Radiofrequency electromagnetic fields (RF-EMFs) are one of the risk factors for male reproductive health and melatonin can be an ideal candidate for therapeutic development against RF-induced male fertility problems due to its antioxidant properties. The possible therapeutic role of melatonin in the destructive effects of 2100MHz RF radiation on rat sperm characteristics is investigated in the present study.
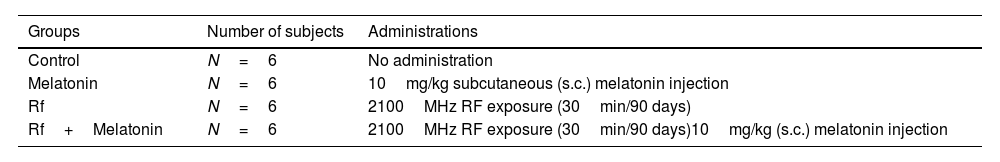
MethodsWistar albino rats were divided into four groups and the experiment continued for ninety consecutive days; Control, Melatonin (10mg/kg, subcutaneously), RF (2100MHz, thirty minutes per day, whole-body), and RF+Melatonin groups. Left caudal epididymis and ductus deferens tissues were placed in sperm wash solution (at 37°C) and dissected. The sperms were counted and stained. Measurements of the perinuclear ring of the manchette and posterior portion of the nucleus (ARC) were performed and the sperms were examined at an ultrastructural level. All of the parameters were evaluated statistically.
ResultsThe percentages of abnormal sperm morphology were significantly increased with RF exposure, while the total sperm count was significantly decreased. RF exposure also showed harmful effects on acrosome, axoneme, mitochondrial sheath, and outer dense fibers at the ultrastructural level. The number of total sperms, sperms with normal morphology increased, and ultrastructural appearance returned to normal by melatonin administration.
DiscussionThe data showed that melatonin may be a beneficial therapeutic agent for long-term exposure of 2100MHz RF radiation-related reproductive impairments.
Los campos electromagnéticos de radiofrecuencia (RF-EMF) son uno de los factores de riesgo para la salud reproductiva masculina y la melatonina puede ser un candidato ideal para el desarrollo terapéutico contra los problemas de fertilidad masculina inducidos por RF debido a sus propiedades antioxidantes. En el presente estudio se investiga el posible papel terapéutico de la melatonina en los efectos destructivos de la radiación RF de 2100MHz en las características del esperma de rata.
MétodosSe dividieron ratas albinas Wistar en 4 grupos y se continuó el experimento durante 90 días consecutivos: grupos control, melatonina (10mg/kg, por vía subcutánea), RF (2100MHz, 30min por día, cuerpo entero) y RF+melatonina. Los tejidos del epidídimo caudal izquierdo y del conducto deferente se colocaron en una solución de lavado de esperma (a 37°C) y se diseccionaron. Los espermatozoides fueron contados y teñidos. Se realizaron mediciones del anillo perinuclear del manchette y de la porción posterior del núcleo (ARC) y se examinaron los espermatozoides a nivel ultraestructural. Todos los parámetros fueron evaluados estadísticamente.
ResultadosLos porcentajes de morfología anormal de los espermatozoides aumentaron significativamente con la exposición a RF, mientras que el recuento total de espermatozoides disminuyó significativamente. La exposición a RF también mostró efectos nocivos en el acrosoma, el axonema, la vaina mitocondrial y las fibras densas externas a nivel ultraestructural. El número total de espermatozoides, los espermatozoides con morfología normal aumentaron y la apariencia ultraestructural volvió a la normalidad mediante la administración de melatonina.
DiscusiónLos datos mostraron que la melatonina puede ser un agente terapéutico beneficioso para la exposición a largo plazo de las deficiencias reproductivas relacionadas con la radiación de RF de 2100MHz.
Male reproductive problems, such as spermatogenesis disorders and infertility have become more prevalent worldwide in recent decades.1 According to worldwide statistics, at least one in six couples has fertility problems and about 30% of the etiology of infertility contributes to the male factor.2 Accumulating evidence has shown that electromagnetic fields (EMFs) are one of the risk factors for male reproductive health.1
The rate of exposure to EMFs, sources from extremely low-frequency microwaves or high-frequency microwaves, has increased during the last century.3 One of the most important sources of EMFs is mobile phones, which generate RF radiation in the range of 800–2600MHz4 and which have achieved higher frequencies through 5G technology. The effects of EMFs emitted by mobile phones on human health have become one of the most important research areas in recent years because of their frequency. It is known that RF waves emitted by mobile phones cause harmful effects on both cellular and molecular levels5 cause detrimental effects on male fertility, in addition to causing certain neurological disorders, and increasing cancer risks.6
Currently available studies show that RF-EMFs have a number of harmful effects on sperm parameters. RF-EMFs emitted from mobile phones may lead to infertility through the following: by triggering single and double DNA breaks; decreasing sperm concentrations, seminiferous tubule diameters, and serum testosterone levels; and by reducing sperm motility, viability, or disturbing the sperm morphology.7–9 RF-EMFs affect cell physiology by altering redox-related processes, producing genotoxicity, genomic instability, and finally causing oxidative stress by increasing the reactive oxygen species (ROS) associated with decreasing ROS scavenging activity during spermatogenesis. Therefore, this state is followed by finding preventions to protect the sperms from the harmful effects of RF-EMFs.10
Because the destructive effects of RF exposure depend on the overproduction of ROS, antioxidant treatments that can be applied in RF exposure conditions are becoming an important research topic.3,11 One of these antioxidants is melatonin, secreted mainly by the pineal gland to regulate the circadian rhythm, but additionally, it is a powerful antioxidant hormone.12 It protects nuclear and also mitochondrial DNA, cytosolic proteins, and membrane lipids from oxidative damage. It also detoxifies ROS, and stimulates antioxidative enzymes.13 Additionally, it has been reported that it inhibits pre-meiotic spermatogenesis arrest through the germ cells, improving sperm vitality and count during assisted fertility treatments. These properties make melatonin an ideal candidate for therapeutic development against RF-induced male fertility problems.14,15
This study aims to investigate the possible therapeutic role of melatonin in the destructive effects of 2100MHz RF radiation on sperm characteristics. This important topic has also been researched at an ultrastructural level by transmission electron microscopic examination in this study, so this case makes our research exceptional and valuable in its field.
Material and methodsAll animal experiments were performed in accordance with ethical norms and approved by the Institutional Animal Ethics Committee Guidelines of Gazi University.
ChemicalsMelatonin (N-acetyl-5-methoxytryptamine) (98.0% purity, Lot: #011M1321V) was purchased from Sigma–Aldrich (St. Louis, USA) and stored at −20°C. Melatonin (100mg) was dissolved in 10% pure ethanol that contained a phosphate buffer saline (PBS) (pH=7.4). All other chemicals were obtained from standard commercial suppliers.
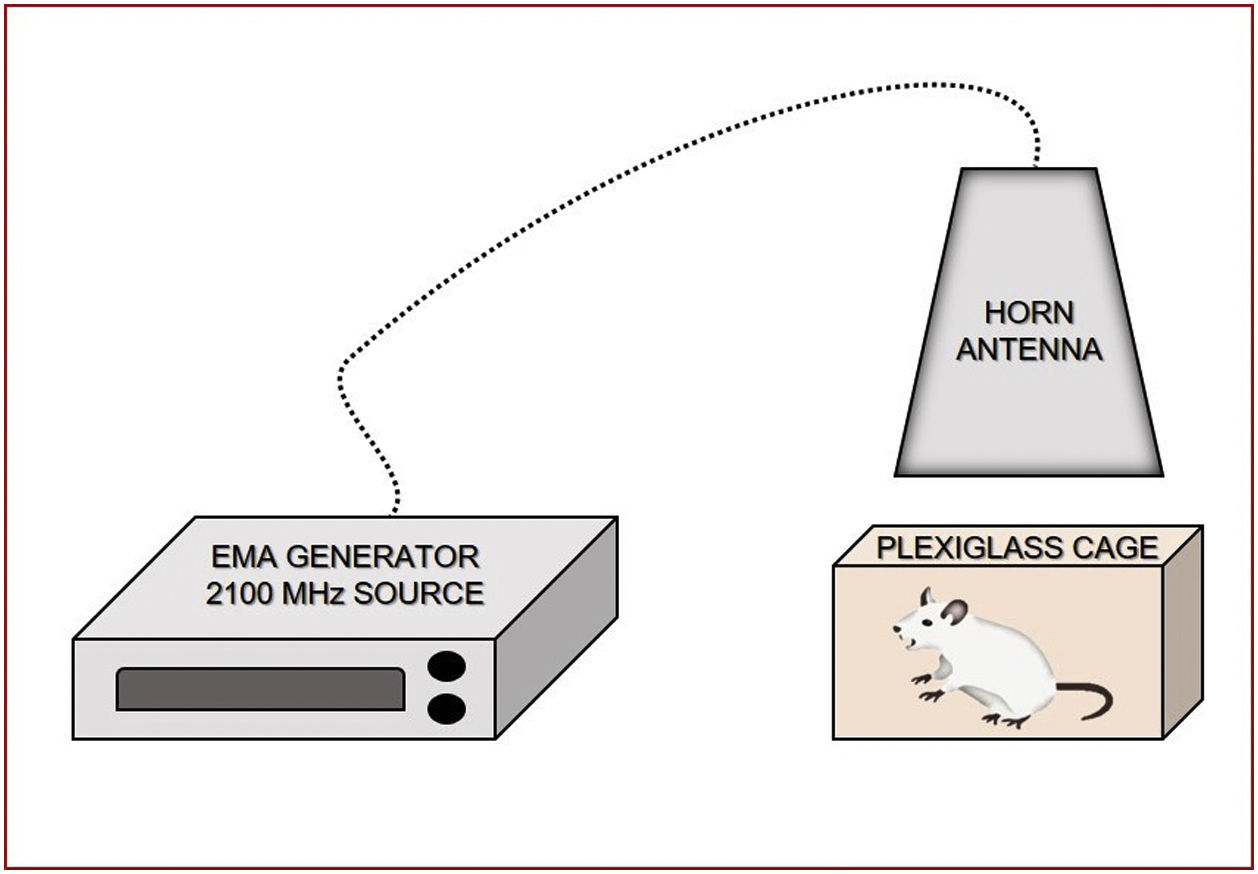
Radiation exposure system2100MHz RF radiation exposures were carried out using a signal generator (frequency range: 9kHz to 3.2GHz, Model SMBV100A; Rohde⪼hwarz, Munich, Germany) with a 3GPP-FDD setting. The exposures were applied with a horn antenna (frequency range: 400MHz to 6GHz, Model 3164-03; ESCO Technologies, Cedar Park, TX). During exposure, rats were placed in plastic cages (15cm×20cm×20cm), three animals in one cage, and exposure was performed by placing the horn antenna on the plastic cage, symmetrically along the perpendicular axis, 11cm below the mid-line of the horn antenna. Exposure was performed approximately 11cm away from the antenna and, because of this, the exposure could be defined as near field exposure, and we could say that this is similar with our exposure from mobile phones. Applied RF radiation was measured with an EMR 300 (Narda, Germany) with an electric field probe type 8.3. For RF fields, the root means square value of electric field (ERMS) was found to be 17.25V/m. The RF environmental background level was around 0.21V/m. The SAR was calculated using the following equation: SAR=σ/ρ[ERMS2] [W/kg], where15 ERMS is the root mean square value of the electric field (V/m), σ is the mean electrical conductivity of the tissues in Siemens/meter (S/m), and ρ is the mass density (kg/m3).16–18 Conductivity (0.87S/m) and mass density (1105kg/m3) were derived for the equivalent tissue using dielectric properties and the mass densities of these tissues. RF exposure in the experiment resulted in a whole-body average SAR of 0.23W/kg, with an ERMS field of 17.25V/m. This SAR value could be accepted as a non-thermal SAR level for RF radiation because it is known that 4W/kg SAR leads to a 1°C increase in the temperature of the exposed tissue. The body temperature of the rats was recorded by rectal measurements before and after exposure sessions. Neither exposure resulted in any rectal temperature increase (Fig. 1).
AnimalsTwenty-four 200–250g weight Wistar albino male rats (Gazi University Medical School Experimental Animal Breeding and Experimental Research Center, Ankara, Turkey) were used in this research. The animals were housed under standard conditions (12-h light/12-h dark cycle with the temperature at 22±2°C). The animals were given tap water as drinking water and fed ad libitum. The animals were acclimatized to laboratory conditions for four weeks prior to the start of the experiments.
The twenty-four male Wistar albino rats were divided into four equal groups (see Table 1).
RF exposure administrations were applied between 4.00 and 5.00pm and subcutaneous melatonin injections were applied at 4.00pm, 40min before radiation exposure. The dose and application time of melatonin was performed following a study by Akbulut et al.19 At the end of the 90-day experimental period, left caudal epididymis and vas deferens samples were collected under ketamine (45mg/kg) and xylazine (5mg/kg) anesthesia.
Preparation of the spermsLeft caudal epididymis and ductus deferens tissues were put into Pure Sperm Wash (Nidacon) solution (maintained at 37°C for 24h) and dissected by sterile needle tips with sperms being transferred to the solution. After that the sperms were counted with the Makler Counting Chamber for spermiogram, and stained with Spermac Stain (FertiPro) for morphological analyses. The perinuclear ring of the manchette and posterior portion of the nucleus (ARC) measurements were performed. All of the parameters were evaluated statistically.
Spermac stain methodThe Spermac stain kit (FertiPro) was used for sperm smears (Lot: FP12S05, Ref. No: SPS050). The smears were air-dried for five minutes and fixed with 4% paraformaldehyde solution immediately for five minutes. Slides were washed by dipping gently seven times into distilled water, stained for one minute in Stain A, then for forty-five seconds in Stain B, and for one minute in Stain C. Between all of the staining steps, the slides were washed by dipping gently seven times into distilled water. After this staining process, the samples were examined under a photographic light microscope (DM4000B Image Analyze System; Leica, Wetzlar, Germany), a Leica DFC280 plus camera, and a LAS software program (Leica) using a 100× objective and immersion oil.
Acrosome=dark green, nucleus=stained red, equatorial region=pale green, midpiece and tail=green.
Transmission electron microscopy methodThe solution with sperm was centrifuged twice at 1200rpm for ten minutes after the fixation process (with 4% glutaraldehyde for thirty minutes). After that, the pellet was gently removed and buried in 1% liquid agar in a petri dish, and stored at room temperature for twenty minutes to freeze. After freezing, 1mm3 pieces were cut and left in a 4% glutaraldehyde solution, again for full fixation. After these preliminary steps, the samples were washed with the same buffer, and post-fixed in 1% osmium tetroxide and 200mM sodium phosphate buffer (pH 7.4) for 1h at 4°C. The samples were again washed with the same buffer for 3h at 4°C, dehydrated with a graded series of ethanol and embedded in Araldite. Thin sections were cut with Leica EMUC7 ultramicrotome using a diamond knife (Leica EMUC7, Hernalser, Germany) mounted on a copper grid and stained with 2% uranyl acetate and lead citrate. The grids were examined under a Carl Zeiss EVO LS 10 TEM-SEM microscope (Germany).3
Statistical analysesAll of the parameters were analyzed using the statistic program. All of the data was presented as mean±standard deviation (SD). The differences between the groups were analyzed by ANOVA tests and p<0.05 was considered to be statistically significant.
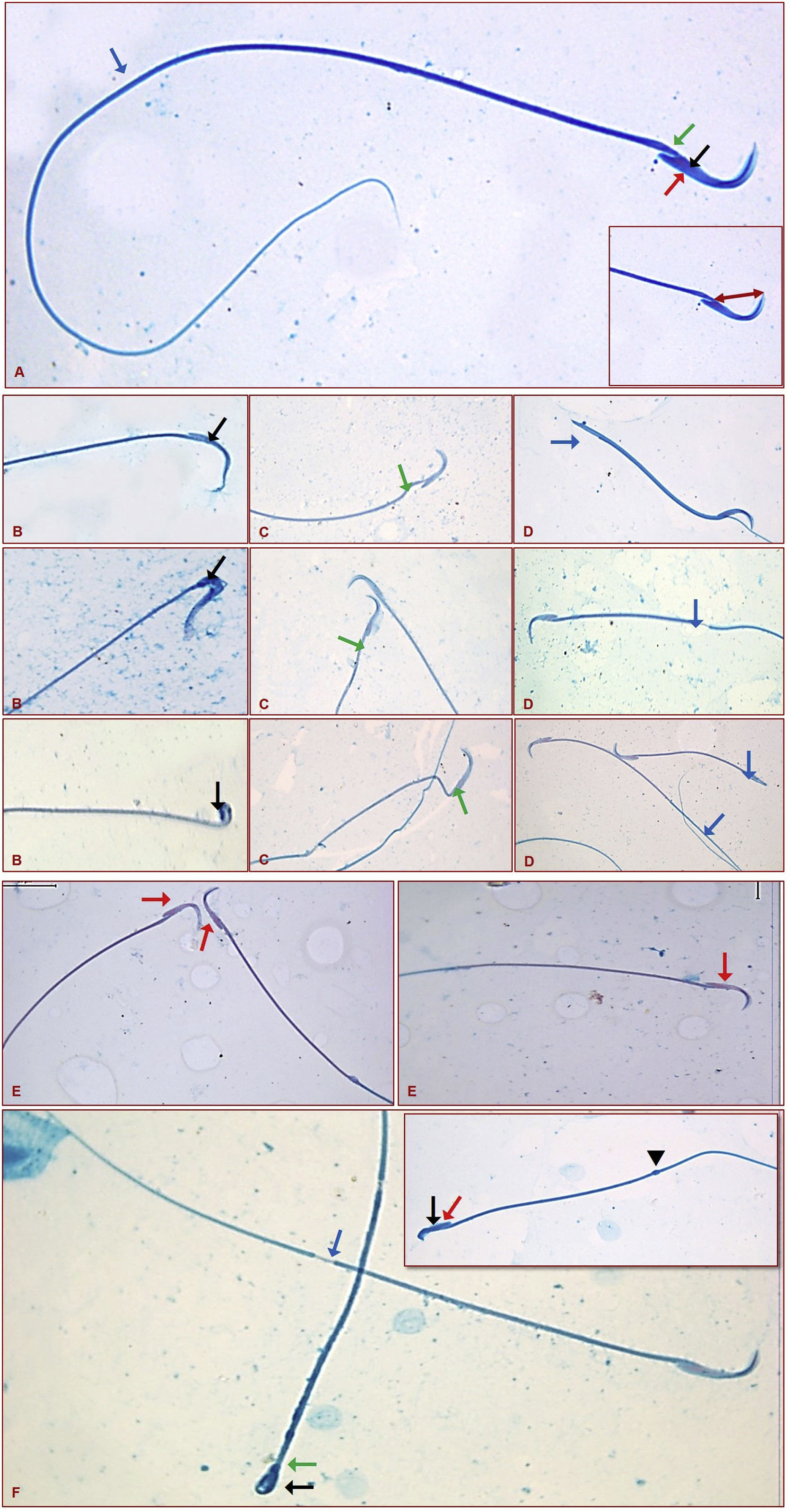
ResultsSpermac stain resultsSperms were distinguished in their normal structures each with a hook or banana shape head, acrosome in the convex part of the head and a nucleus in the central part of the head, and also with a neck and tail of normal appearance in the control group (Fig. 2A).
Normal sperm structure (red arrow: acrosome, black arrow: nucleus, green arrow: neck, blue arrow: tail). Inset shows that how the perinuclear ring of the manchette and posterior portion of the nucleus (ARC) measurement was taken (A). Sperms that showing the head (B-black arrow), neck (C-green arrow), and tail (D-blue arrow) abnormalities. Sperms that showing the acrosome (E-red arrow), and the multiple abnormalities (F and inset-black arrow: head, red arrow: acrosome, green arrow: neck, blue arrow: tail, and arrowhead: cytoplasmic droplet) (Spermac stain ×1000).
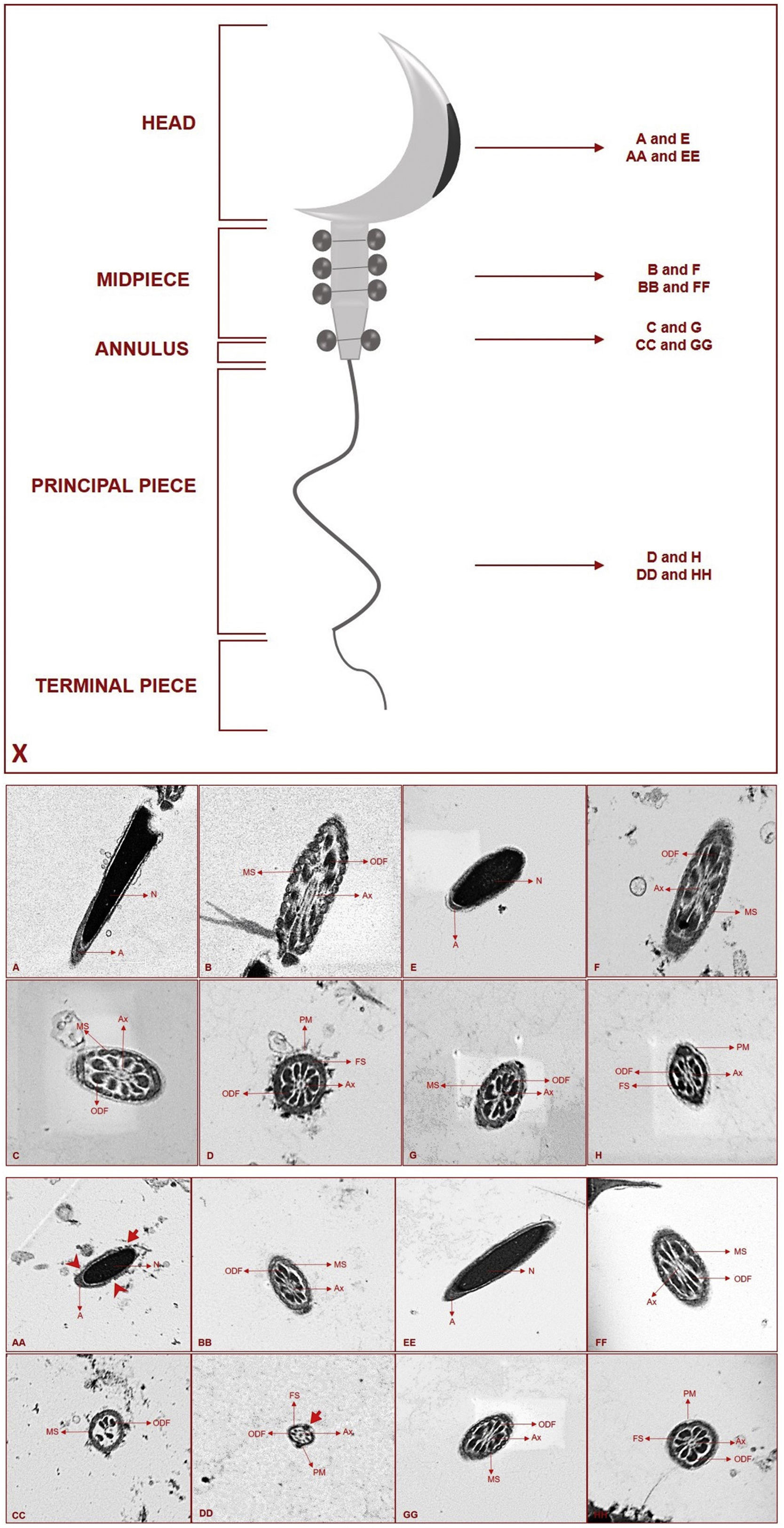
Diagram showing which segments were taken for electron microscopic sections (X). Ultrastructural view of the Control (A–D) and Melatonin (E–H) groups (N: nucleus, A: acrosome, MS: mitochondrial sheath, ODF: outer dense fibers, Ax: axoneme, FS: fibrous sheath, PM: plasma membrane). Ultrastructural view of the RF (AA–DD) and RF+Melatonin (EE–HH) groups (N: nucleus, A: acrosome, MS: mitochondrial sheath, ODF: outer dense fibers, Ax: axoneme, FS: fibrous sheath, PM: plasma membrane) (
: deformed areas of acrosome, : deformed areas of plasma membrane) (Uranyl Acetate-Lead Citrate ×30.14).Abnormalities through sperms were classified as head, acrosome, neck, tail defects, and cytoplasmic droplets. Small and flattened heads, pinheads, wave-looking heads, and distortion in the hook shape were seen within the head defects (Fig. 2B). Folding and thinning in the neck portion of a sperm was considered to be a neck abnormality (Fig. 2C). Detachment or breaking in the distal part, thinning in certain segments, and abnormal bending were observed when the tail abnormalities were evaluated (Fig. 2D).
When the acrosome abnormalities were examined it was seen that the acrosome had completely disappeared in some sperms or disappeared only in the part close to the neck (Fig. 2E). Sperms showing multiple abnormalities were also distinguished, and sperms with a cytoplasmic droplet in the tail region were also seen among these sperms (Fig. 2F and inset).
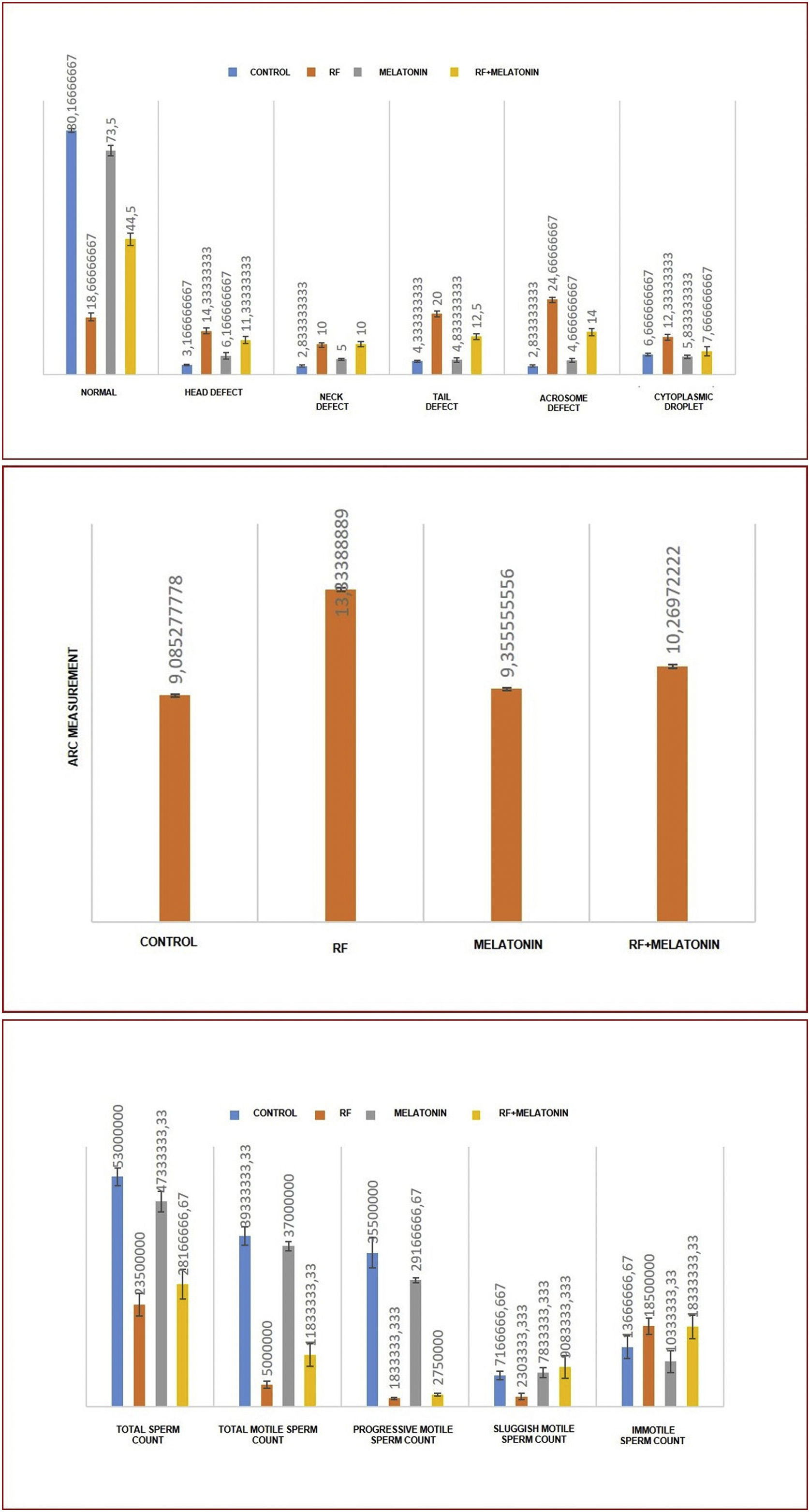
When morphologies were evaluated statistically, significantly lower normal sperms and significantly higher abnormal sperms were observed in the RF group compared to the Control and Melatonin groups. Abnormalities were most commonly found in the tail and acrosome parts. Significantly lower abnormal sperms were seen in the RF+Melatonin group compared to the RF group (p<0.05) (Graphic 1).
Perinuclear ring of the manchette and posterior portion of the nucleus (ARC) measurements were also performed through spermac-stained sperms (Fig. 2A – inset). ARC measurements were significantly higher in the RF and RF+Melatonin groups compared to the Control and Melatonin groups (p<0.05). It was also distinguished that the increase of the RF group was greater than the RF+Melatonin group (Graphic 1).
Spermiogram resultsIn total, total motile, progressive motile, sluggish motile, and immotile sperms were counted for spermiogram analyses for all groups. Significantly lower total, total motile, and progressive motile sperms, but significantly higher immotile sperms, were observed in the RF and RF+Melatonin groups compared to the Control and Melatonin groups. Significantly lower sluggish motile sperms were seen in the RF group compared to the other groups. When the RF and RF+Melatonin groups were evaluated among themselves, a significantly higher total and total motile sperms were observed in the RF+Melatonin group compared to the RF group (p<0.05) (Graphic 1).
Transmission electron microscopic (TEM) resultsSperms are divided into head, midpiece (and annulus), principal, and terminal pieces of a tail. For the ultrastructural examinations, four different sections were taken from four different regions for detailed examination. Longitudinal sections were taken from the head, and cross-sections were taken from three different regions of the sperms; midpiece, the transition zone of midpiece and principal piece (annulus), and toward the end of the principal piece (Fig. 3X).
Condensation nucleus and acrosome were seen in their normal appearance in the Control and Melatonin groups (Fig. 3A and E). Centrally located axoneme, outer dense fibers, and mitochondrial sheath were also seen in their normal structures for the midpiece and annulus sections (Fig. 3B, C and F, G). Axoneme, outer dense fibers, fibrous sheath, and plasma membrane were observed in their normal structure for the sections of the end of the principal piece (Fig. 3D and H).
Deformed areas in the acrosome were distinguished for the RF group. In the head sections, additionally, it was seen that plasma membrane integrity was impaired in a number of regions (Fig. 3AA). Axoneme, outer dense fibers, and mitochondrial sheath were seen in their normal appearance for the midpiece sections (Fig. 3BB). In the annulus part, strict deterioration was observed for axoneme, outer dense fibers, and mitochondrial sheath (Fig. 3CC). In the principal piece, the general appearance was normal except for certain areas where plasma membrane integrity was impaired (Fig. 3DD). It was observed that all regions of the sperms exhibited relatively smaller diameters compared to the other groups.
When the RF+Melatonin group was examined it was seen that the findings generally returned to their normal appearance for all regions due to applied melatonin (Fig. 3EE–HH).
DiscussionRF-EMFs have several detrimental effects on male reproductive health associated with testicular, epididymal damage, and significant changes to sperm count, viability, motility, and morphology.7 Mobile phones are a common source of RF-EMFs. People often keep them so close to the bodies, even while sleeping, so this condition increases their harmful effects on human health.20 The accepted harmful effects of RF-EMFs on biological tissue occur mainly with thermal and nonthermal mechanisms.7
Previous studies have shown that RF-EMFs negatively affect the quality of semen by decreasing the sperm count, motility, morphology, and viability. In addition, they promote spermatozoal cell death, and testicular carcinogenesis by inducing DNA damage due to increased oxidative stress.21,22 Because the mechanism is not fully clarified, the coming possibilities that have been considered for RF-EMFs interactions with male reproductive organs can be summarized as follows: (1) biophysics of RF-EMFs radiation; (2) effect of RF-EMFs on the sperm parameters; (3) role of kinases in the cellular metabolism; (4) RF-induced oxidative stress; (5) genotoxic effects of RF-EMFs; and (6) reproductive endocrine effects of RF-EMFs.8
Gu et al. applied 220MHz radiation exposure to adult rats for one month and, despite the almost low-frequency radiation, they found several detrimental effects for both testis and sperms. According to this study, sperm quality (sperm count, and survival rate of sperms) and the levels of secreted factors of Sertoli and Leydig cells decreased significantly in the RF group. Additionally, abnormal sperm morphology, the levels of caspase-3 and the Bax/Bcl-2 ratio increased markedly in the testis.1 Meanwhile, Aitken et al. found that there was no significant change in the sperm count, survival rate of sperms, and morphology after 900MHz RF exposure.23 Similarly, another study declared that any differences were not observed in the sperm motility and concentration after 900MHz RF exposure to male rats for one year.24 However, previous studies have also shown that 1800MHz RF exposure decreased sperm count, viability, and survival rate of sperms through mice.25 In the present study, we observed a decrease in the percentages of total and total motile sperms with long-term exposure to 2100MHz RF radiation.
Shokri et al. applied 2450MHz exposure to rats for two months. According to this study, the RF group showed a decrease in sperm parameters and an increase in caspase-3 activity in the seminiferous tubules. The percentage of total motile, progressive motile sperms, and sperms with normal morphology parameters reduced significantly in the RF group.14 The possible effects of 3G mobile phone radiation exposure for forty-five days on the male reproductive system were researched by Gautam et al., and they found a significant increase of ROS and lipid peroxidation levels after the exposure caused a decrease in sperm count, and alternate sperm tail morphology.26 According to our study, we distinguished an increase in abnormal sperm morphology in the RF group, especially in the acrosome, head, and tail regions. Additionally, we found a higher ARC measure with long-term exposure to 2100MHz RF radiation that supported abnormalities in the head region of the sperms.
According to another study on humans, semen samples were divided into the normozoospermia, asthenoteratozoospermia, and oligoasthenoteratozoospermia categories. After this, these samples were subdivided into non-exposed and exposed to cell phone radiation for 1-h groups. Finally, researchers revealed a significant decrease in sperm motility, linear velocity, and sperm acrosin activity, whereas a significant increase in the sperm DNA fragmentation percent in exposure groups compared to non-exposure groups. The most serious difference was found in the oligoasthenoteratozoospermic semen samples.27
Because one of the destructive effects of RF exposure depends on the overproduction of ROS, antioxidant treatments can help prevent or reduce certain radiation damage.9 Researchers indicate that melatonin is an effective and non-toxic protective agent for the destructive effects of ionizing radiation through tissue. When the testis is exposed to toxicity, spermatogenesis quickly disrupts, and melatonin has been shown to help prevent sperm damage in the testis both in vivo and in vitro. Additionally, abnormal levels of melatonin in semen are associated with infertility in men. These findings suggest that melatonin is involved in spermatogenesis, and also that melatonin receptors have been found in the sperm of certain species.28 The use of melatonin-containing incubation mediums increased the percentage of motile, progressive motile sperms, and increased mitochondrial activity according to in vitro studies.29 Significantly higher total and total motile sperm quantities were observed in our RF+Melatonin group compared to our RF group.
Moreover, a chemotactic effect of melatonin that is important for ideal fertilization, has been described in the somatic cells, as well as a relationship between melatonin and sperm motility.30 Previous studies have shown that melatonin pretreatment efficiently attenuated the DNA damage effects of mobile phone radiation exposure in a mouse spermatocyte-derived cell line.31 2mg/kg melatonin administration showed a protective effect in the case of 900MHz RF exposure-induced testicular damage.14 Similarly, Meena et al. used 2mg/kg melatonin against 2450MHz microwave radiation for forty-five days. According to this study, it was concluded that melatonin prevents oxidative damage in the testicular tissue and reverses the harmful effects of microwave radiation on sperm count, testosterone level, and DNA fragmentation in testicular cells.13 Pandey and Giri applied 5mg/kg melatonin to mice for thirty-five days under 900MHz RF exposure conditions. They observed that melatonin administration inhibits spermatogenesis arrest, reduced oxidative stress, DNA damage, and abnormal sperm morphology, and that it improves sperm count, and testicular histology compared to the RF group.15 We use 10mg/kg melatonin in our study, and according to this, significantly lower abnormal sperm quantities are distinguished in our RF+Melatonin group than in the RF group.
The current data reveals that there are only a few studies that have researched the possible harmful effects of RF-EMFs on sperm cells at the ultrastructural level. According to this data, Liu et al. and Li et al. revealed an increased number of autophagic vacuoles in the cytoplasm of sperm cells by 1800MHz RF exposure.32,33 Another study showed that the midpiece region, microtubules of axoneme, outer dense fibers of mitochondria, and membranes disrupted, while the nucleus distorted in the acrosome region among the sperm cells after RF exposure.34 Acrosome and plasma membrane among the head, also axoneme, outer dense fibers, mitochondrial sheath of the annulus part were observed as disrupted according to our ultrastructural examinations with long-term exposure to 2100MHz RF radiation. However, there are no studies in the literature that show the effects of melatonin on sperm cells under RF-EMFs conditions at the electron microscopic level. However, the ultrastructural findings are generally returned to their normal appearance for all regions due to applied 10mg/kg melatonin, according to our study. Therefore, this state makes our research unique because it is the first study in this field.
ConclusionsTaken together, long-term exposure to 2100MHz RF radiation causes a decrease in normal-morphology sperms, total sperm count, and also disrupts the cells in the electron microscopic levels, such as acrosome, axoneme, and mitochondrial degenerations. Treatment with melatonin exhibits a beneficial therapeutic agent for long-term exposure of 2100MHz RF radiation-related reproductive impairments. Ours is the first study that examines the effects of melatonin on sperm cells under RF-EMFs conditions at the ultrastructural level. We believe that future molecular studies would extend our research.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingDeclared none.
Conflict of interestDeclared none.
This study was presented as a poster presentation at the 32nd Annual Meeting of the European Society of Human Reproduction and Embryology (ESHRE) between 3 and 6 July, 2016, in Helsinki, Finland. We express thanks to Edward McQuaid, an English language teacher, at Anadolu University for the English language editing of the manuscript.