Cryopreservation has destructive effects on the function and structure of spermatozoa. It is known that leptin and prolactin play an active role in decreasing the rates of reactive oxygen species and DNA fragmentation, as well as enhancing sperm motility. Hence, this experiment aimed to investigate the effects of leptin and prolactin as pro-survival factors on the normozoospermic human semen samples during cryopreservation.
Material and methodsSemen samples were collected from 15 healthy, fertile men ranging from 25 to 40 years. Cryopreservation of the samples was performed in liquid nitrogen over a period of two weeks, using five varying concentrations of leptin/prolactin, 0, 10, 100, 500, and 1000ng/ml respectively. Sperm motility, total caspase activity, and mitochondrial and cytosolic ROS were measured by flowcytometry, TUNEL, and other appropriate tests after thawing of the samples.
ResultsBoth hormones were observed to have positive effects on the motility of the samples post-cryopreservation, the highest improvement being in the 100ng/ml concentration leptin and prolactin in comparison to the control group (P=0.01 and P=0.041, respectively). A significant reduction of mitochondrial ROS was also observed in 100 and 1000ng/ml of leptin (P=0.042), and there was a considerable decrease in the cytosolic ROS in the 100ng/ml of prolactin in comparison to the control group (P=0.048). Total caspase activity was also highly reduced in the 100, 500, and 1000ng/ml of leptin compared to the control group (P=0.039). Interestingly, both hormones also significantly decreased DNA fragmentation in 1000ng/ml compared to the control group (P=0.042).
ConclusionIt can be concluded that leptin and prolactin act as protective agents against cryodamage to spermatozoa during cryopreservation.
La criopreservación tiene efectos destructivos sobre la función y estructura de los espermatozoides. Se sabe que la leptina y la prolactina desempeñan un papel activo en la disminución de las tasas de especies reactivas de oxígeno (ROS) y la fragmentación del ADN, así como en la mejora de la motilidad de los espermatozoides. Por lo tanto, este experimento tuvo como objetivo investigar los efectos de la leptina y la prolactina como factores de supervivencia en las muestras de semen humano normozoospérmico durante la criopreservación.
Material y métodosSe recolectaron muestras de semen de 15 hombres sanos y fértiles de entre 25 y 40 años. La crioconservación de las muestras se realizó en nitrógeno líquido durante un período de 2 semanas, utilizando 5 concentraciones variables de leptina/prolactina: 0, 10, 100, 500 y 1000ng/ml respectivamente. La motilidad de los espermatozoides, la actividad de caspasa total y las ROS mitocondriales y citosólicas se midieron mediante citometría de flujo, TUNEL y otras pruebas apropiadas después de descongelar las muestras.
ResultadosSe observó que ambas hormonas tienen efectos positivos sobre la motilidad de las muestras después de la crioconservación, la mayor mejora se encuentra en la concentración de leptina y prolactina de 100ng/ml en comparación con el grupo de control (p=0,01 y p=0,041, respectivamente). También se observó una reducción significativa de las ROS mitocondriales en 100 y 1000ng/ml de leptina (p=0,042), y hubo una disminución considerable en las ROS citosólicas en los 100ng/ml de prolactina en comparación con el grupo de control (p=0,048). La actividad de la caspasa total también se redujo considerablemente en los 100, 500 y 1000ng/ml de leptina en comparación con el grupo de control (p=0,039). Curiosamente, ambas hormonas también redujeron significativamente la fragmentación del ADN en 1000ng/ml en comparación con el grupo de control (p=0,042).
ConclusiónSe puede concluir que la leptina y la prolactina actúan como agentes protectores contra el criodaño de los espermatozoides durante la criopreservación.
Semen cryopreservation is an extensively used procedure in assisted reproductive technology (ART). Nonetheless, destructive effects of cryopreservation process on the structure and function of spermatozoa such as decreased motility, declined viability, loss of mitochondrial function, and DNA damage are commonly observed after thawing.1,2 Although extreme attempts have been made to avoid cryodamage, it is still undesirable especially for abnormal semen samples that are more liable to be influenced by cryodamage.3
During the freezing process, human spermatozoa are affected by severe chemical and physical stresses which cause apoptosis in these cells.4,5 These devastating effects are often relevant to the rise of reactive oxygen species (ROS) in the cryopreservation.6 Moreover, ROS is a very important factor in male infertility pathogenesis. Current researches have reported that extraordinary doses of ROS are correlated with the induction of damage to sperm DNA.3,7 It is negatively associated with mitochondrial membrane potential (MMP) and sperm motility. One of the most susceptible sperm structures to cryopreservation is mitochondria,4,8 which carry out an important role during the execution phase of apoptosis, in which a decline in MMP discharges cytochrome c and further pro-apoptotic proteins, causing the caspase activation and consequently result in the apoptosis.9,10
Recently, several substances have been applied to prevent cryodamage. For example, various antioxidants including vitamins, enzymes, herbal components, and hormones have been mixed with freezing media to attenuate the detrimental influences of oxidative stresses in both humans and animals.11–16
Leptin a potential satiety hormone is principally secreted by adipose tissue17 and involved in many biological processes such as the neuroendocrine regulation, energy consumption, hematopoiesis, and reproduction.18,19 Gonzalez L.C. in 1999 showed that LH and PRL secretion are stimulated by exogenous leptin in fasted adult male rats.20 In the human, leptin receptors are located in seminiferous tubules.21 Hence, it seems that testicles are the core objective of leptin.22 Recent studies also revealed that leptin has a straight regulatory role in the testicular function control.23 Besides, leptin is expressed in the seminal plasma,21,24 but its cellular origin is not completely defined. Jope in 2003 showed the role of leptin in the male reproductive system through an interaction between leptin and spermatozoa by leptin receptors.24 Aquila in 200525 revealed that human sperm is able to secret leptin which, in turn, suggested that the spermatozoa may regulate its metabolism independently by systemic leptin. These findings made scientists take leptin significance in male fertility into consideration. Interestingly, several studies have confirmed that leptin enhances human sperm motility26,27 and has decreased intracellular ROS levels and DNA fragmentation of adult rat spermatozoa.28 However, several kinds of research confirmed the negative impact of serum leptin in the control of men's gonadal functions.29 Moreover, Glander in 200221 indicated that the leptin concentrations in seminal plasma were significantly lower in the ‘normal’ semen samples than in the ‘pathological’ samples and there was a significant negative association with the percentage of motile spermatozoa.
Prolactin (PRL) is another hormone secreted by adenohypophysis lactotropic cells and participates in the regulation of gonadal related functions.30 PRL has anti-apoptotic effects in several cells comprising lymphoma and mammary cells and contributes to the production and immunoregulation of ovary and steroids.31,32 Recent studies have shown that PRL has important roles in activation and capacitation of sperm by stimulating specific mechanisms.32 Biswas in 1978 proved that just PRL indicated a significant positive association with the motility of spermatozoa.33 Also, several kinds of research revealed that PRL could significantly increase human sperm motility. Suchanek et al. in 1981 reported that there is a great association between the rate of motile sperm in and the PRL concentrations in the ejaculate of normozoospermic men, and in the oligospermia men, the sperm concentration was in correlation with the PRL levels in seminal fluid.34 Chan in 1984 indicated that the seminal plasma PRL concentrations are significantly decreased in azoospermic and suggested that the seminal plasma PRL may play a significant role in the sustaining of sperm motility.35 Aiman in 1988 showed that seminal plasma PRL levels were correlated to sperm concentrations and motilities.36 However, Sueldo et al. in 1985 suggested that high seminal PRL concentrations have a negative effect on sperm capacity.37
Since there is limited and controversial evidence for cryopreservative effects of antioxidants on the human spermatozoa, therefore in this study we decided to investigate the effect of leptin and prolactin as prosurvival factors on the sperm's motility, concentration, DNA fragmentation, ROS, and caspase activity in normozoospermic human samples during cryopreservation.
Material and methodsSample collectionPrior to commencement, a complete description of the study and its associating procedures was provided to the study participants and their informed consent was collected Samples were collected by one-time masturbation into a sterile specimen container who were referred to infertility centers of Zanjan province, Iran. These semen samples were obtained from 15 healthy fertile participants of 25–40 years of age. The WHO protocol and cut of value (2010 edition) were used for semen analysis and to discern a normal sample from abnormal, and their body mass index (BMI) (n=15) were in the normal range. It was ensured that the participants had a history free of smoking, alcohol, or drug abuse before their enrollment in the study. Samples generating ROS, such as those with leuko-cytospermia or varicocele were omitted, as well as samples from participants consuming certain types of medicines including supplementary vitamins. All participants abstained from sexual intercourse for a period of 3–6 days prior to sample collection. Upon collection, the samples were kept at 37°C for approximately 40min, and additionally, any samples which did not liquefy during this period were excluded. This study was fully approved by the Ethics Committee of Zanjan University of Medical Sciences. Each condition of each experiment (motility, ROS, caspase, DNA fragmentation) has been performed on the 15 semen samples of the 15 men.
Semen analysisIn line with the 2010 World Health Organization Guidelines, an optical microscope was employed for the assessment and analysis processes, recording sperm movement as Grade A: fast progressively motile, Grade B: slow progressively motile, Grade C: non-progressively motile and lastly Grade D: immotile.38
Cryopreservation and spermatozoa thawing processesThe experimental hormones, leptin and prolactin, were sourced from Sigma–Aldrich. The stock vial, containing 100μg of the lyophilized peptide in powdered form, was reconstituted by the addition of 1ml of sterile, double-distilled water to make a stock solution. Serial dilutions were then made and stored at −20°C. The semen samples were subdivided into five screw-top cryotubes (Nunc, Denmark) for each group of leptin and prolactin, containing 3×106sperm/ml in each tube. 10, 100, 500, and 1000ng/ml doses of leptin and prolactin were tested26 (Fig. 1). According to Li et al. in 2009, 1ng/ml is the physiological dose (in vivo). However, they tested 10, 100, and 1000ng/ml leptin on the sperm function. Actually, based on this study, we chose 10ng/ml as the lower dose in vitro.39
Non-treated concentration (0ng/ml) was the solvent control group that was added only working solution (Biggerse Whittene Whittingham (BWW) medium) instead of the hormone. Following that, a 0.7:1 ratio of anti-freeze solution (Sperm freezing medium; LifeGlobal® LGSF-020, Single-step freezing medium) was added to each cryotube. The samples were initially exposed to liquid-nitrogen vapor for a period of 10min, upon which they were plunged into liquid nitrogen and stored for 14 days. In line with the protocols of this laboratory, all cryotubes belonging to the same sperm undergone the same thawing procedure, which included transferring samples to room temperature for 5min after the storage period, and eventually placing them in a 37°C water bath for 20min. The final step involved washing the samples with BWW medium with normal range osmolarity, and centrifuging them at 1000rpm for 5min. This was done to ensure the complete removal of the cryoprotective medium prior to subsequent analysis.
Mitochondrial reactive oxygen species (ROS) assayThe MItoSOX™ Red (MSR; Molecular Probes, Invitrogen, USA), a derivative of ethidium bromide, was added to the spermatozoa samples to detect the presence of mitochondrial superoxide anion in live cells. This addition, targeting mitochondria exclusively, was expected to cause fluorescence of the mitochondria when oxidized by the superoxide.40 After dilution in BWW, stock solutions of MSR [5mmol in dimethyl sulfoxide (DMSO)] were added to the spermatozoa at 3×106cells/ml, resulting in a final concentration of 2μmol. To the positive control, 3.6μl of 5mmol arachidonic acid was added. An incubation period of 15min at 37°C without light exposure containing all the samples and their treatment took place before they were centrifuged at 3500rpm for 5min. The resulting supernatant was discarded and the pellet was resuspended with 1ml of BWW in FACS tubes. The prepared samples were then analyzed using flowcytometry (Partec-PAS), with an Argon laser excitation at 488nm combined with emission measurements employing a 585/42 band-pass filter with the FL-2 detector.
Cytosolic reactive oxygen species (ROS) assayTo determine the total superoxide anion regeneration, dihydroethidium (DHE; Molecular Probes, Invitrogen, Eugene, OR, USA) was utilized.41 This assay followed similar processes to the mitochondria assay previously mentioned. BWW solution containing 2μM of DHE was used to dilute a total of 3×106cells/ml of spermatozoa. The samples were then incubated in the dark for 15min at 37°C. Lastly, they were washed once using BWW solution, centrifuged (600g) for 5min, and analyzed on a flow cytometer (Partec-PAS), equipped with emission measurements that used a 585/42 band-pass filter with the FL-2 detector, in combination to an Argon laser excitation at 488nm. Each sample contained tens of thousands of spermatozoa which were inspected following removal of non-sperm-specific events.
Total caspase activityTo assess the levels of active caspases in whole cells, the Fluorescent Labeled Inhibitor of Caspase Assay Kit (FLICA; Immunochemistry Technologies, Bloomington, MN, USA) was used, following protocols described by Pujianto et al.32 The FLICA dye was stored as a 150× as per the instruction of the manufacturer. This solution was added to the spermatozoa samples after being diluted with BWW, resulting in a final concentration of 1× FLICA dye. The samples were incubated with the dye for 90min at 37°C and were then washed twice with 1% apoptosis wash buffer. All of the samples were diluted resulting in a final volume of 500μl in Ham's F10 and were analyzed by flowcytometry (Partec-PAS). The flowcytometry analysis was performed in an identical way to that of the MitoSOX™ Red Assay.
DNA fragmentationTo determine the degree of fragmented DNA in the sperm samples, the In Situ Death Detection Kit, Fluorescein (Roche, Germany) was utilized, according to the manufacturer's instructions. A total of 6×106 spermatozoa were dissolved in a solution of phosphate-buffered saline (PBS, pH 7.4). Subsequently, they were fixed with 100μl of 4% paraformaldehyde at room temperature, without light exposure, for 15min. The samples were then washed with PBS, and permeabilized with a combination of sodium citrate, Triton X-100, and PBS, for 5min on ice. The DNA labeling was performed following the wash, by incubating the samples in 50μl of the TUNEL reaction mixture in a humidified chamber at 37°C without light exposure for one hour. Lastly, the labeled cells were transferred for flowcytometry analysis (Partec-PAS) after another PBS wash. DNase I, as it would produce DNA strand breaks, was used as a positive control, and also analyzed by the TUNNEL assay.
Statistical analysisThe SPSS 16 data software (Chicago, IL, USA) was used for data analysis. The data is offered in the form of mean±standard error of the mean, otherwise known as S.E.M. One-way ANOVA and the LSD post hoc comparison tests were used to compare multiple mean values within different groups of the various experiments. All of the sample analyses were repeated a minimum of two times. P≤0.05 was considered statistically significant.
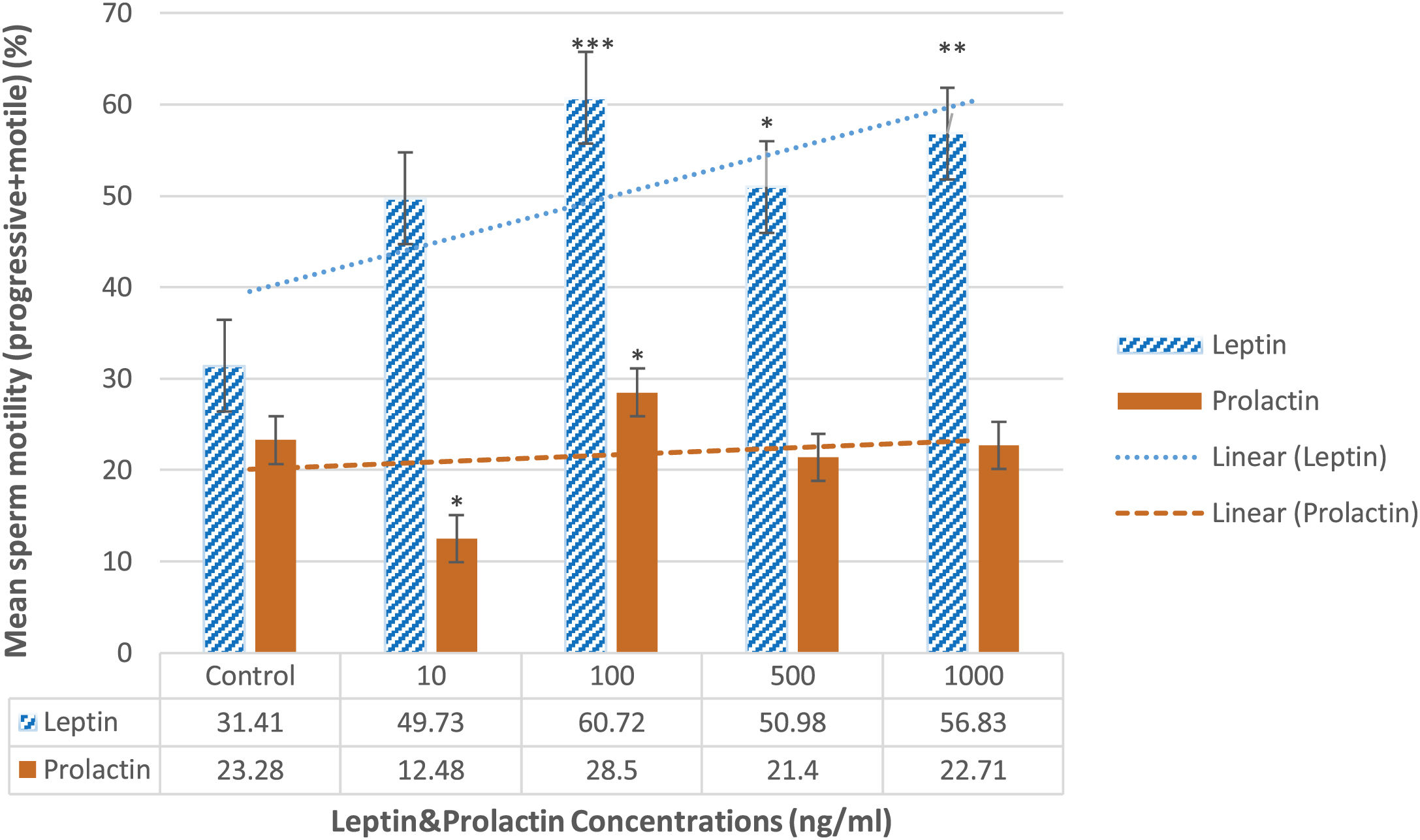
ResultsSperm motilityBased on Fig. 2, after handling with different dosages of leptin, the percentage of total motility of sperm was increased in 10ng/ml compared to the control group (49.73±3.1% vs 31.41±5.2%) although it was not statistically significant. However, it was significantly increased in other doses of leptin 100 (60.72±4.7%; P=0.01), 500 (50.98±2.98%; P=0.045), and 1000ng/ml (56.83±4.98%; P=0.039) in comparison to the control group. Also, prolactin at concentration 100ng/ml significantly increased the motility of sperm compared to the control group (28.5±5.2% vs 23.28±2.99%; P=0.045). The other doses of prolactin had no considerable effects (Fig. 2).
The effects of leptin and prolactin on the sperm motility following treatment with different doses. All data represent mean±SEM. The significant differences compared to the control group, p-value=0.043, p-value=0.041, p-value=0.039 and p-value=0.01 are displayed by (*), (**), and (***) symbols, respectively (n=15).
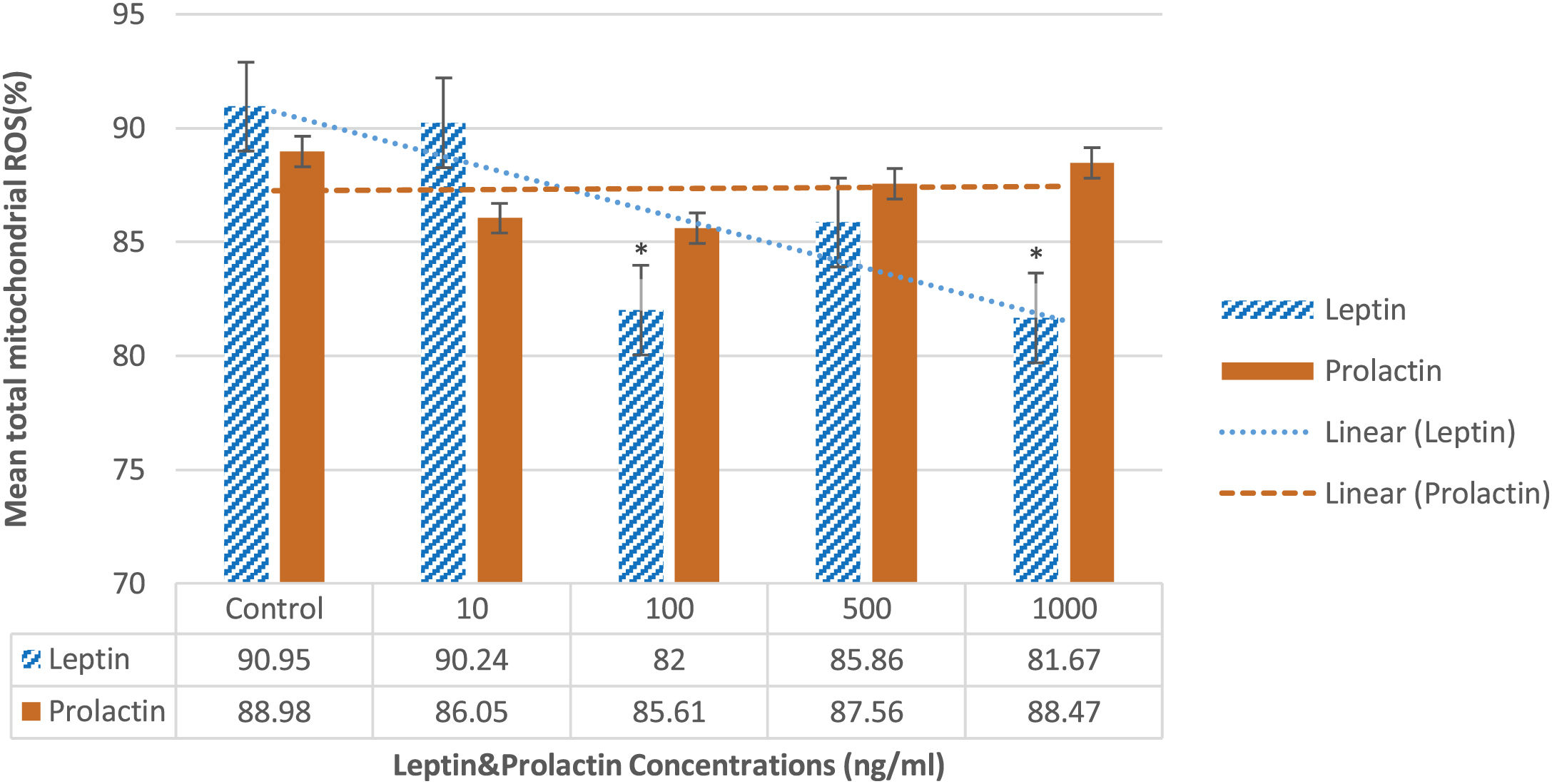
According to Fig. 3, both leptin and prolactin decreased the ROS levels following the cryopreservation in all intervention groups. Leptin 100 and 1000ng/ml meaningfully decreased the mitochondrial ROS compared to the control group (82±0.36%, 81.67±1.83% vs 90.95±1.83%, respectively; P=0.042). However, the other doses of leptin had no significant effects.
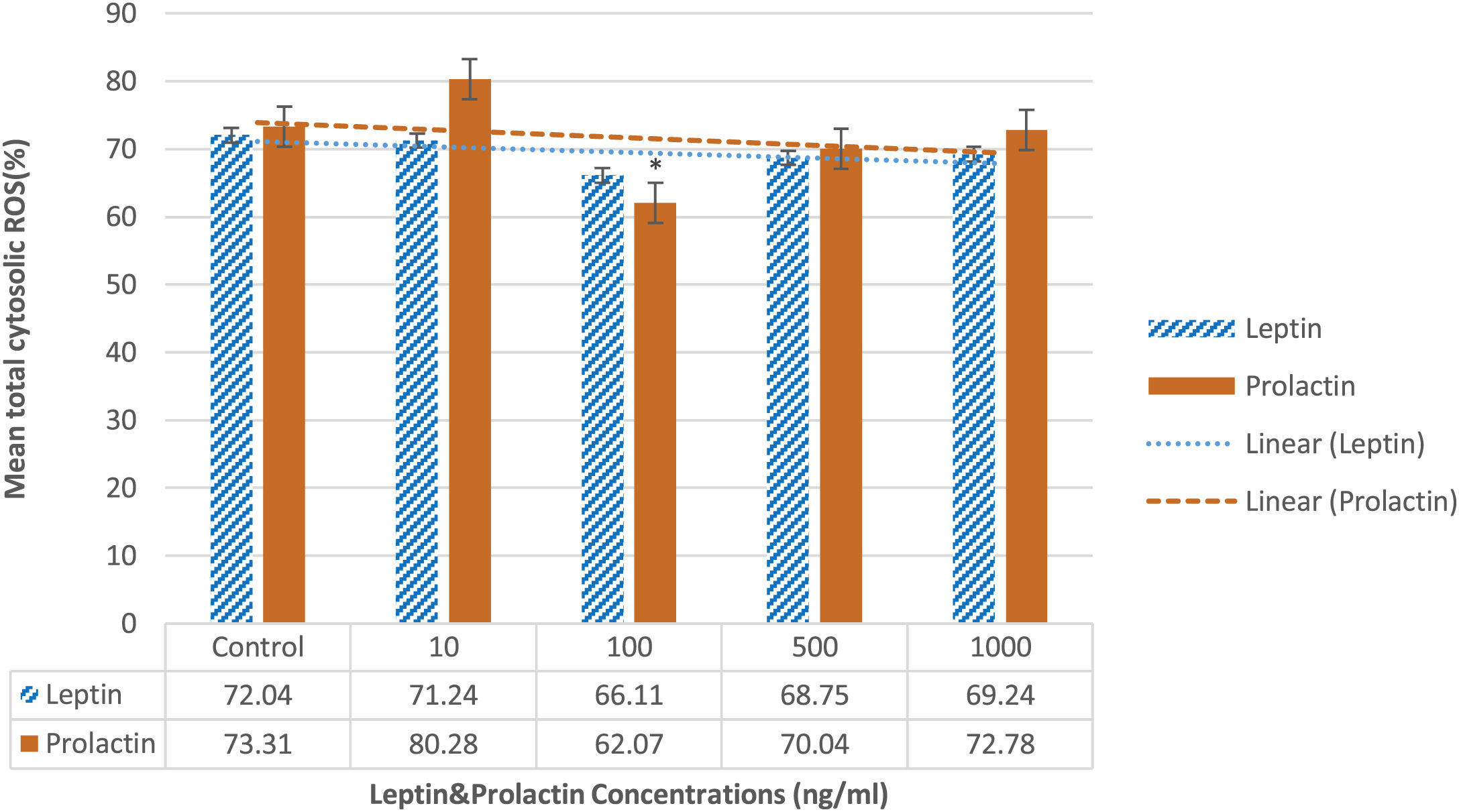
As Fig. 4 displays, prolactin 100ng/ml significantly decreased this parameter compared to the control group (62.07±4.61% vs 73.31±3.98%; P=0.048). The other doses of prolactin had no considerable effects.
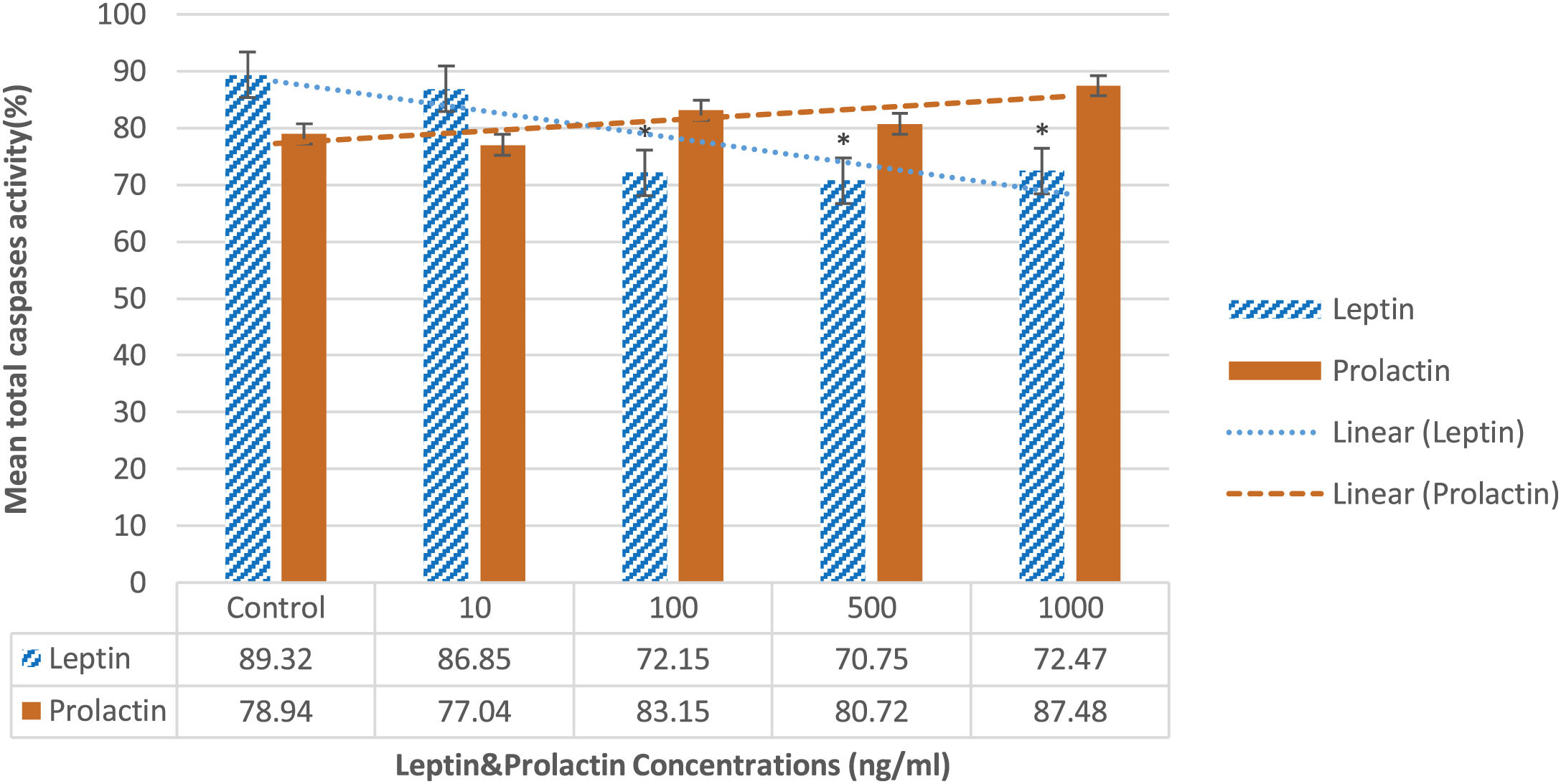
Total caspase activityBased on the findings of flowcytometry and according to the Fig. 5, total caspase activity decreased significantly in 100, 500, and 1000ng/ml compared to the control group (72.15±3.2%, 70.75±2% and 72.47±5% vs 89.32±2.1%, respectively; P=0.039). In contrast, prolactin didn’t cause any considerable changes in the caspase activity at all doses compared to the control group.
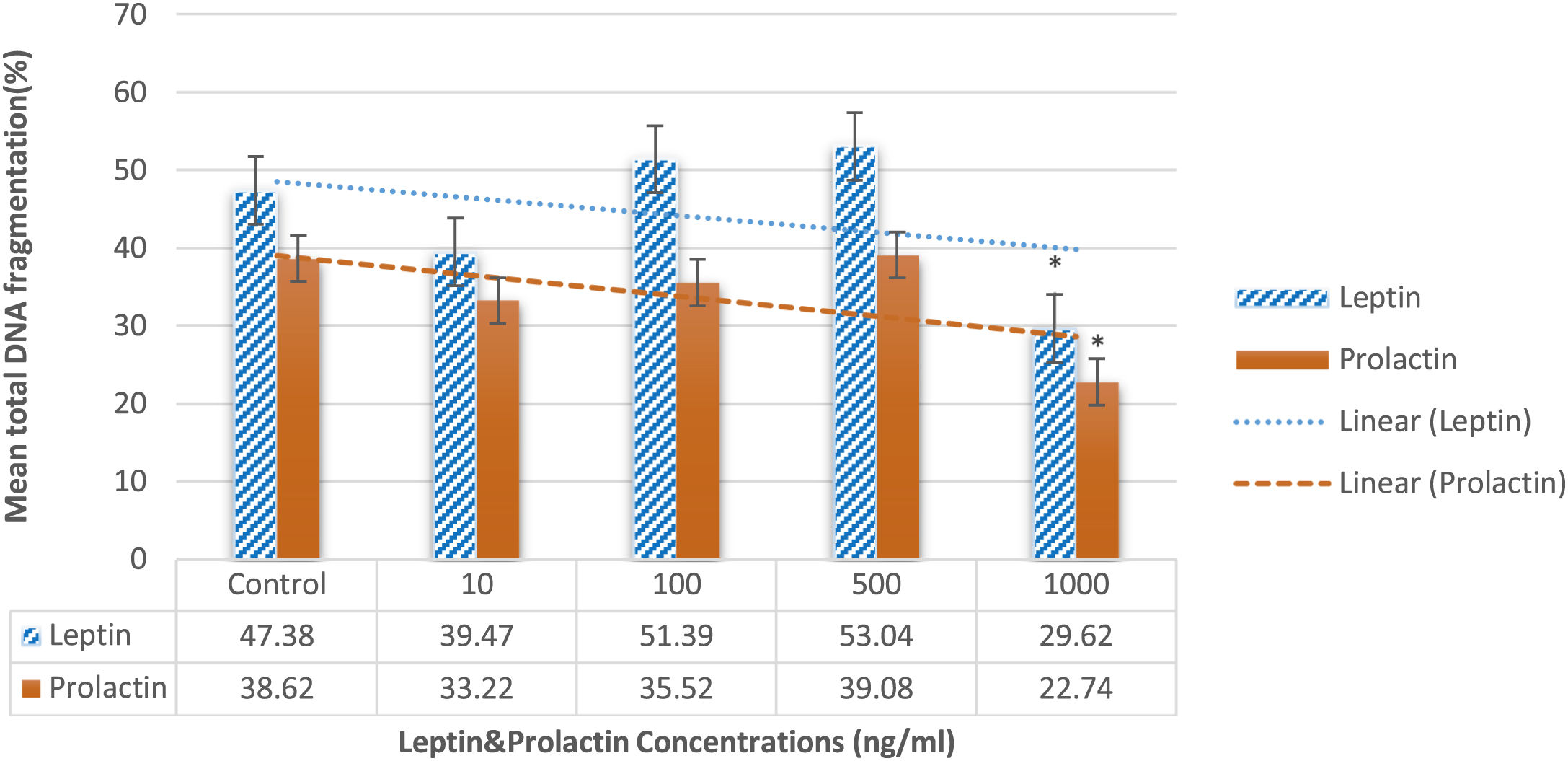
DNA fragmentationAs illustrated in Fig. 6, leptin significantly decreased the DNA fragmentation at does 1000ng/ml in comparison to the control group (29.62±2.83% vs 47.38±5.83%; P=0.042). Interestingly, the same results were revealed about prolactin 1000ng/ml (22.74±2.11% vs 38.62±4.88%; P=0.042). The other doses of both leptin and prolactin had no considerable effects.
DiscussionAlthough human semen cryopreservation is a fundamental procedure in ART, it is recognized that alterations of DNA integrity in bovine cryopreserved normozoospermic are related to synthesis of H2O2 but not to the viability of sperm and generation of other ROSs.42 Moreover, leptin was used as an indicator of sperm motility and DNA fragmentation before and after the cryopreservation. In this study, semen samples from 15 normozoospermic male volunteers were analyzed. There was a negative association between the motility of spermatozoa and seminal leptin. Additionally, there was a positive association between DNA fragmentation index (DFI) and seminal leptin.43
Thomson et al. in 200944 showed that cryopreservation induces DNA damage, oxidative stress, and caspase activation which are the characteristics of the high-quality human spermatozoa and not just the results of poor quality sperm. Moreover, they revealed that the supplementation of the estrogenic compound genistein or a similar antioxidant could be an invaluable addition to the cryoprotectant media. Therefore, in the present study, we treated raw normal semen samples with leptin and prolactin at different concentrations to determine whether these hormones could ameliorate the post-thaw factors affecting the fertilization rate. Although the excess levels of serum leptin in obese males have a detrimental impact on the reproductive system and decrease the androgen concentrations (the inhibitory role), the normal range of leptin might play a positive role in the reproductive activity (the stimulatory role).23,45 Camiña et al. in 2002 demonstrated that human leptin is present in seminal fluid, with at least two charge variants and no binding proteins, the most likely source being either seminal vesicles or prostate tissue.46 Jope et al. in 2003 proved the existence of leptin and its receptors in human seminal plasma and in human ejaculated spermatozoa, and Aquila et al. in 2005 indicated that human ejaculated sperm secrets leptin, which suggests the modulation of sperm metabolism independently by systemic leptin.24,25 Moreover, these studies considered the importance of leptin in male fertility and indicated an interaction between leptin and human spermatozoa through leptin receptors. Glander et al.21 disclosed an adverse association between seminal plasma leptin levels and the motility of spermatozoa, but serum leptin concentration did not indicate any correlation with sperm quality. Zorn et al.47 also found no significant correlation between serum levels of leptin and sperm motility. They concluded a possible correlation between serum levels of leptin and testes function, independently of FSH and LH, engaging testosterone and sex hormone-binding globulin (SHBG) via regulation of Leydig cell function. Nevertheless, Lampiao et al.26 indicated that adding 10nmol leptin to the semen samples can increase human sperm motility. Our finding is in agreement with this in vitro study who indicated that leptin has a positive impact on sperm function and determined that this hormone in high doses could participate in increasing the motility of cryopreserved sperm Although Li. in 2009 showed that different doses of leptin could not alter sperm motility significantly, our results indicated a significant impact of 100, 500, and 1000ng/ml of leptin on the motility of cryopreserved spermatozoa.
Regarding the influence of PRL on the sperm motility, some in vivo studies reported that seminal PRL in contrast to serum PRL is more related to sperm parameters, which seminal PRL decreases are accompanied by sperm motility reductions.48 Chan et al.35 also indicated that seminal PRL levels were similar in both normozoospermic and oligospermia samples, but significantly decreased in azoospermic samples. Seminal PRL levels significantly increased in samples with high sperm motility. Therefore, these studies suggested that the seminal plasma PRL may play an important role in the preservation of sperm motility. Aiman et al. also confirmed that seminal PRL could increase and sustain the motility of spermatozoa due to the existence of its receptors on the neck and middle piece of the human sperm and the scattered points of the spermatozoic tail.35,36 However, Sueldo et al.37 in 1985 showed that higher concentrations of semen PRL were observed in those patients with low sperm concentration and motility as well as in those who showed an abnormal ovum penetration in the hamster. This suggests that high seminal PRL concentrations have a negative influence on the functional capacity of male gamet. These controversial findings could be remarkable evidence for the hypothesis that all these functions follow a dose-dependent manner. Some of the limited in vitro studies such as Pujianto et al., 2009 indicated that 500 and 1000ng/ml of PRL could maintain normal sperm motility during prolonged incubation at 37°C.32 However, our study showed that just 100ng/ml of PRL could significantly increase the motility of cryopreserved spermatozoa. It seems that PRL is not able to maintain the motility of cryopreserved spermatozoa at low levels, but further studies need to examine more samples and other doses of PRL.
To the best of our knowledge, no study has investigated the influence of PRL as a prosurvival factor to reduce the cryodamage to human sperm, and therefore, this is the very first study that assays the effect of PRL as a supplement on the characteristics of human sperm during cryopreservation. Martínez-Fresneda in 201949 showed that either testosterone or PRL added in vitro declined the post-thaw acrosome integrity of ram and buck sperm. Also, the study about leptin and cryopreservation is very limited. Fontoura et al.50 reported that the supplementation of 10ng leptin to capacitated spermatozoa before freezing decreased DNA fragmentation and improved antioxidant enzyme activity, including superoxide dismutase and glutathione peroxidase. These positive effects of leptin on the DNA fragmentation of human sperm during cryopreservation confirmed our findings that 1000ng/ml of leptin decreased DNA fragmentation of cryopreserved sperm (Fig. 6).
Since the sperm mitochondria are very important in the control of apoptosis51 and induce major impacts on the oxidative stress damages, consequently the deleterious effects of oxidative stress can cause structural changes in sperm mitochondria. A great decline in MMP was previously reported following the freezing-thawing process in human spermatozoa.52 Furthermore, mitochondrial ATP production also can be negatively affected by cryopreservation which is straightly correlated to sperm motility. Consequently, it is concluded that without adequate energy sperm is not progressively motile.42 Remarkably, our findings revealed the greater sperm motility in the high doses of leptin and PRL supplementation in normal cryopreserved sperm (Fig. 2). Although at this phase the accurate mechanism of this impact is speculative, it can be already related to energy homeostasis role of these hormones53 or increase in antioxidant factors such as superoxide dismutase, and glutathione peroxidase (GPx), contained in seminal plasma and spermatozoa.50 Interestingly, leptin signaling could induce SOD2 gene expression. Meanwhile, SOD2 is located in mitochondria, not in the cytoplasm.54 Moreover, the highest motility was indicated in supplementation of 100ng/ml leptin/prolactin in our samples which can probably explain a protecting dose-dependent influence on the mitochondrial activity of human spermatozoa (Fig. 2).
As displayed in Figs. 3 and 4, the high doses of leptin (100, 1000ng/ml) and PRL (100ng/ml) reduced the mitochondrial and cytosolic ROS, respectively. Oxidative stress such as cryopreservation induces elevation of lipid peroxidation in the plasma membrane by an excessive upsurge ROS levels in the semen samples. This overproduction, declines ATP concentration via suppression of oxidative phosphorylation or glycolysis, resulting in a decrease in sperm motility.55,56 In other words, specifically, human spermatozoa are susceptible to lipid peroxidation which causes a high level of polyunsaturated fatty acids (PUFA) in these cells. When ROS attacks PUFA, lipid peroxides, and aldehydes are generated, which have an inhibitory effect on sperm motility.57
The presence of these hormones in the human seminal plasma and furthermore localization of their receptors on the spermatozoa, especially to the tail region of spermatozoa suggests that they may exert a local function on spermatozoa. In addition, the sperm has the ability to regulate its functions according to its energy needs, both dependently and independently by systemic leptin/prolactin.25,35,36 When leptin binds to its receptors, a number of signaling pathways such as IRS/P13K is activated in the cell.41 Interestingly, the administration of P13K inhibitor reduced motility in bovine sperm cells.26 Leptin activates P13K and AKT via IRS phosphorylation. Leptin and insulin signaling seems to have similar signaling pathways.58 Unremarkably, the activity of P13K prevented spermatozoa from the entrance to the intrinsic apoptotic pathway. Accordingly, inhibition of P13K induces spermatozoa defaulting to an apoptotic serial which is characterized by losing motility, generation of ROS, and activation of caspase.59
According to Fig. 6, our findings revealed that both leptin and PRL decreased the DNA fragmentation in sperms at their highest doses (1000ng/ml), which confirms the results of Fontoura et al.50 They concluded that leptin supplementation reduced DNA fragmentation during cryopreservation possibly through increasing the activity of superoxide dismutase and glutathione. As illustrated in Fig. 5, although PRL didn’t cause any considerable changes, the caspase activity decreased in supplementation of 100, 500, and 1000ng/ml of leptin. However, it is suggested to assay the activity of PI3K following cryopreservation of human sperm supplemented by leptin and PRL.
Overall, our results indicated that the addition of leptin and PRL to human sperms ameliorate sperm motility and decrease ROS levels, DNA fragmentation, and caspase activation during cryopreservation. Therefore, it seems that applying these antioxidants in the cryopreservation procedure will improve the quality of sperms and consequently could be highly clinically effective and will improve the ART success rate. However, much more detailed information on the basic molecular mechanisms and pathways is still needed, and further studies are suggested. Finally, we do not suggest that leptin and PRL are the major prosurvival factors for human cryopreserved spermatozoa, but they are the first example of such a factor.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThe present study was supported by the Zanjan University of Medical Sciences (grant number: A-10-302-1).
Conflict of interestThe authors declare that they have no conflict of interest.
The authors appreciate the following students for their kind participation in this research: Zahra Ghadimi, Mohammad Ghanimati, Farzaneh Mohammadi, Negin Parsamanesh, and Zahra Shahmohammadi. The authors would also like to acknowledge Parnian Nejatbakhsh, Medical Student at Monash University, Melbourne, Australia, for her big assistance in editing of this manuscript.