To investigate the effect of icariin on the transformation efficiency of germ cell-like cells from mouse induced pluripotent stem cells into sperm cells in vitro.
MethodsFirstly, mouse induced pluripotent stem cells were induced and cultured to transform into germ cell-like cells, and the primordial germ cell-like cells were identified by Western blot and RT-PCR. Then, different concentrations of icariin (0.1μg/mL, 1μg/mL, 10μg/mL and 100μg/mL) were added into the culture medium, and the obtained primitive germ cell-like cells were cultured, Western blot and RT-PCR were used to identify the obtained sperm cells, the transformation efficiency was compared.
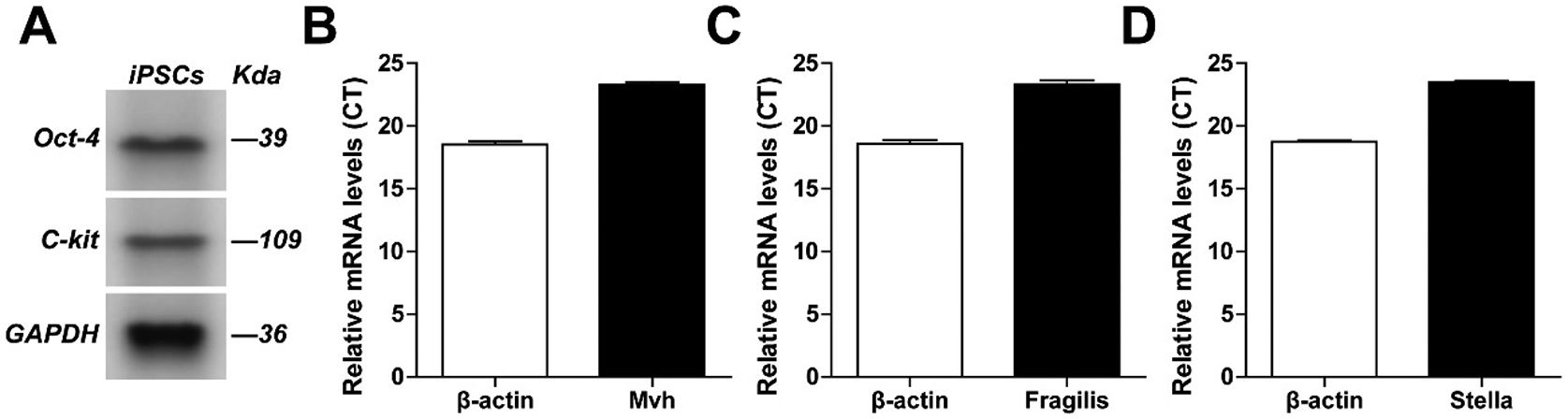
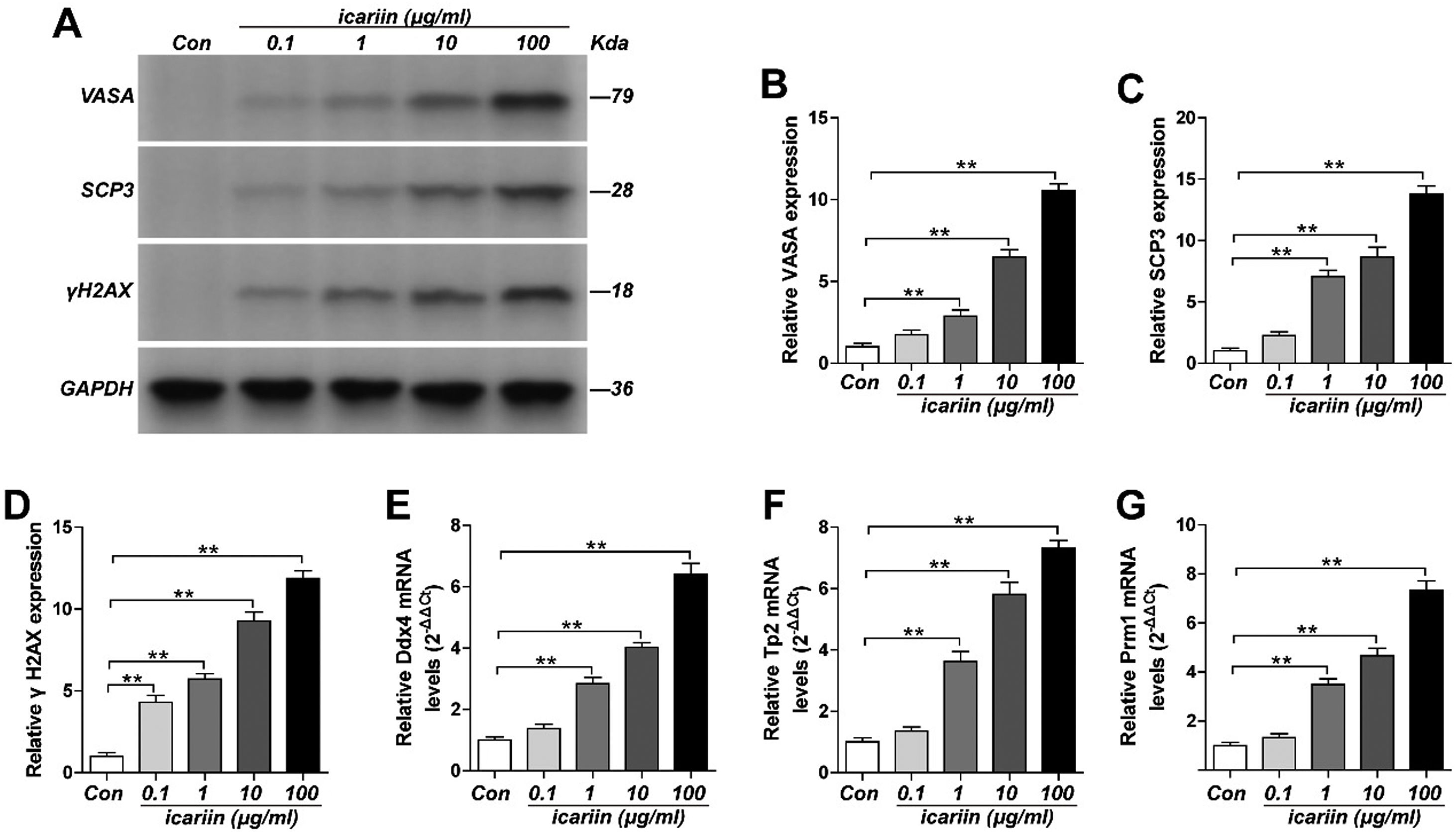
ResultsThe primordium germ cell-like cells obtained from mouse induced pluripotent stem cells in vitro specially expressed Oct-4 protein, C-kit protein, Mvh mRNA, Fragilis mRNA and Stella mRNA. The sperm cells were specially expressed VASA, SCP3 and γH2AX proteins. RT-PCR showed that the sperm cells were specially expressed Ddx4, Tp2 and Prm1 mRNA. Compared with the control group, the expression level of VASA protein (1.744±0.283, 2.882±0.373, 6.489±0.460), SCP3 protein (2.250±0.306, 7.058±0.521, 8.654±0.804), γH2AX protein (4.304±0.433, 5.713±0.339, 9.268±0.545), Ddx4 mRNA (1.374±0.145, 2.846±0.194, 4.021±0.154), Tp2 mRNA (1.358±0.130, 3.623±0.326, 5.811±0.390) and Prm1 mRNA (1.326±0.162, 3.487±0.237, 4.666±0.307) in 0.1μg/mL, 1μg/mL, 10μg/mL icariin experimental groups were all lower than that of VASA protein (10.560±0.413), SCP3 protein (13.804±0.642), γH2AX protein (11.874±0.464), Ddx4 mRNA (6.4005±0.361), Tp2 mRNA (7.314±0.256) and Prm1 mRNA (7.334±0.390) in 100μg/mL icariin experimental group.
ConclusionsIcariin can promote the transformation of mouse induced pluripotent stem cells into sperm cells in vitro, and it is concentration-dependent manner in a certain concentration range.
Investigar el efecto de icariina en la eficiencia de la conversión in vitro inducida en espermatozoides de cultivos de células germinativas derivadas de la transformación de células madre pluripotentes inducidas de ratón.
MétodosPrimero se indujeron y cultivaron células madre pluripotentes inducidas de ratón para transformarlas en células similares a las células germinales, y las células similares a las células germinales primordiales se identificaron mediante Western blot y RT-PCR. A continuación, se añadieron diferentes concentraciones de icariina (0,1μg/mL, 1μg/mL, 10μg/mL and 100μg/mL) al medio de cultivo, y se cultivaron las células primitivas similares a células germinales obtenidas, se utilizaron Western blot y RT-PCR para identificar las células espermáticas obtenidas, y se comparó la eficacia de la transformación.
ResultadosLas células germinales primitivas obtenidas in vitro a partir de células madre pluripotentes inducidas de ratón expresaron especialmente la proteína Oct-4, la proteína C-kit, el ARNm de Mvh, el ARNm de Fragilis y el ARNm de Stella. Los espermatozoides expresaban especialmente las proteínas VASA, SCP3 y γH2AX. La RT-PCR mostró que los espermatozoides expresaban especialmente los ARNm Ddx4, Tp2 y Prm1. En comparación con el grupo de control, el nivel de expresión de la proteína VASA (1,744±0,283; 2,882±0,373; 6,489±0,460), la proteína SCP3 (2,250±0,306; 7,058± 0,521; 8,654±0,804), proteína γH2AX (4,304±0,433; 5,713±0,339; 9,268±0,545), ARNm Ddx4 (1,374±0,145; 2,846±0,194; 4,021±0,154), ARNm Tp2 (1,358±0,130; 3,623±0,326; 5,811±0,390) y ARNm Prm1 (1,326±0,162; 3,487±0,237; 4,666±0,307) en grupos experimentales de 0,1μg/mL, 1μg/mL, 10μg/mL de icariina fueron todos más bajos que los de la proteína VASA (10,560±0,413), proteína SCP3 (13,804±0,642), proteína γH2AX (11,874±0,464), ARNm Ddx4 (6,4005±0,361), ARNm Tp2 (7,314±0,256) y ARNm Prm1 (7,334±0,390) en 100μg/mL icariina grupo experimental.
ConclusionesLa icariina puede promover la transformación de células madre pluripotentes inducidas de ratón en células de esperma in vitro, y es de manera dependiente de la concentración en un determinado rango de concentración.
Infertility, as a global health problem, has been attracting great attention, male factors account for about 40–50% of all infertility cases.1 For azoospermia patients, assisted reproduction and other techniques cannot enable them to successfully produce offspring with their own genetic material. With the extensive research on stem cells, the multidirectional differentiation potential of induced pluripotent stem cells (iPSCs) has been paid more and more attention, and the transformation of iPSCs into germ cells has become a hot research topic.2,3 At present, with the deepening of research, more and more results show that iPSCs can differentiate into germ cells in mice and humans, and eventually obtain haploid sperm cells. Some scholars even successfully produce live mice by combining sperm cells transformed from mouse iPSCs with egg cells.4–7 Although haploid sperm cells can be successfully obtained by iPSC induction culture, the efficiency of induction transformation is low and further research is needed.8,9
Icariin (ICA) is one of the main active components of epimedium in traditional Chinese medicine. Research have shown that ICA can not only promote the proliferation and differentiation of bone marrow mesenchymal stem cells, adipocytes, neural stem cells and other stem cells, but also protect the male reproductive ability. It stimulates sertoli cell proliferation and testosterone secretion in male testis in a dose-dependent manner.10–14
Therefore, this experiment intends to add ICA during the transformation of iPSCs into sperms cells in induction culture to observe whether ICA can improve the transformation efficiency of iPSCs into sperm cells.
Materials and methodsMaterialsMouse iPSCs cell lineBought from Thermo Fisher Scientific (Thermo Fisher Scientific, Pittsburgh, PA, USA).
Main experimental instruments and reagentsIcariin (I1286) (Sigma, St. Louis, MO, USA), high-glucose DMEM medium (contain 100U/mL penicillin, 100μg/mL streptomycin, 0.1mM non-essential amino acids, 2mM l-glutamine, 0.1mM 2-mercaptoethanol, LIF and 15% FBS), fetal calf serum (SH30070.03) (FBS, Hyclone, Logan, UT, USA), LIF (Millipore, Billerica, MA, USA), testosterone (T5411) (Sigma, St. Louis, MO, USA), Star Signal Western Protein Marker (10–200kDa) (M227-01) (GenStar, Beijing, China), Western blot regeneration solution (ZN1923, Biolab, Beijing, China), and PCR primers were synthesized by Shanghai Bioengineering Co., Ltd, Trizol (Takara, Japan), IScript cDNA Synthesis Kit (1708891EDU) (Bio-Rad, Hercules, CA, USA), Model 3K15 low temperature high speed centrifuge (Sigma company), Western blotting system (model: Criterion™ electrophoresis tank, Trans – Blot® transfer trough) (Bio-Rad company), and ABI 7500 real-time fluorescence quantitative PCR instrument (ABI Corporation of American).
MethodsExperimental groupControl group: Mouse iPSCs were transformed into primordial germ cell-like cells (PGCLCs) and then cultured in basal medium (high-glucose DMEM medium) for 2 weeks.
Experimental group: Mouse iPSCs were transformed into primordial germ cell-like cells (PGCLCs) and then cultured in the medium containing ICA (divided into four groups according to the concentration of 0.1μg/mL, 1μg/mL, 10μg/mL and 100μg/mL) for 2 weeks.
Subcultured the IPSCsMouse iPSCs were subcultured in high glucose DMEM medium. When the cells were fused to 90%, the old medium was discarded, mixed digestive solution was added after PBS washing, and the cells were observed under a microscope. Digestion was terminated immediately after the cells became round, and the cells were collected. About 1×106 iPSCs cells were transferred to a 10cm culture dish and incubated with 10mL LIF-free medium. On the 5th day, the cells were continued to be cultured in the complete medium containing testosterone (1μM). After 48h, the medium was changed every 2 days, and the cells were continued to be cultured for 2 weeks.
Western blotTotal cell protein extraction: According to the experimental grouping, each group was prepared as cell suspension and inoculated in 6-well plates with 5×105cells in each well. When the cells were fused to 90%, the supernatant was discarded, washed with PBS, and RIPA was added for full lysis. After centrifugation, the supernatant was immediately absorbed into a pre-cooled Eppendorf tube, which was the extracted cell protein.
Gel electrophoresis and membrane transfer: SDS-PAGE gel was prepared with 10% or 12% separation gel and 5% compression gel according to the molecular weight of proteins to be measured. Proteins were added into swimming lanes for electrophoresis to observe the proteins on the membrane.
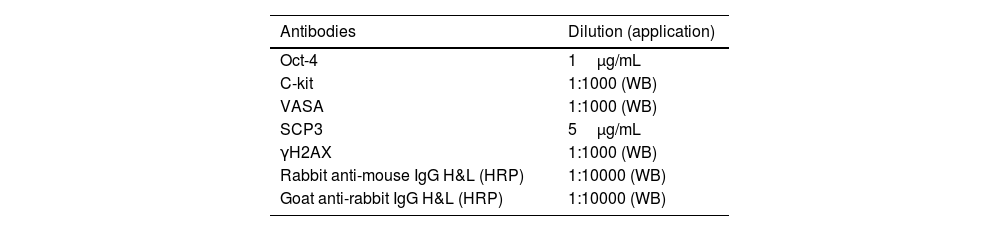
Developing: Tris Buffered Saline with Tween (TBST) was cleaned and sealed, primary antibody and secondary antibody were successively added. Image Pro Plus 6.0 software was used to analyze the optical density value of protein expression level, and the relative protein expression level was calculated as the gray value of target protein/the gray value of internal reference protein (primary antibody, secondary antibody and dilution ratio were shown in Table 1).
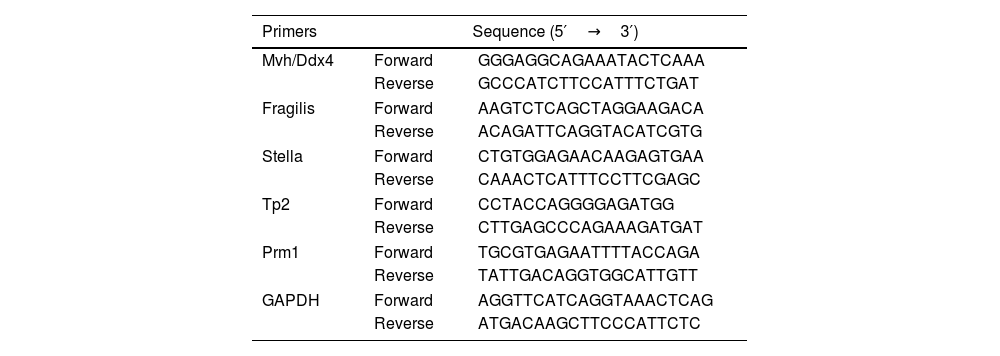
RT-PCRAccording to experimental groups, cell suspensions were prepared for each group and inoculated into 6-well plates with 5×105 cells in each well. When the cells were fused to 90%, the supernatant was discarded, and 0.5mL Trizol was added to each well and repeatedly blown until clarification, and then transferred to EP tube. The RNA was dissolved in DEPC water and then blown and mixed to obtain the total RNA solution. 2μL sample was taken and Nanodrop 2000 was used to determine the mRNA concentration and purity. 20μL system was used for cDNA synthesis and real-time quantitative PCR, RT-PCR was performed according to the kit instructions and 2−ΔΔCt was used to calculate the amplified results (the primers used were shown in Table 2).
Primers used in quantitative real-time PCR.
| Primers | Sequence (5′→3′) | |
|---|---|---|
| Mvh/Ddx4 | Forward | GGGAGGCAGAAATACTCAAA |
| Reverse | GCCCATCTTCCATTTCTGAT | |
| Fragilis | Forward | AAGTCTCAGCTAGGAAGACA |
| Reverse | ACAGATTCAGGTACATCGTG | |
| Stella | Forward | CTGTGGAGAACAAGAGTGAA |
| Reverse | CAAACTCATTTCCTTCGAGC | |
| Tp2 | Forward | CCTACCAGGGGAGATGG |
| Reverse | CTTGAGCCCAGAAAGATGAT | |
| Prm1 | Forward | TGCGTGAGAATTTTACCAGA |
| Reverse | TATTGACAGGTGGCATTGTT | |
| GAPDH | Forward | AGGTTCATCAGGTAAACTCAG |
| Reverse | ATGACAAGCTTCCCATTCTC | |
All data were expressed as means±SD. One-way ANOVA and Tukey's test were used for statistical differences between groups. p values less than 0.05 were considered as significant differences.
ResultsPGCLCs identification resultsWestern blot and RT-PCR were used to identify that the specifically expressed Oct-4 and c-kit proteins of PGCLCs were positive, and the specifically expressed MVH, fragilis and Stella mRNA were positive (as shown in Fig. 1).
Sperm cell identification resultsWestern blot and RT-PCR were used to identify each group. Compared with the control group, the expression levels of VASA protein, SCP3 protein, Ddx4mRNA, TP2mRNA and Prm1 mRNA in 0.1μg/mL ICA group were increased, but the changes were not significant. The expression level of γH2AX protein was significantly increased. The expression levels of VASA protein, SCP3 protein, γH2AX protein, Ddx4 mRNA, TP2 mRNA and Prm1 mRNA in 1, 10 and 100μg/mL ICA group were significantly increased, and the effect was the best when ICA concentration was 100μg/mL (*p<0.05, **p<0.01) (as shown in Fig. 2).
DiscussionAs one of the most common global health problems for couples of reproductive age worldwide, infertility affects about 15% of couples worldwide and has a dramatic psychological impact, leading to a poor quality of life.15 The key to male reproduction is the continuous production of functional sperm in the testes, problems at any stage of spermatogenesis, such as genetic factors, injury-induced azoospermia, exposure to toxins, immune system suppression and anti-cancer can lead to male infertility. According to testicular biopsy, male infertility can be divided into obstruction azoospermia and non-obstructive azoospermia, of which non-obstructive azoospermia is the most serious and common factor.16,17 The development of assisted reproductive technology (ART), especially the technology of intracytoplasmic sperm injection, enable many affected couples (80%) to find a solution.15,18 However, ART is only useful for men with mature sperm and currently cannot help infertile couples lacking functional gametes unless donor gametes are used, and most couples would like to have genetically related children of their own.19
In translational research of regenerative medicine, stem cells are attracting more and more attention as the most promising alternative therapy, and great progress has been made in the biology and function of stem cells, and there are more and more researches on stem cells in infertility. Stem cells mainly include embryonic stem cells, bone marrow mesenchymal stem cells, adipose stem cells and urine-derived stem cells at present. Pluripotent stem cells mainly include embryonic stem cells (ESCs) and iPSCs. ESCs can differentiate into male germ-like cells in vitro, but they are not related to the genes of patients, and human ESCs are of limited source, with ethical issues related to embryo destruction. Therefore, the research and utilization of ESCs is limited to some extent.20 The acquisition of bone marrow mesenchymal stem cells and adipose stem cells will cause certain trauma to the body, and although some researchers have obtained germ cell-like cells through induction transformation of adipose stem cells, haploid sperm cells have not been further transformed.21 IPSCs were first produced by Takahashi K in 2006 by inducing four transcription factors OCt3/4, C-MYC, SOX2 and KLF4 into mouse fibroblasts for transformation. The same transcription factor was used to induce the generation of human induced pluripotent stem cells (hiPSCs) the following year.8,22 Compared with ESCs, iPSCs were derived from patients’ own somatic cells, with fewer ethical problems and abundant sources. They have immune compatibility for autotransplantation and can obtain offspring with their own genes. Therefore, patient-specific sperm can be generated from iPSCs, which provides a basis for the treatment of male infertility.23,24 Therefore, on the basis of relevant researchers, we selected mouse induced pluripotent stem cells for induction culture to obtain sperm cells in this experiment.
Epimedium, the dried leaf of epimedium recorded in the Chinese Pharmacopoeia and described 400 years ago in the Shennong Materia Medica Classic of Traditional Chinese medicine, nourishes the kidneys and significantly enhances Yang. Epimedium has traditionally been used in conjunction with other Chinese herbs and its pharmacological effects are difficult to explain in modern science. In fact, there are at least 260 different compounds present even in a single epimedium. The complexity and diversity of plant chemistry of epimedium have long hindered the research of its pharmacological effects. With the development of isolation science and ethnopharmacology, more than 20 kinds of flavonoids have been identified by chemical reaction and spectral analysis, and these 20 kinds of flavonoids have been isolated from epimedium by systematic separation technology. More and more evidences have proved that icariin is the main bioactive substance in epimedium, and it is also considered as a chemical marker for quality control components of epimedium.25 Although it has been confirmed that icariin can promote the proliferation and differentiation of various stem cells, and also has protective effect on male reproductive ability, the role of icariin in induced pluripotent stem cells is rarely researched.
In this experiment, iPSCs were induced and cultured in mice. Western blot and RT-PCR results showed that ocT-4 protein, C-kit protein, Mvh mRNA, Fragilis mRNA and Stella mRNA were specifically expressed in the cells, indicating that PGCLCs was successfully obtained.26,27 In the process of transformation from iPSCs to sperm cells, the low transformation efficiency mainly lies in the further transformation from germ cell-like cells to sperm cells. Therefore, in the culture of PGCLCs, ICA of 0.1μg/mL, 1μg/mL, 10μg/mL and 100μg/mL were added into the culture medium respectively. The results showed that, the expression of VASA protein and SCP3 protein and the mRNA expression of Ddx4, TP2 and Prml were increased in 0.1μg/mL ICA group, but the change was not obvious. The expression of γH2AX protein was significantly increased.28,29 Compared with the control group, the expression level of VASA protein (1.744±0.283, 2.882±0.373, 6.489±0.460), SCP3 protein (2.250±0.306, 7.058±0.521, 8.654±0.804), γH2AX protein (4.304±0.433, 5.713±0.339, 9.268±0.545), Ddx4 mRNA (1.374±0.145, 2.846±0.194, 4.021±0.154), Tp2 mRNA (1.358±0.130, 3.623±0.326, 5.811±0.390) and Prm1 mRNA (1.326±0.162, 3.487±0.237, 4.666±0.307) in 0.1μg/mL, 1μg/mL, 10μg/mL icariin experimental groups were all lower than that of VASA protein (10.560±0.413), SCP3 protein (13.804±0.642), γH2AX protein (11.874±0.464), Ddx4 mRNA (6.4005±0.361), Tp2 mRNA (7.314±0.256) and Prm1 mRNA (7.334±0.390) in 100μg/mL icariin experimental group.
In conclusion, icariin can promote the transformation of mouse induced pluripotent stem cells into sperm cells in vitro, and it is concentration-dependent manner in a certain concentration range. This study can provide some reference for improving the transformation efficiency of iPSC into sperm cells.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingHainan Health Industry Scientific Research Project (19A200048); project supported by Hainan Province Clinical Medical Center (QWYH202175).
Conflict of interestThe authors declare that they have no conflict of interest.