Erectile dysfunction (ED) is the inability to achieve or maintain erection sufficient for satisfactory sexual performance. Although the definition is well known, there are controversial issues about the effects of hormones and inflammation on ED.
ObjectivesWe aimed to compare the clinical value of the hormonal and inflammation parameters in sexual dysfunction.
Materials and methodsA total of 152 patients diagnosed with erectile dysfunction between September 2018 and March 2019 and 101 healthy males were included in this prospective study as case group and control group, respectively. The 152 patients were divided into three groups based on their total International Index of Erectile Function (IIEF) scores: (I) severe ED, (II) mild-moderate ED and (III) mild ED. All groups were compared in terms of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and total testosterone (TT), estradiol, prolactin, testosterone-to-estradiol ratio and 25 (OH) vitamin D.
ResultsPatient and control groups differed significantly in term of NLR, PLR, prolactin and vitamin D (p<0.001, p=0.004, p=0.002, p<0.001, p<0.001, respectively). NLR was more significant in determining the severity of ED (p<0.001). It was observed that libido score (the total score of IIEF items #11 and #12) was negatively associated with prolactin and NLR (p<0.001, p=0.023, respectively), was positively associated with vitamin D and TT (p<0.001, p=0.02, respectively), and was lower in severe ED patients.
ConclusionsAlthough more clinical studies are needed, we think that our findings may be useful on these controversial issues of ED.
La disfunción eréctil (DE) es la incapacidad de lograr o mantener una erección suficiente para mantener relaciones sexuales satisfactorias. Aunque su definición es bien conocida, existen cuestiones controvertidas acerca de los efectos de las hormonas y la inflamación en la DE.
ObjetivosNuestro objetivo fue comparar el valor clínico de los parámetros hormonales e inflamatorios en la disfunción sexual.
Materiales y métodosIncluimos en este estudio prospectivo a un total de 152 pacientes diagnosticados de DE entre septiembre de 2018 y marzo de 2019, y 101 varones sanos como grupo de estudio y grupo control, respectivamente. Dividimos a los pacientes en 3 grupos, basándonos en las puntuaciones totales del International Index of Erectile Function (IIEF): I) DE grave, II) DE de leve a moderada y III) DE leve. Comparamos los grupos en términos de índice neutrófilo-linfocito (NLR), índice plaqueta-linfocito (PLR) y testosterona total (TT), estradiol, prolactina, índice testosterona-estradiol y 25 (OH) vitamina D.
ResultadosLos grupos de estudio y control difirieron significativamente en términos de NLR, PLR, prolactina y vitamina D (p<0,001, p=0,004, p=0,002, p<0,001 y p<0,001, respectivamente). El índice NLR fue más significativo para determinar la gravedad de la DE (p<0,001). Se observó que la puntuación de la líbido (puntuación total de los ítems 11 y 12 del IIEF) guardó una asociación negativa con prolactina y NLR (p<0,001 y p=0,023, respectivamente), una relación positiva con vitamina D y TT (p<0,001 y p=0,02, respectivamente), y fue inferior en los pacientes con DE grave.
ConclusionesAunque son necesarios más estudios clínicos, creemos que nuestros hallazgos podrían resultar útiles para estas cuestiones controvertidas de la DE.
Erectile dysfunction (ED) is the inability to achieve or maintain erection sufficient for satisfactory sexual performance, affecting up to 52% of men aged 40–70 years.1 Libido is the sexual impulse forming the basis of human life energy and behavior. ED and decreased libido often present as sexual dysfunction in clinical practice.2
Potency is an indicator of cardiovascular system health, and ED appears as an early warning of disorder in this system.3 Chronic low-level inflammation causes endothelial damage and atherosclerosis, causing myocardial infarction, coronary artery disease, and ED.4,5The neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) are recently introduced biomarkers of inflammation based on neutrophil, platelet and lymphocyte counts.6 Despite studies showing that NLR and PLR are associated with cardiac adverse events and ED, and that these values have decreased in impotence patients who respond to treatment, there is still no clear decision about their place in clinical use.5,7–9
In addition to neurogenic and psychogenic factors, hormonal disorders are an important factor causing ED.2 Despite the fact that Total Testosterone (TT), Estradiol (E2), Prolactin and Vitamin D levels have been studied in many studies in the field of andrology, controversial issues remain. Although TT has been shown to have a positive effect on sexual functions in the literature, it has been shown that high levels do not always cause this and there is a threshold and also that the use of testosterone preparations with low, low-normal and normal TT levels has a low effect on ED and a mild effect on libido.10,11 Estradiol is synthesized from testosteron in the adipose tissue with aromatase enzymes in the periphery and works in balance with Gonadotropin – releasing hormone (GnRH) by suppressing the primary production of testosterone by inhibiting GnRH synthesis. It was shown in some studies that Testosterone/Estradiol (T/E2) ratio has no effect on sexual function, while in some publications, decreased estradiol level caused by decreased fat mass has been shown to increase erectile function, especially in obese patients.12 Although it has been shown that increased prolactin levels decrease libido, there is still no clear decision on its association with ED.13 25 (OH) vitamin D levels have been shown to play a role in the regulation of endothelial functions other than bone metabolism, and its deficiency has been shown to cause ED by affecting vascular system health, but it is still unclear about its place in the approach to sexual dysfunction.14
In this study, we aimed to elucidate the etiology of ED, to compare inflammatory and hormonal values and, unlike other studies, to find out how these relate not only to ED, but also to libido and which may be more important.
Materials and methodsThis prospective cohort study was approved by the Ankara Dışkapı Yıldırım Beyazıt Training and Research Hospital ethics committee (No: 62/17). The patients gave a written informed consent according to the Institutional Review Board Guidelines and the Declaration of Helsinki. A total of 152 male patients who presented to our urology clinic between September 2018 and March 2019 and were diagnosed with ED were enrolled in this study as the study group. 101 healthy male patients without sexual dysfunction were included in the study as a control group. Age, history, International Index of Erectile Function (IIEF) scores and libido scores (the total score of IIEF items #11 and #12 [IIEF11, IIEF12]) were recorded for each patient. All patients were ED-treatment naive. Comorbidities were scored using the Charlson Comorbidity Index (CCI). The 152 patients were divided into three groups based on their total IIEF score: (I) severe ED (0–10), (II) mild-moderate ED,11–19 and (III) mild ED.20–25 The three ED groups and the control group were compared in terms of NLR, PLR, and serum levels of total testosterone, estradiol, prolactin, testosterone-to-estradiol ratio, and 25 (OH) vitamin D. Blood samples of the patients were obtained from the antecubital vein between 08:00 and 10:00AM after overnight fasting. Samples were centrifuged at 3000rpm for 10min. The serum was separated and stored at −80°C until analysis. Complete blood count (CBC), total testosterone, estradiol, prolactin and 25 (OH) vitamin D levels were measured using Cobas e 601 modular system (Roche Diagnostics) via chemiluminescence microparticle immunoassay. NLR was calculated as the absolute neutrophil count divided by the absolute lymphocyte count and PLR was calculated as the absolute thrombocyte count divided by the absolute lymphocyte count in complete blood count (CBC) test.
Patients with a history of percutaneous coronary intervention, surgical myocardial revascularization, stable angina pectoris, acute infection, rheumatic diseases, hypertension, diabetes mellitus, dyslipidemia, malignancy, chronic obstructive lung disease, hepatopathy, renal insufficiency, current smoking, abnormal body mass index (BMI) and patients who were receiving vitamin D supplementation were excluded from the study.
Statistical analysisWhether the distributions of continuous variables were normally or not being determined by Kolmogorov–Smirnov test. The assumption of homogeneity of variances was examined by Levene test. Descriptive statistics for continuous variables were expressed as mean±SD or median (25–75th) percentiles, where appropriate.
The differences in continuous variables between healthy controls and patients with ED were compared by Student's t or Mann–Whitney U test, where applicable. While, the mean differences among more than two independent groups were compared by One-Way ANOVA, otherwise, Kruskal–Wallis test was applied for the continuous variables where parametrical test assumptions were not met. When the p-values from the One-Way ANOVA or Kruskal–Wallis test statistics were found as statistically significant to know which group differ from which others by using post hoc Tukey HSD or Dunn–Bonferroni multiple comparison test. Degrees of association between continuous variables were evaluated by Spearman's Rank Correlation analyses.
Receiver operating characteristic (ROC) curve analyses were performed to find the cut-off levels for NLR and PLR as a predictor of diagnosis for ED. Youden's index method was used to find the best cut-off values for NLR and PLR. Sensitivity, specificity, positive and negative predicted values and accuracy levels at the best cut-off values for NLR and PLR were also calculated.
Multiple logistic regression analyses were performed to examine whether NLR or PLR was a statistically significant predictor or not to discriminate controls and patients with ED after adjustment for confounding factors. Any variable whose univariable test had a p value less than 0.10 was accepted as a candidate for the multivariable model along with all variables of known clinical importance. Odds ratios, 95% confidence intervals and Wald statistics for each independent variable was also calculated.
Data were analyzed using SPSS for Windows version 22.0 (SPSS Inc. Co., Chicago, IL, USA). A p-value less than 0.05 was considered as statistically significant.
ResultsWhen the erectile dysfunction patients and the control group were compared it was observed that the mean age was similar in the groups and the median CCI score was higher in the ED group (p<0.001, Table 1). Patients were divided into severe ED (n=60), mild-moderate ED (n=72), and mild ED (n=20) groups. In ED subgroups, it was seen that there were differences in mean age and median CCI scores (p=0.009, p=0.003), respectively, mean age of severe ED group was higher than mild and mild-moderate ED groups, and median CCI score was lower in mild ED group than mild-moderate ED groups (Table 2). There was a negative correlation between age and CCI score and IIEF (p<0.001, Table 3).
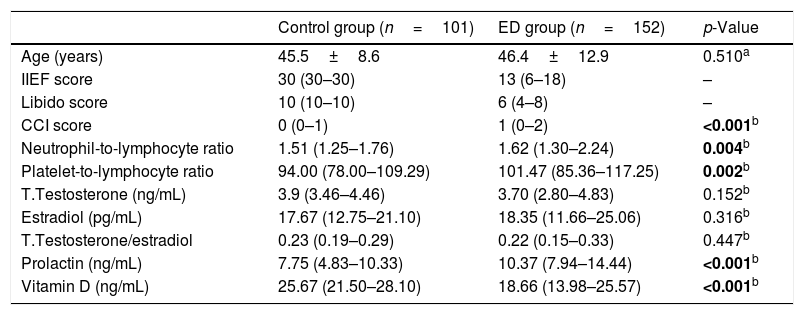
Clinical and laboratory features of controls and patients with erectile dysfunction.
| Control group (n=101) | ED group (n=152) | p-Value | |
|---|---|---|---|
| Age (years) | 45.5±8.6 | 46.4±12.9 | 0.510a |
| IIEF score | 30 (30–30) | 13 (6–18) | – |
| Libido score | 10 (10–10) | 6 (4–8) | – |
| CCI score | 0 (0–1) | 1 (0–2) | <0.001b |
| Neutrophil-to-lymphocyte ratio | 1.51 (1.25–1.76) | 1.62 (1.30–2.24) | 0.004b |
| Platelet-to-lymphocyte ratio | 94.00 (78.00–109.29) | 101.47 (85.36–117.25) | 0.002b |
| T.Testosterone (ng/mL) | 3.9 (3.46–4.46) | 3.70 (2.80–4.83) | 0.152b |
| Estradiol (pg/mL) | 17.67 (12.75–21.10) | 18.35 (11.66–25.06) | 0.316b |
| T.Testosterone/estradiol | 0.23 (0.19–0.29) | 0.22 (0.15–0.33) | 0.447b |
| Prolactin (ng/mL) | 7.75 (4.83–10.33) | 10.37 (7.94–14.44) | <0.001b |
| Vitamin D (ng/mL) | 25.67 (21.50–28.10) | 18.66 (13.98–25.57) | <0.001b |
Charlson Comorbidity Index=(CCI); International Index of Erectile Function=(IIEF).
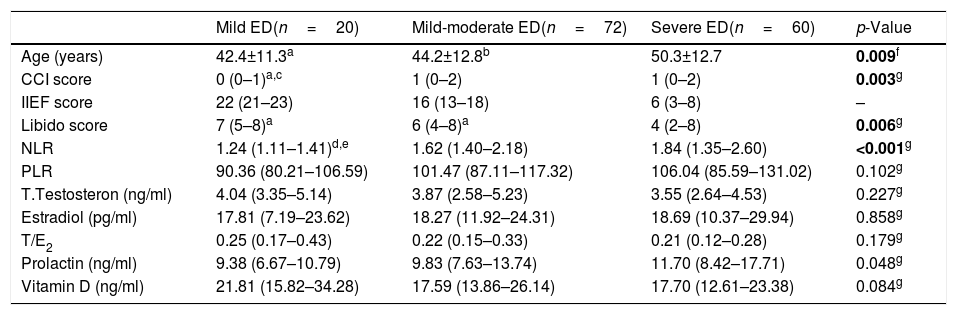
Clinical and laboratory features of patients according to groups.
| Mild ED(n=20) | Mild-moderate ED(n=72) | Severe ED(n=60) | p-Value | |
|---|---|---|---|---|
| Age (years) | 42.4±11.3a | 44.2±12.8b | 50.3±12.7 | 0.009f |
| CCI score | 0 (0–1)a,c | 1 (0–2) | 1 (0–2) | 0.003g |
| IIEF score | 22 (21–23) | 16 (13–18) | 6 (3–8) | – |
| Libido score | 7 (5–8)a | 6 (4–8)a | 4 (2–8) | 0.006g |
| NLR | 1.24 (1.11–1.41)d,e | 1.62 (1.40–2.18) | 1.84 (1.35–2.60) | <0.001g |
| PLR | 90.36 (80.21–106.59) | 101.47 (87.11–117.32) | 106.04 (85.59–131.02) | 0.102g |
| T.Testosteron (ng/ml) | 4.04 (3.35–5.14) | 3.87 (2.58–5.23) | 3.55 (2.64–4.53) | 0.227g |
| Estradiol (pg/ml) | 17.81 (7.19–23.62) | 18.27 (11.92–24.31) | 18.69 (10.37–29.94) | 0.858g |
| T/E2 | 0.25 (0.17–0.43) | 0.22 (0.15–0.33) | 0.21 (0.12–0.28) | 0.179g |
| Prolactin (ng/ml) | 9.38 (6.67–10.79) | 9.83 (7.63–13.74) | 11.70 (8.42–17.71) | 0.048g |
| Vitamin D (ng/ml) | 21.81 (15.82–34.28) | 17.59 (13.86–26.14) | 17.70 (12.61–23.38) | 0.084g |
Charlson Comorbidity Index=(CCI); International Index of Erectile Function=(IIEF);Erectile Dysfunction=(ED); Neutrophil-to-lymphocyte ratio=(NLR); Platelet-to-lymphocyte ratio=(PLR); Total Testosterone/Estradiol ratio=(T/E2).
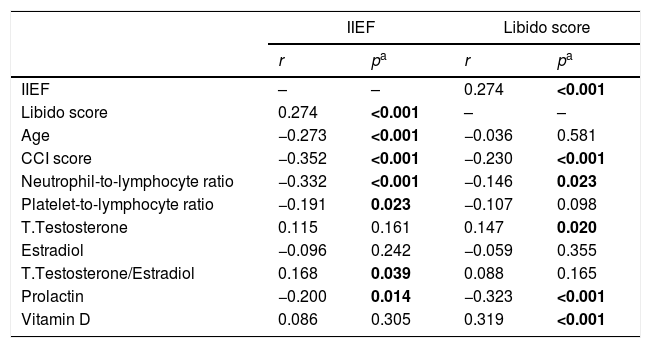
The results of correlation analyses.
| IIEF | Libido score | |||
|---|---|---|---|---|
| r | pa | r | pa | |
| IIEF | – | – | 0.274 | <0.001 |
| Libido score | 0.274 | <0.001 | – | – |
| Age | −0.273 | <0.001 | −0.036 | 0.581 |
| CCI score | −0.352 | <0.001 | −0.230 | <0.001 |
| Neutrophil-to-lymphocyte ratio | −0.332 | <0.001 | −0.146 | 0.023 |
| Platelet-to-lymphocyte ratio | −0.191 | 0.023 | −0.107 | 0.098 |
| T.Testosterone | 0.115 | 0.161 | 0.147 | 0.020 |
| Estradiol | −0.096 | 0.242 | −0.059 | 0.355 |
| T.Testosterone/Estradiol | 0.168 | 0.039 | 0.088 | 0.165 |
| Prolactin | −0.200 | 0.014 | −0.323 | <0.001 |
| Vitamin D | 0.086 | 0.305 | 0.319 | <0.001 |
r: Coefficient of correlation.
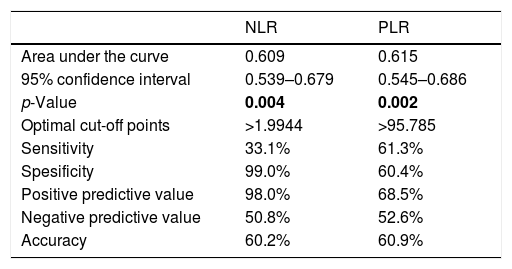
Median NLR and PLR were higher in the ED group when compared to the control group (p=0.004, p=0.002, respectively, Table 1). There was a difference between ED subgroups in terms of median NLR (p<0.001). Median NLR was lower in the mild ED group when compared to the other groups (p<0.001). It was seen that there was no difference between the groups in terms of median PLR (Table 2). There was a negative correlation between the IIEF score and NLR and PLR, respectively (Table 3). The optimal cut-off point for NLR and PLR in separating the control group and ED group was 1.9944 (AUC=0.609, 95% CI: 0.539–0.679 and p=0.004), 95.785 (AUC=0.615, 95% CI: 0.545–0.686 and p=0.002) respectively (Table 4).
The results of ROC analyses.
| NLR | PLR | |
|---|---|---|
| Area under the curve | 0.609 | 0.615 |
| 95% confidence interval | 0.539–0.679 | 0.545–0.686 |
| p-Value | 0.004 | 0.002 |
| Optimal cut-off points | >1.9944 | >95.785 |
| Sensitivity | 33.1% | 61.3% |
| Spesificity | 99.0% | 60.4% |
| Positive predictive value | 98.0% | 68.5% |
| Negative predictive value | 50.8% | 52.6% |
| Accuracy | 60.2% | 60.9% |
NLR: neutrophil-to-lymphocyte ratio, PLR: platelet-to-lymphocyte ratio.
When the ED – control group and ED subgroups were compared within themselves, no difference was found in terms of median TT, T/E2, E2 (Tables 1 and 2). Median prolactin levels were higher in the ED group than in the control group (p<0.001, Table 1), but there was no difference between the ED subgroups (Table 2). Median vitamin D levels were lower in the ED group than in the control group (p<0.001, Table 1), but there was no difference between the ED subgroups (Table 2). The IIEF score was found to have a positive relationship with T/E2 and a negative relationship with prolactin, respectively (Table 3).
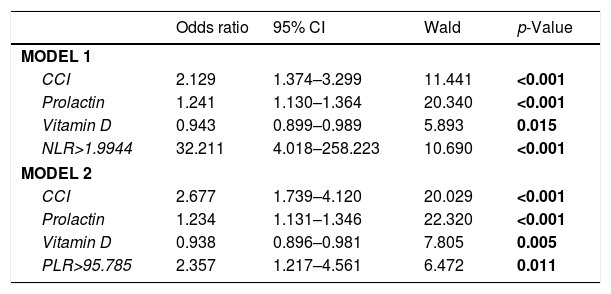
Multivariate logistic regression analysis was used to determine whether NLR was a statistically significant predictor independent of other factors in distinguishing the control group and the ED group. In model 1, when the correction was made according to other factors, the probability of ED continued to increase statistically in cases with NLR level (according to the optimal cut-off point obtained from ROC analysis) higher than 1.9944 compared to cases with NLR level lower than 1.9944, [OR=32.211, 95% CI: 4.018–258.223 and p<0.001]. Regardless of other factors, the possibility of ED increased statistically significantly, as prolactin levels increased [OR=1.241, 95% CI: 1.130–1.364 and p<0.001]. Each 1-point increase in CCI score increased the probability of ED 2.129 times (95% CI: 1.374–3.299), independent of other factors (p<0.001). However, as the Vit D level increased, the possibility of ED declined independently from other factors [OR=0.943, 95% CI: 0.899–0.989 and p=0.015] (Table 5).
The results of multiple logistic regression analyses.
| Odds ratio | 95% CI | Wald | p-Value | |
|---|---|---|---|---|
| MODEL 1 | ||||
| CCI | 2.129 | 1.374–3.299 | 11.441 | <0.001 |
| Prolactin | 1.241 | 1.130–1.364 | 20.340 | <0.001 |
| Vitamin D | 0.943 | 0.899–0.989 | 5.893 | 0.015 |
| NLR>1.9944 | 32.211 | 4.018–258.223 | 10.690 | <0.001 |
| MODEL 2 | ||||
| CCI | 2.677 | 1.739–4.120 | 20.029 | <0.001 |
| Prolactin | 1.234 | 1.131–1.346 | 22.320 | <0.001 |
| Vitamin D | 0.938 | 0.896–0.981 | 7.805 | 0.005 |
| PLR>95.785 | 2.357 | 1.217–4.561 | 6.472 | 0.011 |
NLR: neutrophil-to-lymphocyte ratio, PLR: platelet-to-lymphocyte ratio, CI: confidence interval.
Multivariate logistic regression analysis was used to determine whether PLR was a statistically significant predictor independent of other factors in distinguishing the control group and the ED group. In model 2, when the correction was made according to other factors, the probability of ED continued to increase statistically in cases with PLR level (according to the optimal cut-off point obtained from ROC analysis) higher than 95.785 compared to cases with PLR level lower than 95.785, [OR=2.357, 95% CI: 1.217–4.561 ve p=0.005]. Regardless of other factors, the possibility of ED increased statistically significantly, as prolactin levels increased [OR=1.234, 95% CI: 1.131–1.346 and p<0.001]. Each 1-point increase in CCI score increased the probability of ED 2.677 times (95% CI: 1.739–4.120), independent of other factors (p<0.001). However, as the Vit D level increased, the possibility of ED declined independently from other factors [OR=0.938, 95% CI: 0.896–0.981 and p=0.011] (Table 5).
The median libido score was lower in the severe ED group when compared to the mild and mild-moderate ED group (Table 2). Libido was observed to be positively correlated with IIEF score and negatively correlated with CCI score (p<0.001). There was a negative correlation between the inflammatory parameters NLR and PLR and libido. Libido was found to be positively related to TT and vitamin D, respectively, and negatively to prolactin (Table 3).
DiscussionIn our study, we investigated the effects of age and comorbidities on the sexual health, and parallel to the negative correlation between age and CCI score and IIEF in ED subgroups (p<0.001), we found that these values were higher in severe ED group (p<0.05). When we examined the effects on libido, we found that comorbidities decreased libido (p<0.001) and parallel to the relationship with IIEF (p<0.001), we found that libido was lower in the severe ED group (p<0.05). It has been shown in the literature that ED is generally seen after 40 years of age and can be seen in young men with a rate as low as 1–10%, and is associated with diseases such as diabetes mellitus, hypertension, hyperlipidemia, metabolic syndrome and depression.2,15 A previous study evaluated two components of sexual dysfunction, ED and decreased libido, and found that the improvement of symptoms led to the improvement of libido.16 In our study, in addition to these data in the literature, we compared these effects in ED subgroups, as well as between case and control groups.
Although the increase in total testosterone level was positively correlated with libido and the increase in testosterone/estradiol ratio with ED, no difference was observed in terms of median TT, E2 and T/E2 in the comparisons of case-control groups and ED subgroups (p>0.05). Relationship between total testosterone levels and sexual dysfunction has been investigated in numerous studies. The studies indicated that reduced total testosterone levels were correlated with decreased sexual desire, and ED was improved by testosterone replacement monotherapy particularly in patients with mild ED.16 A previous systematic review evaluating a total of 14 randomized controlled studies reported that testosterone regulates both nitric oxide (NO) and PDE5 levels, men with hypogonadism may have a partial deficiency of the PDE5 enzyme, and the achievement of normal levels of the PDE5 enzyme during testosterone therapy is highly important.17 On the other hand, the effects of the T/E2 ratio have also been investigated in the literature. A previous study evaluated a total of 230 patients with a mean age of 66.32±8.17 years and reported that ED and decreased libido were present in 60.9% and 46.5% of the patients, respectively. The authors also noted that age exhibited a positive correlation with both of these parameters while the T/E2 ratio had no effect on sexual dysfunction.18 Another retrospective study evaluated a total of 878 male patients and reported that the ED patients, compared to healthy controls, had higher estradiol levels (p<0.001) and a higher estradiol/testosterone ratio (7.45±3.09×10 (−3); p<0.001).19 Meaningfully, the presence of contradictory findings in the literature implicates that further prospective studies are needed.
When we look at the prolactin levels, although a negative correlation between IIEF score and libido and prolactin, which were higher in our case group compared to the control group, were observed, it was also seen that the values of the ED subgroups were similar. Literature indicates that increased prolactin has a negative impact on sexual desire as it inhibits the secretion of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH). Additionally, increased prolactin has also been shown to cause ED by inhibiting smooth muscle relaxation of the penile corpus cavernosum and reducing testosterone concentrations.20 Interestingly, a recent study revealed that hyperprolactinemia was associated with arteriogenic ED as shown by color Doppler ultrasound and the males with moderate or severe ED had lower prolactin levels.13 The European Male Ageing Study (EMAS) evaluated a total of 2948 males aged 40–79 years reported that the patients with low prolactin levels had poor CSF (change in sexual functioning) scores.21 On the other hand, both reduced and elevated prolactin levels are known to have a negative impact on sexual function and therefore are worthy of further investigation.
Cardiovascular diseases and ED share the same risk factors.22 ED is considered as a warning sign of early cardiovascular diseases.23 Moreover, hypovitaminosis D is considered to cause endothelial dysfunction, thereby leading to increased risk of atherosclerotic cardiovascular disease.24 In our study, our patients were selected from among patients with no history of cardiovascular disease and it was seen that vitamin D levels were lower in ED patients than in the control group (p<0.001), but no difference was observed between ED subgroups. However, correlation analysis showed a positive effect with libido (p<0.001). A previous study evaluated 143 ED patients and reported that vitamin D deficiency (<20ng/mL) was present in 45.9% of the patients, vitamin D levels were lower in patients with severe ED compared to patients with mild ED (p=0.02), and patients with arteriogenic ED had lower Vitamin D levels compared to patients with non-arteriogenic ED as assessed by penile Doppler ultrasound.14 Another study evaluated 3390 male patients and revealed the incidence of 25(OH) vitamin D deficiency and ED as 30% and 15.2%, respectively. Moreover, the 25(OH) vitamin D levels were lower in the ED patients compared to non-ED individuals.24 We think that studies examining the effect of vitamin D replacement therapies on clinical improvement in patients with erectile dysfunction will be useful in literature to demonstrate the importance of our knowledge of this premedication period.
In our study, NLR and PLR values, which we examined as an indicator of systemic inflammation, were found to be higher in ED patients compared to the control group. NLR was found to be effective in determining ED groups and also, it was found to have a negative relationship with libido. Numerous studies reporting on the use of NLR and PLR have indicated that systemic inflammation results in vascular damage and atherosclerosis, and some other studies have investigated the effectivity of these two parameters in ED patients as well.4,25 A previous study evaluated a patient group of 101 male ED patients and a control group of 31 sexually active men and reported that the NLR, PLR, and CRP levels were significantly higher in the patient group compared to the control group, the NLR, PLR, and CRP levels established a negative correlation with the IIEF-5 scores, PLR was found to be independent predictor for ED, and both NLR and PLR could serve as practical parameters.5 Another study suggested that NLR can be an independent predictor for ED (OR [CI] 2.43 [1.06; 5.63]).26 Based on these findings, it is tempting to consider that NLR and PLR, which are inexpensive and easily calculable parameters, can be promising in the clinical approach to vascular wellbeing and, hence, to ED as well.
The limitations of our study include the lack of hormones such as DHEA-S, 17a Hydroxyprogesterone, androstendione, and CRP as an inflammation marker, as well as the fact that most of our patients had Vit D deficiency and that the number of patients participating in the study was low.
ConclusionSexual dysfunction is associated with numerous factors including age, comorbidities, systemic inflammation, and abnormal hormone profile. Our results indicated that older age, increased prevalence of comorbidities led to lower IIEF scores and these scores were higher in patients with severe ED compared to patients with mild and mild-moderate ED. While NLR and PLR values may be practical markers in determining patient groups, we think that NLR may be more effective when evaluating ED severity and libido. We found that total testosterone, estradiol and testosterone/estradiol ratio had no effect on determining the patient group and the severity of ED, but total testosterone may have a positive relationship with libido. We found that prolactin was higher in the patient group and had a negative effect on libido, whereas vitamin D had a lower and positive effect on the libido in the patient group. Although more clinical studies are needed, we think that our results may be useful in approaching sexual dysfunction.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThe authors have no conflict of interest to disclose.