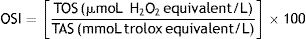This study was performed to evaluate the effect of ethanolic extract of Turkish propolis (EEP) on testicular ischemia/reperfusion (I/R) damage in rats in terms of biochemistry and histopathology, for the first time.
MethodsA total of 18 male Sprague-Dawley rats were divided into three groups with six rats in each group: control, torsion/detorsion (T/D), and T/D+EEP (100mg/kg). Testicular torsion was performed by 720° rotating the left testicle in a clockwise direction. The duration of ischemia was 4h and orchiectomy was performed after 2h of detorsion. EEP was applied only once 30min before detorsion. Tissue malondialdehyde (MDA), total oxidant status (TOS) and total antioxidant status (TAS) levels were determined using colorimetric methods. Oxidative stress index (OSI) was calculated by proportioning tissue TOS and TAS values to each other. Tissue glutathione (GSH) and glutathione peroxidase (GPx) levels were determined using enzyme-linked immunosorbent assay (ELISA) kits. Johnsen's testicle scoring system was used for histological evaluation.
ResultsIn the T/D group, it was determined that statistically significant decreasing in TAS, GSH, GPx levels and Johnsen score, and increasing in TOS, OSI and MDA levels (p<0.05) compared with control group. EEP administration statistically significantly restored this I/R damage (p<0.05).
ConclusionThis is the first study to show that propolis prevent I/R-induced testicular damage through its antioxidant activity. More comprehensive studies are needed to see the underlying mechanisms.
Este estudio se realizó para evaluar por primera vez el efecto del extracto etanólico de propóleo turco (EEP) sobre el daño por isquemia/reperfusión (I/R) testicular en ratas en términos de bioquímica e histopatología.
MétodosUn total de 18 ratas macho Sprague-Dawley se dividieron en 3 grupos con 6 ratas en cada grupo: control, torsión/detorsión (T/D) y T/D+EEP (100mg/kg). La torsión testicular se realizó con una rotación de 720° del testículo izquierdo en el sentido de las agujas del reloj. La duración de la isquemia fue de 4h y la orquiectomía se realizó a las 2h de la detorsión. EEP se aplicó solo una vez 30min antes de la detorsión. Los niveles de malondialdehído tisular (MDA), estado oxidante total (TOS) y estado antioxidante total (TAS) se determinaron mediante métodos colorimétricos. El índice de estrés oxidativo (OSI) se calculó proporcionando los valores de TOS y TAS del tejido entre sí. Los niveles de glutatión tisular (GSH) y glutatión peroxidasa (GPx) se determinaron utilizando kits de ensayo inmunoabsorbente ligado a enzimas (ELISA). Se utilizó el sistema de puntuación de testículos de Johnsen para la evaluación histológica.
ResultadosEn el grupo T/D, se determina una disminución estadísticamente significativa en los niveles de TAS, GSH, GPx y puntuación de Johnsen y un aumento en los niveles de TOS, OSI y MDA (p<0,05) en comparación con el grupo control. La administración de EEP restauró de forma estadísticamente significativa este daño I/R (p<0,05).
ConclusiónEste es el primer estudio que demuestra que el propóleo previene el daño testicular inducido por I/R a través de su actividad antioxidante. Se necesitan estudios más completos para ver los mecanismos subyacentes.
Testicular torsion (TT) is an emergency caused by the sudden bending of the spermatic cord around its axis in the scrotum. This condition causes cessation of blood flow to the affected testicle and ischemic damage, often irreversible.1 The two most important factors determining the degree of testicular damage are the duration and degree of twisting of the spermatic cord.2 Surgical intervention of TT includes detorsion of the twisted testicle and restoration of testicular blood flow.3 TT causes potentially leading to male subfertility when misdiagnosed and/or incomplete treated.4 The incidence of TT is very high. It is revealed that 1 out of 158 men before the age of 25 has TT, and sperm quality is decreased in approximately 35% of these individuals.5 The main pathophysiology of TT is ischemia/reperfusion (I/R) injury. I/R injury causes infertility by causing neutrophil recruitment, increased reactive oxygen species (ROS), pro-inflammatory cytokines/adhesion molecules, lipid peroxidation and apoptosis.2 There are many experimental studies reported the protective role of various pharmacological agents, such as natural product extracts, phosphodiesterase inhibitors, vitamins, minerals, hormones and non-steroidal anti-inflammatory drugs, in alleviating I/R injury by reducing oxidative stress, inflammation and apoptosis during the surgical treatment of TT.1,2
Propolis is the name given to the resinous substance that bees collect from different plant species, also known as “bee glue”.6 It is used to close the holes and cracks in beehives, to soften the inner surface of the hive, to maintain the internal temperature of the hive and to prevent the invasion of predators.7 Although the majority of propolis composition is composed of resin (50%), there are also wax (30%), essential oils (10%), pollen (5%) and other organic compounds (5%) in the structure.8 Phenolic compounds, esters, flavonoids, terpenes, beta steroids, aromatic aldehydes and alcohols are the structures that make up the organic compounds content of propolis.6 The antiseptic, anti-inflammatory, antioxidant, antibacterial, antimycotic, antifungal, anti-ulcer, anticancer, neuroprotective and immunomodulatory properties of propolis extracts have been demonstrated by various experimental studies.6–8
Numerous studies have investigated the protective effects of propolis extracts on various tissue, such as brain, spinal cord, and ovary, in experimental I/R injury models. Shimazawa et al. reported that propolis protects brain tissue against I/R-induced injury through decreasing the level of oxidative stress9, while Kasai et al. demonstrated that propolis extract prevents I/R-induced spinal cord damage in rats through decreasing inflammation and apoptosis.10 Koc et al. recently reported that propolis has ovary protective effect in I/R-induced experimental injury model through decreasing oxidative stress, apoptosis and inflammation.11 However, no previous study has examined the protective effect of propolis extract in I/R-induced testicular damage model. The aim of this study was therefore to investigate the testicle protective effect of ethanolic extract of Turkish propolis (EEP) in an I/R-induced rat model together with the mechanisms for the first time.
Materials and methodsChemicals and reagentsFolin&Ciocalteu's phenol reagent, phosphate buffered saline (PBS) tablet, phosphoric acid, thiobarbituric acid, 1,1,3,3-tetramethoxypropane, ethanol, sodium carbonate and gallic acid were purchased from Sigma–Aldrich (St. Louis, MO, USA).
Propolis sample and preparation of extractThe propolis samples were collected from a rural area of Eastern Black Sea region of Turkey (Yomra, Trabzon). The samples were converted to fine powder using a laboratory grinder (Retsch ZM200, Haan, Germany). For preparing stock EEP (5×104μg/mL), 1g of the powdered sample was then mixed with 20mL of ethanol. The mixture was incubated with continuous shaking at 150rpm at 45°C for 24h. The resulting supernatant was then filtered through filter paper and finally passed through 0.2μm filters. The prepared stock extract was stored in the dark at −20°C and used in experiments by serial dilution with ethanol.12
Determination of total phenolic contentThe total phenolic content (TPC) of the EEP was determined using the previously described colorimetric method.13 Gallic acid was used as a reference compound, and the TPC value was calculated as mg gallic acid equivalent (GAE)/g sample.
AnimalsThe experimental procedure of this research were approved by the Animal Care Ethical Committee of Karadeniz Technical University (Trabzon, Turkey) (Protocol no: 2019/8) and were conducted in conformity with US National Institutes of Health guidelines. Healthy adult male Sprague-Dawley rats with proven fertility, 3 months of age and weighing 200–250g were supplied from the Surgical Practice and Research Center of Karadeniz Technical University (Trabzon, Turkey). Animals were maintained in this center in steel cages under controlled temperature (23±2°C), 12-h light/dark cycle and 50±5% relative humidity. Water and food were available ad libitum. General anesthesia was achieved with intraperitoneal (i.p.) administration of 60mg/kg ketamine hydrochloride (Vem Pharmaceuticals, Ankara, Turkey) and 12mg/kg xylazine (Bayer, Leverkusen, Germany).
Experimental designAfter the acclimation period, the animals were divided into three groups of 6 animals each. Experimental interventions was summarized in Table 1. Group 1 (Control group): In this group, a scrotal opening was created by making a small incision in the scrotum on the left ilioinguinal side. After 360min, the testicles were removed without any application. Group 2 (T/D group): A scrotal hole was made by making a small incision on the left ilioinguinal side. The removed testicle was rotated 720° clockwise and secured into the scrotum with sutures. After 240min, detorsion was performed, and the testicle was placed in the scrotal sac. After 120min, orchiectomy was performed.14Group 3 (T/D+EEP group): Although the procedure in the I/R group was followed the same, 30min before detorsion 100mg/kg EEP application (i.p.) was made. The dose of the EEP was determined according to the literature data15,16 and our preliminary data. Half of the testicular tissues removed after the surgical procedure were placed in micro volume tubes for use in biochemical analysis and stored at −80°C. The other half of the tissues were fixed in Bouin's solution for histological analysis.
Biochemical analysisThe tissue samples were homogenized at 9500rpm in 1mL of PBS using a homogenizer (IKA, T25 Ultra-Turrax, Staufen, Germany). The supernatant portions were separated by means of centrifugation at 1800×g for 10min at 4°C and used in the biochemical analysis.
Protein levels in supernatants were determined using a commercial kit (Pierce BCA Protein Assay Kit, Thermo Scientific, Rockford, IL) according to the manufacturer's instructions. Bovine serum albumin was used as a standard and protein levels of the supernatants were calculated in mg/mL using the albumin standard graph. All biochemical parameters measured in supernatants were proportioned to the amount of protein and expressed per mg of protein.
Malondialdehyde (MDA) levels of tissue samples were determined according to the method developed by Mihara and Uchiyama.17 Briefly, 500μL of supernatant was mixed 3mL of 1% phosphoric acid and 1mL of 0.672% thiobarbituric acid. After vortexing, the mixture was incubated in a boiling water bath for 1h. The tubes were then allowed to cool at room temperature and centrifuged at 1800×g for 10min. 200μL of the each supernatants were taken and transferred to a 96-well plate. Absorbances were then read at 532nm using a microplate reader spectrophotometer (Versamax, Molecular Devices, CA, USA). 1,1,3,3-tetramethoxypropane was used as a standard and tissue MDA levels were expressed as nmol/mg protein.
Tissue glutathione (GSH) and glutathione peroxidase (GPx) levels were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (BT Lab, Zhejiang, China) according to the manufacturer's recommendations. GSH and GPx levels were expressed as μg/mg protein and ng/mg protein, respectively.
Tissue total oxidant status (TOS) and total antioxidant status (TAS) levels were determined using commercial colorimetric kits (Rel Assay Diagnostics, Gaziantep, Turkey) according to the manufacturer's recommendations. The TOS/TAS ratio was used as the oxidative stress index (OSI) and was calculated using the formula18:
Histological analysisFor histological examinations, the tissue samples were fixed in Bouin's solution, dehydrated in graduated alcohol series and embedded in paraffin. Tissue samples in paraffin were cut at 5μm thickness for histological examination using an automated microtome (Leica RM 2255, Tokyo, Japan) and stained with hematoxylin eosin (H&E). The slides were evaluated under a light microscope (Olympus BX51 Tokyo, Japan) to evaluate histological changes in the testicles.19 Johnsen's testicle scoring system was used to categorize spermatogenesis.20 It was evaluated 20 seminiferous tubules in each section, giving a score from 1 (very poor) to 10 (perfect) to each tubule according to the Johnsen's criteria. Scoring was done by a histologist who was blind to the study groups.
Statistical analysisData were presented as the mean±standard deviation for six rats per group in the biochemical and histological analysis. The compliance of the data to normal distribution was evaluated with the Kolmogorov–Smirnov test. Comparisons between groups regarding the in biochemical and histological analyses were carried out using one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons test. Data were analyzed with Statistical Package for the Social Sciences (Version 23.0, NY, USA). Statistical significance was set at p<0.05.
ResultsTable 2 showed the levels of oxidative stress markers and histological Johnsen's scores of all groups. The MDA, TOS and OSI levels of the T/D group were significantly increased compared to the control group (p=0.0001). On the other hand, the TAS, GPx and GSH levels of the I/R group was significantly decreased compared to the control group (p=0.0001, p=0.015 and p=0.019, respectively). The EEP treatment significantly restored the levels of MDA, TOS, TAS, OSI, GPx and GSH (p=0.0001, p=0.0001, p=0.004, p=0.0001, p=0.022, and p=0.046, respectively). The histopathological Johnsen score was significantly lower in the T/D group compared to the control group (p=0.0001), but the level of histopathological Johnsen score was significantly restored by EEP treatment (p=0.01).
A comparison of biochemical parameters and histopathological scores in the groups.
| Control | T/D | EEP+T/D | |
|---|---|---|---|
| TOS (μM H2O2 Equi/L) | 22.8±6.22 | 65.3±15.9a | 19.6±10.4b |
| TAS (mM trolox Equi/L) | 1.02±0.54 | 0.64±0.21a | 0.95±0.38b |
| OSI (arbitrary unit) | 2.25±0.67 | 11.5±5.35a | 2.05±1.13b |
| MDA (nmol/mg protein) | 7.88±0.69 | 22.7±2.77a | 8.70±1.37b |
| GPx (ng/mg protein) | 1.92±0.25 | 1.29±0.10a | 1.89±0.33b |
| GSH (μg/mg protein) | 12.9±4.04 | 5.68±0.65a | 11.6±2.90b |
| Johnsen score | 9.70±0.09 | 6.52±0.34a | 8.32±0.92a,b |
T/D: torsion/detorsion, EEP: ethanolic extract of Turkish propolis, TOS: total oxidant status, TAS: total antioxidant status, OSI: oxidative stress index, MDA: malondialdehyde, GPx: glutathione peroxidase, GSH: glutathione.
p-Values according to one-way ANOVA test, post hoc Tukey test. Data were expressed as mean±SD.
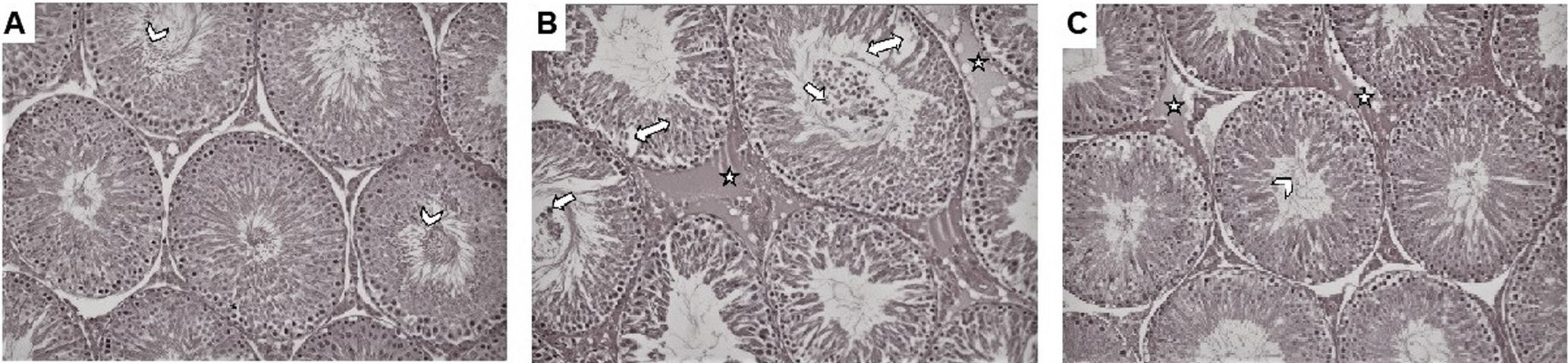
Photomicrographs of H&E stained sections of testicle of rats were presented in Fig. 1. In Group 1, it was observed that the seminiferous tubule epithelium structure, peritubular tissue was regular and there were many spermatozoa in the lumen of the seminiferous tubule (A). In Group 2, it was observed that the seminiferous tubule structure was significantly deteriorated, and there were separations and distinct vacuolizations in the epithelium lining the tubule. In addition, there was a large number of immature germinal epithelial cells in the lumen of the seminiferous tubule and marked edema in the peritubular tissue around the tubules (B). In Group 3, it was observed that the seminiferous tubule structure had improved considerably compared to Group 2, the number of spermatozoa increased and peritubular edema decreased in the seminiferous tubules, which were generally seen in a smooth structure (C).
Photomicrographs of hematoxylin and eosin stained sections of testicle of rats (200×). (A) Seminiferous tubule epithelial structure was observed in normal morphology in control group and there were many matured spermium (arrowhead) in lumen. (B) In T/D group, there were many immature germinal epithelial cells (white arrow) in the lumen of the seminiferous tubule. In addition vacuolization of the tubule epithelium (double-headed arrow), and edema (star) in the peritubular connective tissue were observed. (C) In T/D+EEP group, it was observed that the seminiferous tubule structure had improved considerably compared to T/D group, the number of spermatozoa increased (arrowhead) and peritubular edema (star) decreased in the seminiferous tubules.
Testicular torsion occurs due to impaired blood flow in the testicle as a result of the rotation of the spermatic cord structures.21 Immediate surgical treatment is mandatory as ischemia of the testicle longer than 6h may cause irreversible damage.22 The first application that should be done urgently in the clinic is to provide reperfusion with detorsion.21 While the detorsion process is essential for re-oxygenation of the tissue, paradoxically it causes more damage to the tissue than ischemia. This process “called I/R injury”, is characterized by oxidative stress, which is the main cause of organ damage.15 Excessive amounts of ROS that occur duration of I/R cause the release of inflammatory cytokines, accumulation of neutrophils and macrophages, resulting in testicular atrophy, germ cell apoptosis, and disruption of spermatogenesis.22 Therefore, it is very important to find new agents that reduce testicular I/R damage in order to prevent the infertility.15
Propolis is a resinous mixture created by honey bees blending the extracts collected from leaves and flower buds with their saliva.8 It is rich in phenolic compounds that exhibit antioxidant, anti-inflammatory, antimicrobial, anticancer, immunomodulatory and wound healing activities.6 It is regarded as a potential source of natural antioxidants that can counter the effects of oxidative stress underlying many pathogenesis, such as cancer, diabetes, ischemia, cardiovascular and neurodegenerative diseases.8 Although numerous studies have investigated the protective effects of propolis on various tissue, including brain, spinal cord, and ovary9–11, in experimental I/R injury models, no previous study has examined the protective effects of propolis extract in I/R-induced testicular injury. Thus, the main aim of this present study was to explore whether the propolis effects on the I/R-induced testicular damage recovering their normal biochemical and histological status. It is well known that oxidative stress induced as a result of increased reactive oxygen species (ROS) is an important pathological component of testicular ischemic injury.23 The potential protective effect of EEP on testicular injury was therefore investigated biochemically using oxidative stress biomarkers and the results were presented in Table 2. MDA is an end product formed during lipid peroxidation and is considered an important marker of cell dysfunction caused by oxidative stress.16 Among the antioxidant enzymes, GPx is one of the first lines of defense against oxidative damage. The biological function of GPx is to reduce the conversion of lipid hydroperoxides to the alcohols and the free hydrogen peroxide reaction.24 In the case of increased oxidative stress, free radicals are scavenged by the GPx activity and the reducing power of GSH, after a while the pool of oxidized GSH in the cell is depleted. The decrease in the pool of oxidized GSH decreases the cell's ability to resist free radicals over time, and lipid peroxidation reactions are accelerated. Therefore, GSH is considered a sensitive marker in the assessment of oxidative stress and inversely correlates with the formation of MDA.23 It is well known that two of the crucial parameters for evaluating redox balance in biological systems are TAS and TOS. While TAS determines the overall ROS scavenging ability in a biological sample, TOS can be defined as the cumulative amount of total oxidants in the sample. For the quantitative assessment of redox homeostasis disorders, the OSI, which is called the “gold indicator of oxidative stress”, is used.25 The increased MDA, TOS and OSI levels and decreased TAS, GPx and GSH levels in the T/D group were found to be statistically significantly than the control group. These results showed that the I/R model is worked successfully and testicular damage is occurred. The statistically significant correction of the disturbed redox balance by EEP application indicates that EEP has a testicular protective effect. Histological evaluation using a microscopic Johnsen scoring system is considered the gold standard in the assessment of I/R injury.14 The statistically significant decrease in the Johnsen scores in the T/D group compared with the control group indicates that the I/R damage was successfully achieved. The statistically significant improvement of this decrease with the application of EEP shows its testicular protective effect.
Similar to these results, in studies showing that propolis improves I/R-induced damage in other tissues, da Costa et al. demonstrated that propolis extract exhibits renoprotective effect in an I/R-induced kidney damage model through decreasing MDA levels and increasing GSH levels26, while Geyikoglu et al. reported that propolis extract reduces the levels of myeloperoxidase (MPO), MDA, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), 8-hydroxy-2′-deoxyguanosine (8-OHdG) and caspase-3 in an experimental I/R induced ovary damage model.15 Doganyigit et al. demonstrated that propolis extract has renoprotective effect in a lipopolysaccharides-induced experimental I/R injury model through decreasing the level of MDA27, while Abdel-Rahman et al. reported that propolis has neuroprotective effect in a experimental cerebral I/R injury model through increasing the efficacy of antioxidant systems, such as GPx, GSH, catalase (CAT) and superoxide dismutase (SOD).23 Recently, Bazmandegan et al. reported that propolis exhibits neuroprotective effect in a experimental cerebral I/R injury model through decreasing the levels of oxidative stress.16 Furthermore, there are also studies showing the protective effect of propolis against testicular damage caused by various chemicals, such as chlorpyrifos, mitomycin C, cisplatin and copper by reducing oxidative stress.19,24,28,29
In this study, we found the TPC value of EEP as 115.1±1.62mg GAE/g sample using the Folin-Ciocaltau method. Consistent with our results, TPC values of propolis extracts vary between 30 and 200mg GAE/g sample according to the literature data.8 It is reported that propolis samples collected from Trabzon, Turkey are rich in benzoic acid (4.3%), ferulic acid (0.12%), caffeic acid (0.61%), quercetin (1.1%), chrysin (9.86%), pinocembrin (16.26%), pinostrobin (2.26%), naringenin (6.2%), pinobanksin (7.6%), galangin (1.6%) and apigenin (2.6%).30 In recent years, it has been reported that compounds of propolis, such as caffeic acid, chrysin, quercetin, apigenin and naringin, have potential testicle-protective activity.5,21,22,31,32 The antioxidant effect of these compounds is attributed to their ability to donate electrons to ROS, chelate metal ions, and stimulate antioxidant and detoxifying enzymes.33,34 It is thought that the protective effect of EEP against I/R-induced testicular damage is not due to a single phenolic compound, but to the synergistic effect of all phenolics contained within it.
Our study has a few limitations that need to be explored in detail in future studies. First, more effective results can be observed by differentiating the propolis preparation technique and the used solvents. Second, the testicle protective effect of propolis can be re-evaluated by focusing on more detailed mechanisms. Third, we did not investigate the application of EEP at different times or in prolonged I/R periods in this study. Considering that these factors may affect the protective effect of propolis, additional studies are needed to determine the optimal dose and duration of administration.
ConclusionThis study provides strong evidence of the beneficial effects of propolis on testicular I/R injury for the first time. EEP administration at the concentrations of 100mg/kg before the reperfusion period improved T/D-induced testicular damage. This improvement has been demonstrated by the evidence of biochemical oxidative stress markers and histological evaluation. However, in order to obtain more reliable results and reveal the underlying mechanisms, it is recommended that further clinical studies be conducted and supported by different animal uses.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNone.
Conflict of interestThe authors declare that they did not have conflict of interests.
We would like to acknowledge animal, biochemistry and histology laboratory staffs for their effort during the study.