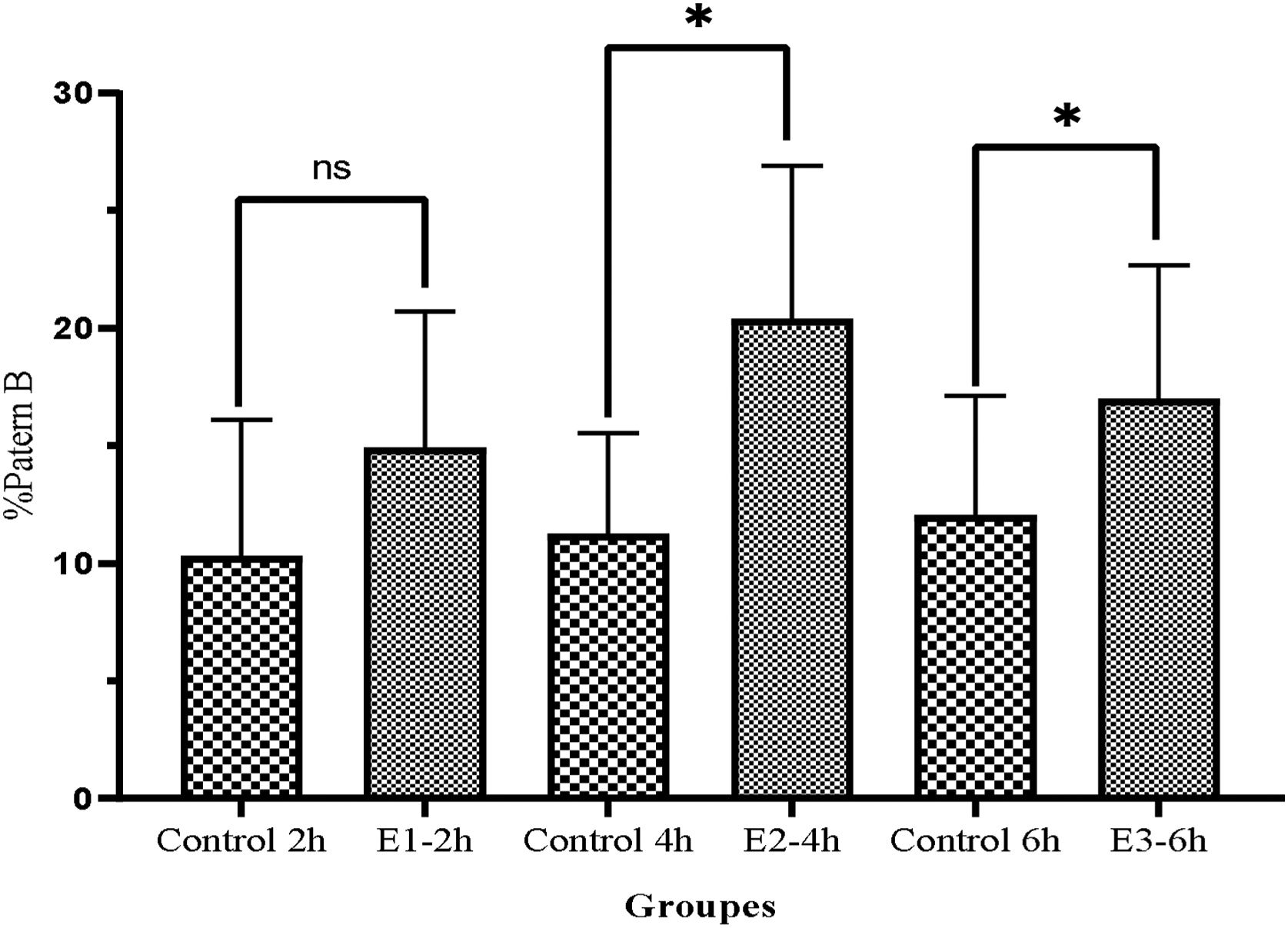
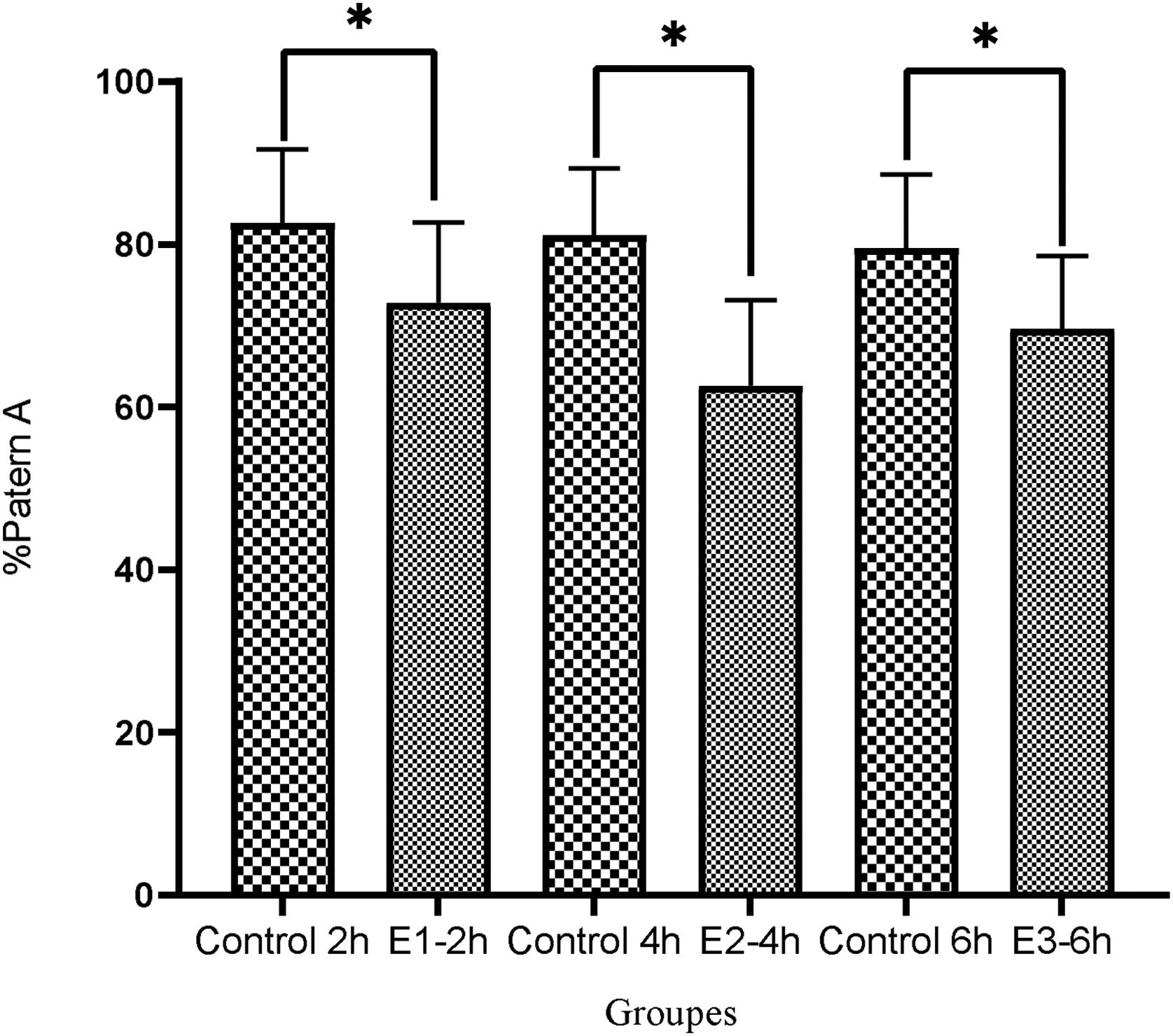
ResultsAfter incubation with a cAMP analog and IBMX, the levels of progressive motile sperms considerably improved in all experimental groups compared to the control group (E1=18.89±7.1, E2=30±9.7, E3=26.3±9.6 vs Control=10.28±6.2, P<0.05) especially in E2 group (P<0.05), indicating a greater effect of db cAMP (5mmol) and IBMX (0.2mmol) for 4h compared to the same doses at 2 and 6h. Also, non-progressive motile sperms significantly decreased in E2 group compared to the other groups (P<0.05). Moreover, both patterns C and B were substantially improved in all experimental groups especially in E2 group (P<0.05).
ConclusionOur findings support that the supplementation of sperm with db cAMP+IBMX specially for 4h, could be useful for men with asthenozoospermia to improve the success of assisted reproductive technology.
La calidad del esperma humano está disminuyendo progresivamente. Una de las principales razones de infertilidad es la falla en la capacitación de esperma. Examinamos la influencia de un AMPc (análogo de monofosfato de adenosina cíclico)+IBMX (3-isobutil-1-metilxantina) sobre la motilidad y la tasa de capacitación del esperma humano a lo largo del tiempo.
Material y métodosSe obtuvieron muestras de 20 pacientes infértiles astenozoospérmicos que se referían a la unidad del Centro Académico de Educación, Cultura e Investigación del centro de investigación de infertilidad, Qom, Irán. Las muestras se procesaron con una centrifugación en gradiente de densidad. Los espermatozoides se dividieron en 4 grupos: control, experimental 1, 2 y 3 (E1, E2, E3) en función de los horarios de dosis/tiempo (AMPc 5mmol+IBMX 0,2mmol/2, 4 y 6h, respectivamente). El análisis de esperma asistido por computadora y los ensayos de clortetraciclina se usaron para medir la movilidad y la capacidad de los espermatozoides.
ResultadosDespués de la incubación con un análogo de AMPc e IBMX, los niveles de espermatozoides móviles progresivos mejoraron considerablemente en todos los grupos experimentales en comparación con el grupo de control (E1=18,89±7,1, E2=30±9,7, E3=26,3±9,6 vs. Control=10,28±6,2, p<0,05) especialmente en el grupo E2 (p<0,05), lo que indica un mayor efecto de db AMPc (5mmol) e IBMX (0,2mmol) durante 4h en comparación con las mismas dosis a las 2 y 6h. Además, los espermatozoides móviles no progresivos disminuyeron significativamente en el grupo E2 en comparación con los otros grupos (p<0,05). Además, ambos patrones C y B mejoraron sustancialmente en todos los grupos experimentales, especialmente en el grupo E2 (p<0,05).
ConclusiónNuestros hallazgos respaldan que la suplementación de esperma con db AMPc+IBMX, especialmente durante 4h, podría ser útil para hombres con astenozoospermia para mejorar el éxito de la tecnología de reproducción asistida.
As a matter of fact, sperm capacitation is a basic process of fertility.1 Capacitation is the postejaculatory modification of molecular and cellular events in the plasma membrane such as deletion of superficial components like glyco-proteins, acrosome-stabilizing agent, decapacitation factor, and inhibitor of acrosin2,3; it is a vital event for sperm fertility. The primary purpose of capacitation is to warrant the penetration of the spermatozoa to the cumulus cells and zona-pellucida. The spermatozoa endures an exocytotic procedure named the acrosome reaction.4 The reversibility of the events resulting in sperm capacitation may be a significant feature of its well regulated and impeccable timing. Sperm capacitation can occur by several signaling pathways. A basic pathways is the modification in the form of movement termed hyperactivation, which spermatozoa displays at the fertilization site.5
In the course of capacitation, sperms movement changes from progressive to hyperactive.6 Hyperactivation impairment was previously reported in infertile humans.7 Wiser et al. (2014) revealed that improvement of sperm motility leads to greater fertilization success in humans.8
Capacitation is started and sustained by several factors including calcium, bicarbonate, soluble adenylyl-cyclase,8 protein-kinase A (PKA), cyclic adenosine monophosphate9 and metabolic substances.10 PKA stimulation at capacitation depends mostly on cAMP made by the SAC. The adenylyl-cyclase leads to production of cAMP, which is a key factor in the beginning and preservation of capacitation.11,12 Besides, PKA activation results in polymerization of actin, a critical event for the progress of hyperactivation, which is required for effective fertilization.13 The activation of PKA is facilitated by the Ca2+ and HCO3− dependent SAC.14
Supplementation of incubation media with cAMP analogs and 3-isobutyl-1-methylxanthine (IBMX) may lead to sperm hyperactivation and capacitation.5,10 Extracellular cAMP persuades sperm capacitation by the activation of some signaling pathways that include phospholipase-c (PLC), and an increase in sperm Ca2+, as well as SAC and cAMP/PKA, signaling.10,15 Additionally, cAMP is regulated by SAC and cyclic-nucleotide phosphodiesterase (PDE), which can reduce cAMP value. IBMX as a PDE inhibitor has been revealed to surge capacitation16 and motility.17
In previous researches, the inhibitors commonly have been presumed to impress specially on PDEs leading to increase of cAMP through PKA. Nonetheless, As far as our information, evidence concerning the exact effectiveness of PDE-inhibitors and cAMP supplements at different times on human sperm capacitation and motility is lacking. Therefore, our purpose was to explore the influence of cAMP analog, db cAMP and IBMX at different doses and time periods on the capacitation rate and motility patterns.
Material and methodsStudy designA total of 20 infertile asthenozoospermic men of mean age range 25–40 years, referred to the ACECR unit of the infertility research center of Qom, Iran, in 2018 were enrolled in the study. This prospective clinical trial was accepted by the Ethics Committee of The Islamic Azad University of Qom (approval code: IR, ACECR.JDM.REC.1397.001) and informed permission was obtained from all patients.
Sample collectionAll samples met the WHO standards for asthenozoospermia.18 Semen samples were obtained through masturbation after 3–5 days of sexual abstinence and allowed to liquefy at room temperature. Semen parameters (volume, sperm count, progressive and non-progressive motility and normal morphology) were evaluated according to WHO guidelines (2010). Semen samples were subjected to the density gradient centrifugation technique (DGC). Briefly, Sperm was prepared by standard DGC using 45% and 90% Isolate (Irvine Scientific; Santa Ana, CA, USA). Only spermatozoa migrating in the lower plate were selected. Spermatozoa were collected, washed and divided into four groups for evaluating capacitation and sperm motility. For this purpose, sperm samples were incubated in different time and different concentrations, so that in the control group, sperm samples were incubated only by mixed culture medium(HamF10). However, in different treatment groups, each of the samples was first incubated with different concentrations of cAMP (dbcAMP: N6,2′-O-dibutyryladenosine 3′:5′-cyclic monophosphate; Sigma) and IBMX (3-isobutyl-1-methylxanthine; Sigma) for different time.19 In the following groups, the amount of concentration used and the required incubation time are well shown.
- a)
Control: Semen samples after processing by the DGC method and incubated with culture medium (HamF10) for (2, 4, 6h).
- b)
Experiment 1 (E1-2h): Samples were incubated with 5m/mol dbcAMP and 0.2m/mol IBMX for 2h.
- c)
Experiment 2 (E2-4h): Samples were incubated with 5m/mol dbcAMP and 0.2m/mol IBMX for 4h.
- d)
Experiment 3 (E3-6h): Samples were incubated with 5m/mol dbcAMP and 0.2m/mol IBMX for 6h.
sperm motility was assessed by the Computer Aided Sperm Analysis (CASA) system (LABOMED, SDC313B, Germany), which defined sperm as progressive motile sper) PMS), Non-progressive motile sperm(NPMS) and Non-motile sperm (NMS).
Measurement of sperm capacitationChlortetracycline (CTC, sigma) staining was used to evaluation of sperm capacitation. Briefly the sperm samples were washed in (PBS) and incubated with of Chlortetracycline. CTC solution (750mM CTC, 5mM cysteine in 130mM NaCl and 20mM Tris–HCl) was prepared freshly, pH adjusted to 7.8 and stored at 4°C under dark condition. Then, each suspension was positioned on a glass slide, smeared and overlaid by a coverslip. The slides were stored in a wet chamber to prevent evaporation and CTC fading. A EUROStar III Plus fluorescent microscope (equipped with a 40×ZEISS Plan 40/0.65 and a Lumenera 375 camera; Inc. Electronics Division, GERMANY) was used to analyze the samples. At the end 100 sperm were analyzed for each determination of the percentage of the different CTC patterns in each sample. Sperm were categorized as the succeeding acrosomal staining patterns: (A) Uniform bright fluorescence over the whole head (uncapacitated sperm); (B) Fluorescence-free (dark) band in the post-acrosomal region(capacitated sperm); (C) Low fluorescent signal throughout the sperm head (acrosome reacted spermatozoa).
Statistical analysesData were compared by paired t-test via the statistical package for social studies (SPSS software, Version 20, Chicago, IL, USA). Also, Pearson correlation was applied for comparisons of total motility and capacitation. P-value<0.05 was considered significant. Data were described as mean±SD.
ResultsThe effect of cAMP and IBMX on sperm motilityAs Fig. 1 displays, there was an increase in progressive motility sperm (PMS) in all subjects tested compared to the control groups (E1-2h 18.89±7.1 vs 10.28±6.2; P<0.05),
(E2-4h 30±9.7 vs 8.01±3.2; P<0.001), (E3-6h 26.3±9.6 vs 3±1.2; P<0.001). Interestingly, the increase of PMS was significant difference in the E2 group in comparison to the E1 group (P<0.05, Fig. 1). Although, the percentage of PMS was much more obvious in E2 group compared to E3 group, but there was no significant difference between two groups (P>0.05, Fig. 1).
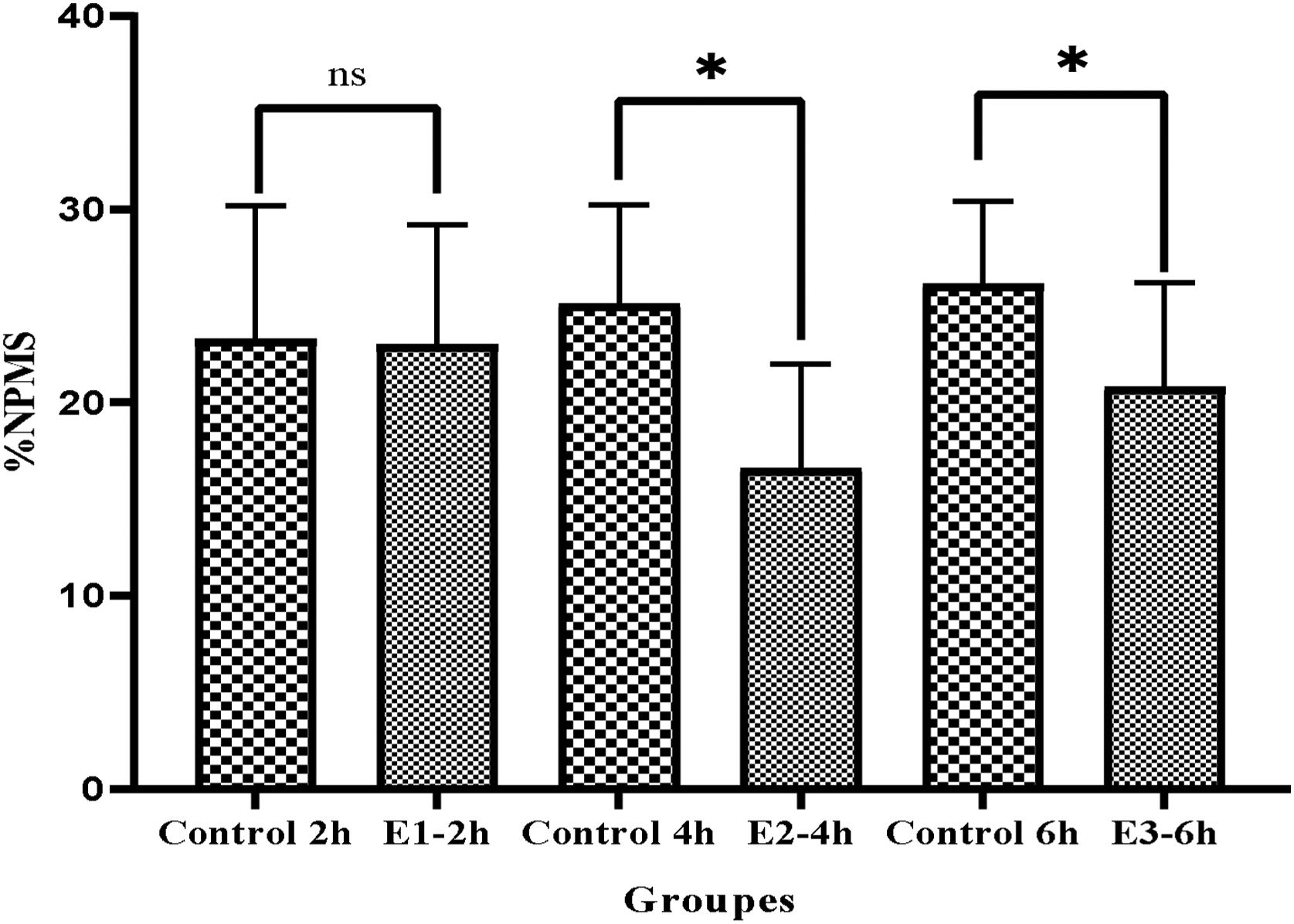
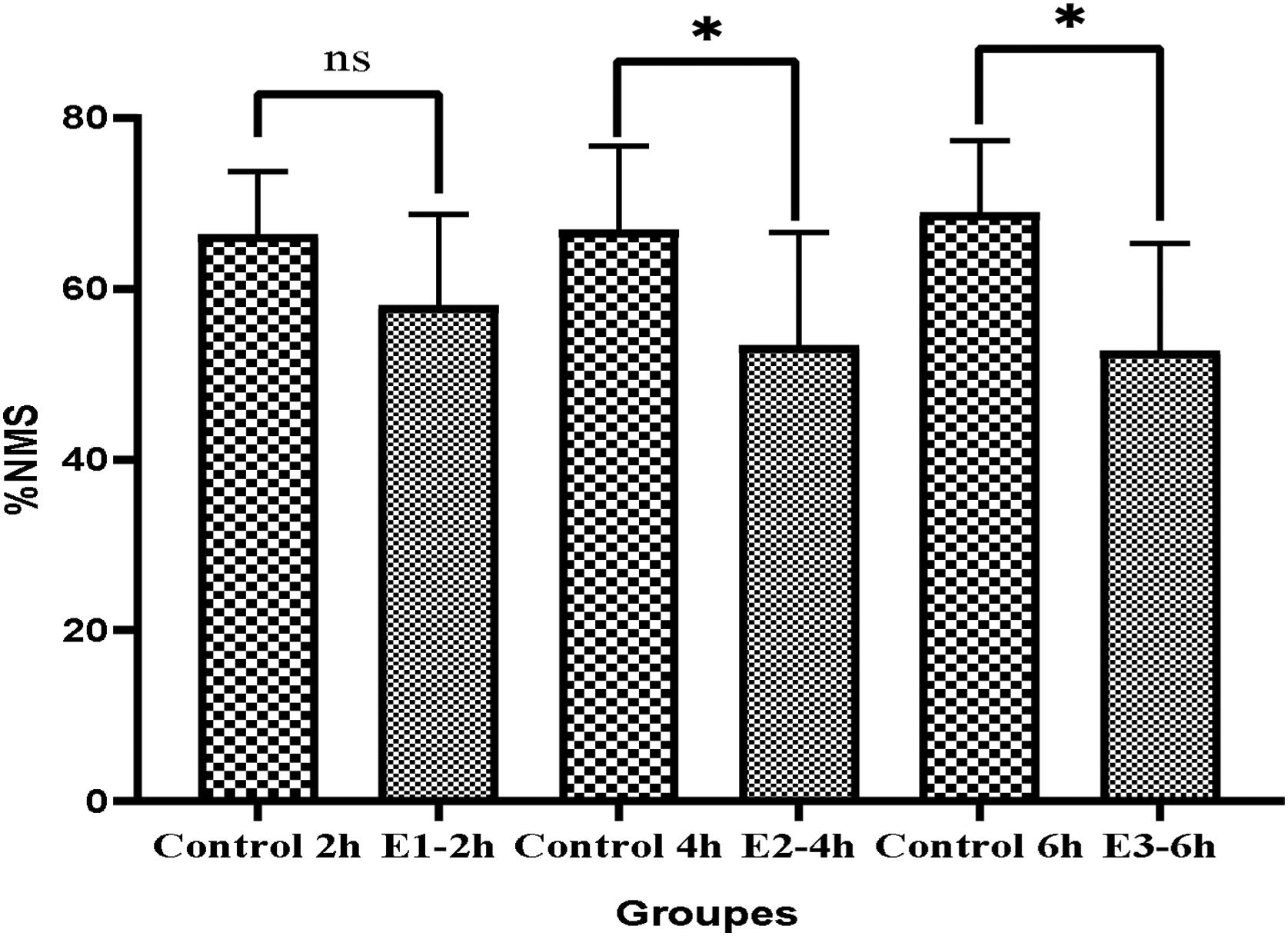
Moreover, the results showed the percentage of non-progressive motility sperm (NPMS) significantly decreased in E2-4h and E3-6h in comparison to the control groups. E2-4h (16.6±5.4 vs. 25.12±5.12), and E3-6h (20.8±5.4 vs. 28.14±4.25) (P<0.05) (Fig. 2). The Mean of NPMS percentage between showed that there was no significant in E1-2h compared to control group (23±6.21 vs. 23.3±6.86) (P>0.05) (Fig. 2). Examination of non-motility sperm (NMS) between different groups showed that the mean of (NMS) in groups E2-4h and E3-6h decreased significantly compared to control groups (E4h-53.3±13.3vs, 66.87±9.8; P<0.05) (E6h 52.7±12.6 vs. 68.86±8; P<0.05), respectively. Also in group E2-4h, the mean of NMS decreased compared to groups E1-2h and E3-6h but, it was no significantly different (E1-2h 58.0±10.7, E2-4h 53.3±13.3, E3-6h; P>0.05) respectively (Fig. 3).
Fig. 4 represented that the pattern C considerably increased in all experimental groups in comparison to the control groups (E1-2h=12.7±4.4, E2-4h=16.8±5.1, E3-6h=13.3±5.3 vs, Control 2h=7±4.5, Control 4h=6.1±4.2 and Control 6h=4.9±3.2, P<0.05). Remarkably the surge was more significantly higher in E2-4h group even compared to E1-2h group (P<0.05).
As illustrated in Fig. 5, the pattern B also improved in the all three experimental groups compared to the control group (E1-2h=14.9±5.8, E2-4h=20.4±6.8, E3-6h=17±5.7 vs, Control 2h=10.3±5.8, Control 4h=11.25±4.3 and Control 6h=12.5±51 (P<0.05).
In contrast, Fig. 6 displayed that the pattern A was considerably reduced in the E1-2h, E2-4h and E3-6h groups in comparison to the control group, respectively (72.8±9.9, 62.6±10.5 and 69.6±982.6±9 vs. Control 2h=82.6±9.1, Control 4h=81.1±8.2 and Control 6h=79.5±9.1) (P<0.05).
DiscussionData outlined in this study support that the adding of cAMP analogs and IBMX increased human sperm capacitation and following that increased sperm motility. Regarding sperm motility and hyperactivation, our findings are somewhat compatible with the studies of Bajpai et al. (2003) with the difference that they did not directly investigate the effect of cAMP analog and IBMX on sperm movement and did not compare the doses at different times.6
Therefore, it is concluded that cAMP has a substantial controlling role at the beginning of flagellar movement20,21 and in changes of the flagellar twist,22,23 it is likely that such cAMP-dependent variations might be associated to the motility pattern alterations.
The present results confirm the findings of previous researches stating that cyclic AMP is critical for the capacitation related events like the motility activation, changes in the movement pattern and the acrosome reaction.6,20,24 Then again, Lefievre et al. (2000) achieved similar results showing that use of IBMX could improve the sperms’ motility.25
On the same path, present study revealed that using a cAMP analogue+IBMX increased human sperm capacitation which confirmed the outcomes of Martinez-Leon et al. (2015) and Thundathil et al. (2002) with the difference that they did not compare the doses at different times.5,26 Similarly, studies using semen from bovines, rams and hamsters reported similar results.10,16
It is known that the cAMP analog's effects on sperm are arbitrated by the stimulation of PKA.24 Additionally, cAMP and its targets alter several capacitation-induced signaling actions including: 1 – membrane fatty acids transformation,27 2 – membrane hyperpolarization,28 3 – intra-cellular pH and Ca2+ surge29 and 4 – protein tyrosine-phosphorylation increase.30 Although the acrosome reaction is an imperative part of capacitation, but it is not usually considered at the capacitation process.31 Therefore, cAMP actions on the acrosome reaction are generally cited not in association with capacitation.
In Figs. 4–6, we illustrated the foremost sperm capacitation paths where cAMP analogs were engaged. Although cAMP analogs could entirely substitute for specific media components in the backup of capacitation, their effects were amplified by the PDE-inhibitors like IBMX. Moreover, capacitation can be imitated in the medium comprising a mixture of cAMP analogs instead of Na+, Cl− or etc.32 One of the primary signaling actions detected after exposing to capacitating settings is the quick surge in intracellular cAMP levels.33 Remarkably, using cAMP analogs rapidly increased these initial high cAMP levels in sperm. It is known that the cAMP increase instantly activates PKA.34 The quick regulation of cAMP is induced by cAMP analog identifying its consumption in the capacitation medium.
Although cAMP+IBMX significantly increased sperm capacity as well as motility in all experimental groups, sperm capacity was substantially higher in the E2 group compared to the E1 and E3 groups suggesting that time after stimulation with the agents is an important factor in altering sperm capacity. Incubating sperms for 4h was the most suitable time for altering sperm capacity that agrees with Parrish et al. (1995) study on the semen of bulls.19
Finally, our outcomes confirmed that the addition of cAMP analogs and IBMX could increase the human sperm capacitation and motility, specially after incubation for 4h. The kinases/phosphatases, as the critical factors during evolution of sperm motility regulate the protein phosphorylation.11 Especially, the cAMP/PKA-dependent pathway is very important in tyrosine-phosphorylation of various flagellar proteins, which are related to sperm motility.35 Hence more studies investigating the incubation time of human semen is required to making comparative assessments. It is well established that using pharmacological supplements of cAMP analogs and IBMX are necessary for the hyperactivated motility and capacitation, essential for fertilizing the egg. However, more researches are needed to understand the effect and underlying mechanisms of cAMP and IBMX on human sperm motility and capacitation. Besides, time is very important in this process and to achieve better results, the sperm should be incubated for 4h. Therefore, as a result, the fertility success rate will increase substantially.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors would like to express their gratitude towards the Islamic Azad University of Qom, as well as The Iranian Academic Centre for Education, Culture and Research for funding this project. The authors would also like to acknowledge Parnian Nejatbakhsh, medical student at Monash University, Melbourne, Australia, for her assistance in editing sections of this manuscript. The authors are grateful to all participants of this study, without whom this work would have been impossible.