It is necessary to be able to predict sperm retrieval before microdissection testicular sperm extraction (mTESE) in azoospermic men. This study established the importance of proliferating cell nuclear antigen (PCNA) and LIM15 gene expression levels in predicting the success of sperm retrieval by mTESE.
Materials and methodsOne hundred and forty-three men who were diagnosed with non-obstructive azoospermia (NOA) were included in the study. Patients’ age, total testosterone and follicle stimulating hormone values, testicular volume and testicular histology were recorded by prospectively. PCNA and LIM15 gene expression levels were determined by real-time PCR in the materials from both ejaculate and testicular specimens.
ResultsTestis volume and histology were the most important factors in predicting the sperm retrieval rate (SRR). The PCNA and LIM15 gene expression levels measured in testicular tissues and the LIM15 gene expression levels measured in ejaculate significantly correlated with the SRR in mTESE (p=0.038, p=0.022, and p=0.004, respectively). Although the PCNA gene expression level measured in ejaculate was higher in men with successful sperm retrieval, the difference was not statistically significant (p=0.061). According to the multivariate logistic regression analysis, testicular volume and LIM15 gene expression level in ejaculate were independent predictive parameters for sperm retrieval.
ConclusionThe data showed that LIM15 gene expression level in ejaculate is a useful molecular marker to predict the SRR before mTESE.
Es necesario poder predecir la recuperación de espermatozoides antes de la extracción de esperma testicular por microdisección (mTESE) en hombres azoospérmicos. Este estudio estableció la importancia del antígeno nuclear de células en proliferación (PCNA) y los niveles de expresión del gen LIM15 para predecir el éxito de la recuperación de espermatozoides mediante mTESE.
Materiales y métodosSe incluyeron en el estudio 143 hombres que fueron diagnosticados con azoospermia no obstructiva. La edad de los pacientes, los valores de testosterona total y de la hormona estimulante del folículo, el volumen testicular y la histología testicular se registraron de forma prospectiva. Los niveles de expresión génica de PCNA y LIM15 se determinaron mediante PCR en tiempo real en los materiales de muestras de testículos y eyaculados.
ResultadosEl volumen de los testículos y la histología fueron los factores más importantes para predecir la tasa de recuperación de espermatozoides. Los niveles de expresión del gen PCNA y LIM15 medidos en tejidos testiculares y los niveles de expresión del gen LIM15 medidos en la eyaculación se correlacionaron significativamente con la tasa de recuperación de espermatozoides en mTESE (p=0,038, p=0,022 y p=0,004, respectivamente). Aunque el nivel de expresión del gen PCNA medido en la eyaculación fue mayor en los hombres con recuperación exitosa de espermatozoides, la diferencia no fue estadísticamente significativa (p=0,061). Según el análisis de regresión logística multivariante, el volumen testicular y el nivel de expresión del gen LIM15 en la eyaculación fueron parámetros predictivos independientes para la recuperación de espermatozoides.
ConclusiónLos datos mostraron que el nivel de expresión del gen LIM15 en la eyaculación es un marcador molecular útil para predecir la tasa de recuperación de espermatozoides antes de mTESE.
Azoospermia is seen in 1% of men and in 10–15% of infertile men.1 Obstructive azoospermia (OA) accounts for 15–20% of azoospermia in men; the rest is non-obstructive azoospermia (NOA).2 Intracytoplasmic sperm injection (ICSI) with microdissection testicular sperm extraction (mTESE) has been a great achievement in the treatment of these cases. The sperm-retrieval rate (SRR) after mTESE in patients with NOA is approximately 40–60%.3 To prevent high cost and potential complications, such as ovarian hyperstimulation syndrome of assisted reproductive techniques (ARTs) and TESE-related surgical complications and psychological problems that may arise from failure, predicting the SRR of TESE is essential to provide patients with correct information before surgery.
Although testicular histology as a model for predicting SR has been reported to increase diagnostic accuracy, precise, non-invasive prognostic methods are still needed.4 Recently, genetic markers have been proposed as potentially useful tools for predicting the presence of testicular sperm in azoospermic individuals,5 so exploring the presence of testis-specific markers in seminal plasma may help estimate the prognosis of mTESE.
LIM15, also known as DNA meiotic recombinase 1 (DMC1), is a meiosis-specific recombinase.6 RecA, a protein involved in the DNA-repair mechanism in prokaryotes, transfers the sequence found in the undamaged complementary chain by a recombination exchange process7 and is considered the ancestor of LIM15 and Rad51,8 which provide recombination in eukaryotes. While both meiotic and somatic cells express Rad51,9 LIM15 is specifically expressed in the spermatogenesis as meiotic recombination protein.10 Proliferating cell nuclear antigen (PCNA) is a nuclear protein that plays a role in DNA replication and repair as the cofactor of the DNA polymerase delta. Its expression levels are associated with the prognosis and survival of various types of cancer, especially since it plays a key role in cell proliferation.11–13 In men with NOA, spermatagonial DNA synthesis can also be evaluated by measuring the amount of PCNA expression.14 We studied this, considering that the expression of PCNA, a proliferation marker, would be higher in TESE-positive infertile men than in TESE-negative ones.
In this study, we aimed to determine the predictive potential of PCNA and LIM15 expression levels measured in ejaculate and testicular tissue for the chance of successful SR during mTESE.
Material and methodsPatient selectionOne hundred and forty-three infertile men who were diagnosed with NOA and decided to undergo mTESE were included in the study with their informed consent and approval from a local ethical committee (registration number 03-154-18) between February 2018 and July 2019. After the ejaculates were centrifuged at 3000×g for at least 15min, diagnoses of azoospermia were confirmed by repeating the tests at 2-week intervals. Spermiogram results were evaluated on the basis of the World Health Organization (WHO) 2010 guidelines. Forty-six patients who did not want to include in the study or who were diagnosed with obstructive azoospermia were excluded from the study. Patients’ data were recorded prospectively. Patients’ urological and systemic disease histories were questioned. Afterward, patients were examined to evaluate testicular consistency, epididymal distension, palpable vas deference, varicoceles, and secondary sexual characteristics. The patients did not receive pre-op hormone manipulation. Cases with non-palpable vas deferens were excluded from the study. Transrectal ultrasonography was performed to all patients with ejaculate volume lower than 2ml to exclude OA.
To compare testicular volume with SRR, testicular volume of testis from which sperm was obtained in TESE-positive patients and testicular volume of large testis in TESE-negative patients were used. Testicular volumes were calculated using ultrasonography as described previously.15 Blood samples were taken from antecubital vein at morning in fasting. Serum FSH (reference range 1.27–19.26mIU/ml) and serum total testosterone (TT) (reference range 175–781ng/dl) values were measured on a Beckman Coulter device (Beckman Coulter, Brea, California [CA], United States of America [USA]) with the chemiluminescent immunoassay method. In all patients, Y chromosome microdeletion analysis and karyotyping were performed. PCR was performed on genomic DNA extracted from peripheral blood leukocytes, and the products were visualized by electrophoresis as previously described.16
Tissue samples were taken by mTESE as described previously.3 Each sample was placed in a petri dish filled with 0.5ml of human tubal fluid (HTF) medium. The samples were examined by a specialist embryologist, and the process continued until mature spermatozoa were found. The presence of at least one mature spermatozoon from mTESE was accepted as procedural success. During the procedure, one part of the sample was placed into a Bouins’ solution and sent for histopathological investigation. Histopathological subgroups of NOA patients were determined in the pathology laboratory according to their Johnsen scores. The patients were diagnosed with Sertoli cell-only (SCO), maturation arrest (MA), and hypospermatogenesis (HS) according to the features of testis histology. The material taken for genetic examination was also placed in sperm-washing solution (G-IVF, Vitrolife, Gothenburg, Sweden).
RNA isolation, cDNA synthesis, and PCR array—qRT-PCR analysisFor LIM15 and PCNA gene-expression measurements, RNAs were isolated from the residual tissue of the mTESE samples (3–5mg) taken for routine diagnosis and treatment; RNA was extracted from fresh tissue using Zymo Quick-RNA™ Miniprep Kit (Catalog Nos. R1054 and R1055). Before complementary DNA (cDNA) synthesis, the amount of RNA was measured with a Nanodrop ND1000 (Thermo Scientific, USA), approximately 500ng of which was used in the cDNA synthesis. The cDNA from purified RNA was synthesized using an Ipsogen RT cDNA kit (Catalog No./ID 679913).
Custom qPCR probes from Custom Desing Probes (Roche) for LIM15, PCNA, and actin beta (ACTB) were used for real-time PCR (RT-PCR) with a LightCycler® 480 Probes Master Mix according to the manufacturer's instructions (Catalog No. 04 707 494 001). RT-PCR was conducted in triplets for each sample per gene, and ACTB was used for internal control. The relative expression levels of LIM15 and PCNA were calculated and normalized using the advanced relative quantification method relative to ACTB.17
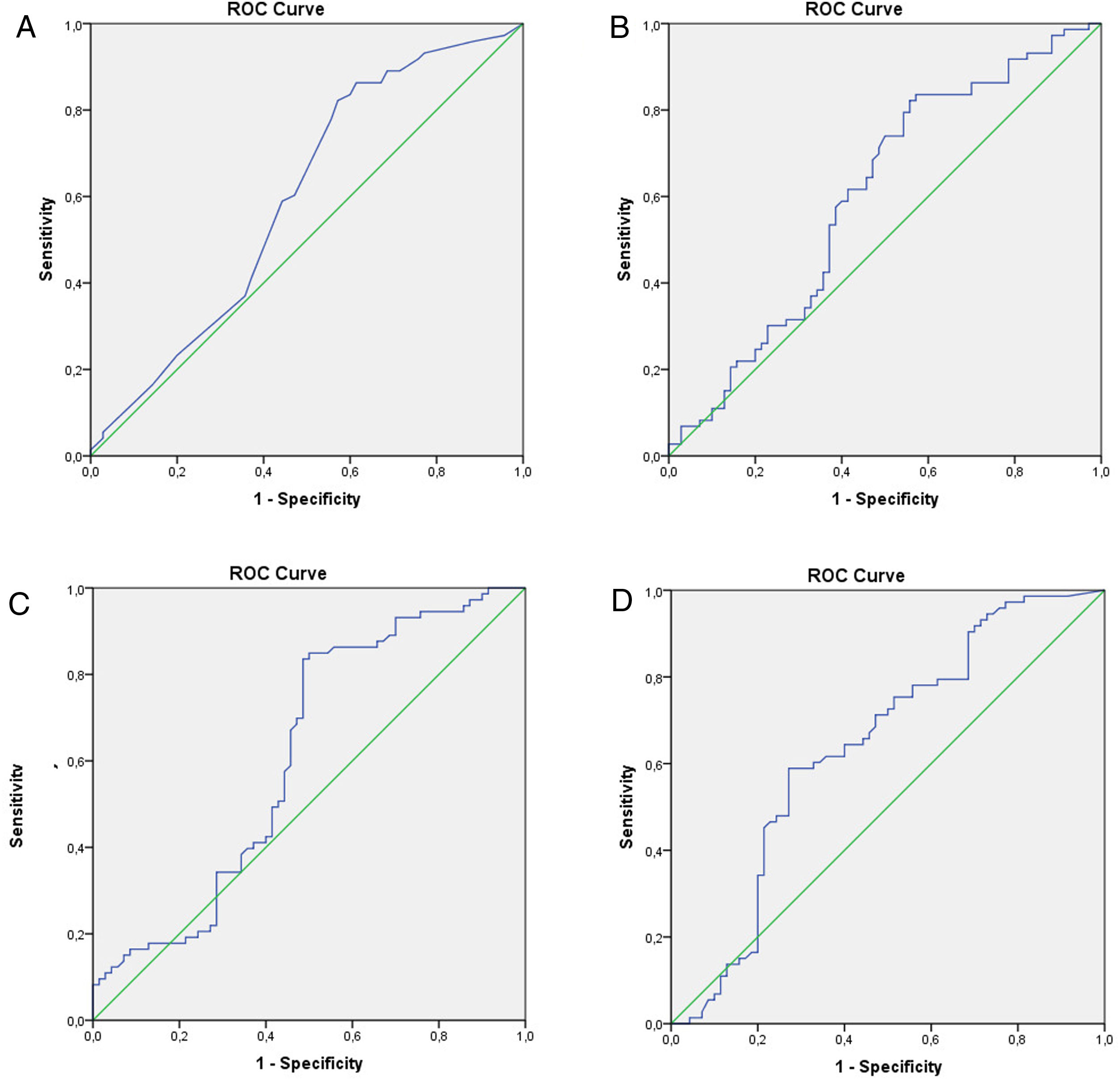
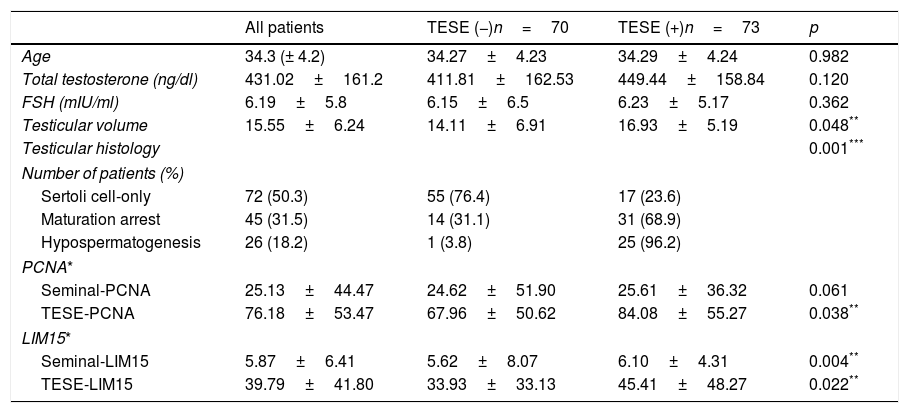
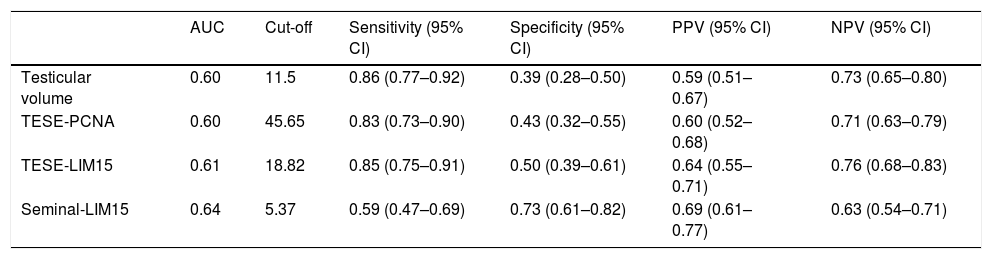
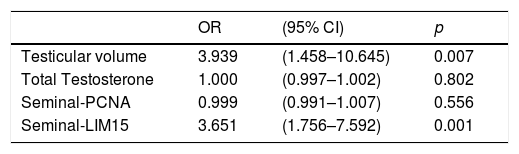
Statistical analysisAll statistical analysis was performed using SPSS 20 software (SPSS Inc., Chicago, IL, USA). Power analysis was performed. Normal distribution assumption for continuous variables was analyzed with the Kolmogorov–Smirnov test. The clinical factors were analyzed with the independent sample t-test, chi-square, and multivariate regression analysis. A value of p<0.05 was considered statistically significant. The best cutoff point in each clinical factor was determined using the Youden index. The difference in Youden index was calculated using the Z score. For each factor, the receiver operating characteristic (ROC) curve was obtained. The difference in ROC was calculated using the Z score.
ResultsTable 1 summarizes the clinical and laboratory characteristics of 143 patients according to their SR results, which were successful in 73 patients (51%). The mean age of TESE-negative and -positive patients were 34.27±4.23 and 34.29±4.24, respectively. Three factors, which were age, serum FSH and TT levels, were not statistically significant between TESE-negative and -positive patients (p=0.982, p=0.362 and p=0.120, respectively).
Comparison of clinical and laboratory parameters of 143 NOA patients according to sperm-retrieval success.
| All patients | TESE (−)n=70 | TESE (+)n=73 | p | |
|---|---|---|---|---|
| Age | 34.3 (± 4.2) | 34.27±4.23 | 34.29±4.24 | 0.982 |
| Total testosterone (ng/dl) | 431.02±161.2 | 411.81±162.53 | 449.44±158.84 | 0.120 |
| FSH (mIU/ml) | 6.19±5.8 | 6.15±6.5 | 6.23±5.17 | 0.362 |
| Testicular volume | 15.55±6.24 | 14.11±6.91 | 16.93±5.19 | 0.048** |
| Testicular histology | 0.001*** | |||
| Number of patients (%) | ||||
| Sertoli cell-only | 72 (50.3) | 55 (76.4) | 17 (23.6) | |
| Maturation arrest | 45 (31.5) | 14 (31.1) | 31 (68.9) | |
| Hypospermatogenesis | 26 (18.2) | 1 (3.8) | 25 (96.2) | |
| PCNA* | ||||
| Seminal-PCNA | 25.13±44.47 | 24.62±51.90 | 25.61±36.32 | 0.061 |
| TESE-PCNA | 76.18±53.47 | 67.96±50.62 | 84.08±55.27 | 0.038** |
| LIM15* | ||||
| Seminal-LIM15 | 5.87±6.41 | 5.62±8.07 | 6.10±4.31 | 0.004** |
| TESE-LIM15 | 39.79±41.80 | 33.93±33.13 | 45.41±48.27 | 0.022** |
The testicular volume of TESE-negative and -positive patients were 14.11±6.91ml and 16.93±5.19ml, respectively. The best cutoff value of the testicular volume for discriminating between TESE-negative and -positive patients was 11.5ml (sensitivity 86%, specificity 39%, p=0.048), with an AUC of 0.60 (Table 2). According to the histological evaluations, the SRRs in the SCO, MA, and HS groups were 23.6%, 68.9%, and 96.2%, respectively. Comparing the groups in terms of SR success showed that meaningful correlation between SRRs and testis histology (x2=48.590, p=0.001) (Table 1).
Cut-off point, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) and area under the curve (AUC) for testicular volume, PCNA and LIM15 genes.
| AUC | Cut-off | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | |
|---|---|---|---|---|---|---|
| Testicular volume | 0.60 | 11.5 | 0.86 (0.77–0.92) | 0.39 (0.28–0.50) | 0.59 (0.51–0.67) | 0.73 (0.65–0.80) |
| TESE-PCNA | 0.60 | 45.65 | 0.83 (0.73–0.90) | 0.43 (0.32–0.55) | 0.60 (0.52–0.68) | 0.71 (0.63–0.79) |
| TESE-LIM15 | 0.61 | 18.82 | 0.85 (0.75–0.91) | 0.50 (0.39–0.61) | 0.64 (0.55–0.71) | 0.76 (0.68–0.83) |
| Seminal-LIM15 | 0.64 | 5.37 | 0.59 (0.47–0.69) | 0.73 (0.61–0.82) | 0.69 (0.61–0.77) | 0.63 (0.54–0.71) |
The relationship between TESE-PCNA and TESE-LIM15 and the SRR was statistically significant (p=0.038 and p=0.022, respectively) (Table 1). According to the receiver operating characteristic (ROC) curve extracted from TESE-PCNA and TESE-LIM15, the maximal sensitivity (83%) and specificity (43%) were obtained for PCNA expression with a cut-off point of 45.65 and an area under the curve (AUC) of 0.60, while the maximal sensitivity (85%) and specificity (50%) were obtained for LIM15 expression with a cut-off point of 18.82 and an AUC of 0.61 (Fig. 1 and Table 2).
Seminal-LIM15 was statistically higher in TESE-positive men (p=0.004). As shown in Fig. 1 and Table 2, the maximal sensitivity (59%) and specificity (73%) were obtained for seminal-LIM15 gene expression with a cut-off point of 5.37 and an AUC of 0.64. Although seminal-PCNA was higher in TESE-positive men, the difference was not statistically significant (p=0.061). Since seminal-PCNA did not differ statistically between the 2 groups, the ROC curve was not plotted.
Independent predictive factors were detected by multivariate logistic regression analysis for the presence of sperm in the TESE. The model included four parameters (testicular volume, TT, Seminal-LIM15 and Seminal-PCNA) which known before TESE. According to the model, testicular volume and Seminal-LIM15 were independent predictive parameters for SR (Table 3).
Multivariate logistic regression analysis of the factors determined before TESE and independent predictive factors that reflect possibility of SR.
| OR | (95% CI) | p | |
|---|---|---|---|
| Testicular volume | 3.939 | (1.458–10.645) | 0.007 |
| Total Testosterone | 1.000 | (0.997–1.002) | 0.802 |
| Seminal-PCNA | 0.999 | (0.991–1.007) | 0.556 |
| Seminal-LIM15 | 3.651 | (1.756–7.592) | 0.001 |
SR: sperm retrieval, OR: odds ratio, CI: confidence interval.
The most effective treatment for patients with NOA is obtaining sperm by mTESE and using them for assisted conception by ICSI. The goal of surgical search for sperm is to find enough mature sperm for ICSI. However, the literature reports SRRs of 45–63% by mTESE.18 In other words, in half of men with NOA, the first mTESE procedure fails. Measuring predictive genetic markers in testicular tissue and/or ejaculate may provide patients with better consultation and prevent both surgery-related expenses and drawbacks. This study is one of the few comparing clinical, laboratory, and genetic variables to predict the chance of successful SR by mTESE. In our study, besides the expression levels of PCNA and LIM15 in testicular tissue and seminal fluid, TT, FSH, testicular volume, and testicular histology were investigated as predictive factors.
Many past studies have tried to predict SRR before TESE. The reported predictive potentials of variables such as age, TT and FSH values, and testicular volume are controversial.19,20 The most predictive factor of SRR in men with NOA today is testicular histology, which is an invasive procedure that requires a second surgical intervention to collect sperm. For this reason, a non-invasive method to estimate the success of finding sperm with TESE is needed. In fact, our data showed that, excepting testicular volume, no statistically significant correlations exist between age, TT, and FSH values with SRR (Table 1). Although a significant relationship exists between testicular volumes and SR rates, studies in this area have been controversial.21,22 Ziaee et al. reported that the best cut-off value of testicular volume is 9.5ml as a predictive factor of SR.23 However, previous studies demonstrated that, although testicular volume tends to be higher in successful TESE groups, it cannot be regarded as an independent predictive factor in multivariate analysis.21 Topographical variations in testicular pathology may be responsible for this inconsistency.24 However, in our study, we determined that testicular volume was an independent predictive factor for SR (p=0.007). The sperm retrieval rate in men with testicular volume>11.5ml was approximately four times higher than those with testicular volume<11.5ml (Table 3). Nevertheless, as in other studies,25,26 the SRR in our study group was significantly higher in patients with HS and MA than those with SCO (96.2% and 68.9% vs. 23.6%, respectively, p=0.001). Similarly, HS and MA statistically differed (p=0.001).
Some studies have investigated the roles of various markers in determining the presence of sperm in testicles before TESE. CD24 and CD133, which are accepted as stem-cell markers, ADAMTS1 and ADAMTS5 proteins, which play roles in separating germ cells from Sertoli cells, and proteomics, such as ECM1, TEX101, WBSCR28, ADCY10, TMEM225, and SPEM1, have been studied.27–29 The authors indicated their potential uses as predictive markers for successful SR in NOA patients scheduled to undergo testicular sperm extraction.
Demonstrating meiosis in testicular tissue by the expression of meiosis-specific markers can provide important information about sperm production in azoospermic men. LIMl5 contains sequences common to the meiosis-specific genes “box A” and “box B,” which are nucleotide-binding regions, thus suggesting the important role of LIM15 in chromosomal pairing and recombination.30 In mice, LIM15-deficiency causes sterility due to defective meiotic recombination and chromosomal synapsis with the histological phenotype arrested in the meiotic prophase without postmeiotic spermatids.31 Accumulated data imply that LIM15 may have essential role in meiotic progression, and meiosis in LIM15 mutant men arrests in pacynema due to incomplete chromosome synapsis and no crossing-over.32 In this study, the expression level of LIM15 and PCNA genes in seminal fluid were higher in TESE-positive men. However, while seminal-LIM15 was statistically higher in the TESE-positive group, the level of seminal-PCNA gene expression did not reach statistical significance (Table 1). For this reason, in our cases where LIM15 expression is reduced, we can explain the absence of spermatozoa with TESE by meiotic arrest. In addition, we determined that seminal-LIM15 was an independent predictive factor for SR (p=0.001) (Table 3). In brief, LIM15 gene expression in seminal fluid can help predict the success of TESE interventions. Since about 10–15% of couples are infertile due to defects in meiosis, genetic screening of LIM15 expression may identify the etiology of some male-related infertility cases associated with impaired meiosis. In fact, our data support this phenomenon with lowered LIM15 expression levels in the ejaculate of NOA patients. Recently, statistically significant differences in LIM15 gene expression and histologic phenotypes have been found by expression-profiling various DNA-repair genes in testicular biopsy samples with PCR arrays; these differences gradually decrease toward HS, MA, and SCO and are highest in normal spermatogenesis.33 Although we could not detect this compatibility for LIM15 in this study, we showed that the LIM15 gene expression level was higher in patients where sperm was obtained. This may be due to the random distribution of histological foci in which the biopsy samples were taken. Anyway, measuring LIM15 gene expression in azoospermic patients may reduces concerns about the presence of spermatogenesis and whether treatment will be effective.
It has been proposed that the interaction between LIM15 and PCNA mediates recombination-associated DNA synthesis during meiosis. PCNA expression is an index of proliferative activity in testicular tissues34 and lowered PCNA levels are well-correlated with impaired spermatogenesis in humans.35 Shinjo et al. determined PCNA expressions in the testicular tissue of NOA patients by immunohistochemical analysis and calculated the PCNA labeling index. According to their results, there was a marked increase in the PCNA labeling index of men successfully treated with gonadotrophin injections.14 Salama et al. also evaluated PCNA expressions in varicocele patients by assessing their staining intensity and found a significant correlation with sperm concentration.35 They concluded that PCNA is a useful molecular marker for determining germ-cell kinetics in infertile men. However, these studies evaluated PCNA function in testicular tissue samples; to find a non-invasive method, we measured PCNA not only in testicles but also ejaculate and found a statistically significant correlation between TESE-PCNA expression levels and successful SRR. Although a similar correlation was also found for the seminal-PCNA results, it did not reach a statistically significant level (Table 1).
Regarding our data, since LIM15 and PCNA gene expression levels in testicular tissue were found significantly higher in patients with successful SR by TESE, their measurements can help to predict the success of TESE interventions. As testicular sampling is not available prior to TESE, investigating these markers in tissue applies only to patients with previous testicular surgery. Beside this, our trial faces several limitations. First, the number of patients was low. If it had been higher, values that differed numerically might also have differed statistically. Second, in TESE-negative patients, the SR in recurrent TESEs would have changed the results, and the data would consequently have to be revised. Third, gene expression levels of NOA patients could be compared to gene expression levels in men with normal spermatogenesis. However, this analysis could not be performed due to the low number of patients with vasectomy or OA in our country. Fourth, depending on testicular heterogeneity, it is possible to detect gene products belonging to spermatogenesis at different rates in different samples taken at different times, which potentially affects the results. Spermatogenesis occurs by an overly complicated mechanism that has not yet been fully revealed. The clinical use of the genetic markers of spermatogenesis still remains elusive, as the pathophysiology is multifocal and often not limited to a few genes or loci. We believe that it would be of more practical value to study the association of NOA with a panel of genes. This may provide improved sensitivity of prediction of successful mTESE and the authors need to note this in the limitations. Many genomic, proteomic, and metabolomic molecular interactions, as well as epigenetic factors, determine the proliferative capacities of sperm stem cells and meiosis. However, with technical advancement, more successful results will certainly be obtained in inexplicable azoospermia cases. The AUC for seminal-LIM15 is quite modest but is statistically significant; theoretically, this could provide some ground work for a non-invasive seminal test in the decision algorithm for patients prior to considering mTESE. Our findings will shed light on other research in this area.
ConclusionAccording to our results, the down-regulation of PCNA and LIM15 genes is related to insufficient spermatogenesis. PCNA and LİM15 gene expression levels in TESE tissue and LIM15 gene expression level in seminal fluid can help predict TESE success in patients with NOA.
Ethical disclosuresThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors have no conflicts of interest to disclose.