Carpolobia lutea root extract (CLRE) has been reported to enhance penile erection. However, the mechanism involved is poorly understood. We investigated in vitro mechanisms of CLRE action on contractile activity of rabbit corpus cavernosum (CC).
MethodsCorpus cavernosum strips from four healthy male New Zealand rabbits (2.5–3.0kg) were mounted on an organ chamber and contracted with phenylephrine (PE) (10−9 to 10−5M) and Potassium Chloride (KCl) (10–50mM) before treatment with various concentrations of CLRE (0.1–1.2mg/ml). Interactions between CLRE and a Nitric Oxide Synthase (NOS) inhibitor (N-nitro-l-arginine methyl ester – l-NAME 10−4M); guanylyl cyclase inhibitors (Oxalodiazolo 4,3-a quinoxalin-1-one – ODQ 10μM, 20μM, 30μM), and (methylene blue 10–30μM); a cyclooxygenase inhibitor (10−4M indomethacin); potassium-channel inhibitors (100μM tetraethyl ammonium TEA), (100ηM apamin) and (glibenclamide 10μM and 20μM); and a calcium-channel inhibitor (−10−4M nifedipine) were investigated.
ResultsMaximal contractions of KCl and PE contracted CC strips were significantly reduced in a concentration-dependent manner (40.8±3.6% and 38.6±4.0% from 64.6±2.9% and 98.1±4.2% respectively). Relaxant effect of CLRE was significantly reduced by ODQ (38.6±4.0% to 6.4±1.3% and 38.6±4.0% to 7.2±1.2%), nifedipine (38.6±4.0% to 21.1±2.7%) and glibenclamide (40.8±3.6% to 31.5±3.3%). However l-NAME, indomethacin, methylene blue, TEA and apamin did not inhibit relaxation by CLRE.
ConclusionConcentration-dependent relaxant effect of CLRE in rabbit CC involves the soluble guanylate cyclase/cyclase Guanosine Monophosphate system, and activation of ATP-dependent K+ channels.
Se ha informado que el extracto de raíz de Carpolobia lutea (CLRE) mejora la erección del pene. Sin embargo, el mecanismo involucrado es poco conocido. Investigamos mecanismos in vitro de acción CLRE sobre la actividad contráctil del cuerpo cavernoso de conejo (CC).
MétodosSe montaron tiras de cuerpo cavernoso de 4 conejos sanos de Nueva Zelanda machos (2,5-3kg) en una cámara de órganos, y se contrajeron con fenilefrina (PE) (10 −9 −10 −5M) y cloruro de potasio (KCl) (10-50mM) antes del tratamiento con diversas concentraciones de CLRE (0,1-1,2mg/ML). Se observaron interacciones entre CLRE y un inhibidor de la sintasa de óxido nítrico (NOS) (éster metílico de N-nitro-L-arginina –L-NAME 10 -4M); inhibidores de la guanilil ciclasa (oxadiazol 4,3-a quinoxalin-1-ona -ODQ 10, 20, 30μM) y (azul de metileno 10-30μM); un inhibidor de la ciclooxigenasa (indometacina 10-4M); inhibidores del canal de potasio (TEA de tetraetilamonio 100μM), (apamina 100ηM) y (glibenclamida 10 y 20μM), y se investigó un inhibidor del canal de calcio (−10 −4M nifedipina).
ResultadosLas contracciones máximas de las tiras CC contraídas con KCl y PE se redujeron significativamente de una manera dependiente de la concentración (40,8±3,6% y 38,6±4% de 64,6±2,9% y 98,1±4,2%, respectivamente). El efecto relajante de CLRE se redujo significativamente por ODQ (38,6±4% a 6,4±1,3% y 38,6±4% a 7,2±1,2%), nifedipina (38,6±4% a 21,1±2,7%) y glibenclamida (40,8±3,6% a 31,5±3,3%). Sin embargo, l-NAME, indometacina, azul de metileno, TEA y apamina no inhibieron la relajación por CLRE.
ConclusiónEl efecto relajante dependiente de la concentración de CLRE en CC de conejo implica el sistema de guanilato ciclasa/ciclasa guanosina monofosfato soluble y la activación de los canales de K+ dependientes de ATP.
Erectile dysfunction (ED) is the inability to achieve and sustain an erection sufficient for satisfactory sexual performance.1 ED is a common problem and it occurs in up to 40–52% of men aged 40 years and above.2 It has long been postulated that men with erectile dysfunction will rise to 322 million by the year 2025.3 Sildenafil, a phosphodiesterase type 5 (PDE-5) inhibitor is recommended as a first-line therapy for ED of varying etiology and severity.4,5 Although many drugs are now available for treating ED, finding a new drug for treating ED and understanding its mechanism of action is still important as patients are often dissatisfied with the available therapies due to their associated high cost, adverse effects and at times lack of efficacy.6
Many plant extracts are traditionally used to improve sexual performances.7,8 In this regard, the World Health Organization (WHO) has estimated that about eighty percent of the world populations rely chiefly on traditional medicines.9 People suffering from ED have continued to explore medicinal plant as alternative therapy due to its accessibility and affordability.10
In Africa, several indigenous plants with medicinal potentials have been used in the treatment of various ailments. Carpolobia lutea G.Don (family polygalaceae) is a small tree growing up to 15ft in height.11 It is an important local plant widely used in Western and Southern Nigeria as a bedtime chewing sticks. It is reputed locally as “ogunaleko” meaning “sexual invigorator” or “aphrodisiac”.12,13
We have previously reported on the comparative aphrodisiac efficacy of CLRE with sildenafil in vivo in male rabbits.14 Although Carpolobia lutea is widely acknowledged in Nigeria for its aphrodisiac potentials, its effectiveness as well as possible mechanism of action in erectile tissue contractile activity is poorly understood.
The present study was designed to investigate the effects of methanolic extract of Carpolobia lutea on erectile function of corpus cavernosum smooth muscle in vitro. The interactions between Carpolobia lutea root extract with a Nitric Oxide Synthase (NOS) inhibitor: (N-nitro-l-arginine methyl ester – l-NAME), guanylyl cyclase inhibitors: (Oxalodiazolo(4,3-a) quinoxalin-1-one – ODQ and methylene blue), Potassium channel inhibitors: (tetraethyl ammonium TEA-, a voltage operated K+ channel blocker, apamin, a small conductance Ca2+-activated K+ channel blocker and glibenclamide an ATP-sensitive K+ channel blocker), indomethacin: a non-selective inhibitor of prostacyclin production and nifedipine: a voltage-gated Ca2+ channel blocker were all investigated.
Materials and methodsProcurement of Carpolobia lutea plant rootFresh roots ofCarpolobia lutea were collected from Bodija market, Oyo State, Nigeria, in December 2014. The plant was identified and authenticated at the Department of Botany University of Ibadan. The plant was assigned voucher number FHI 109755 at the Forestry Research Institute of Nigeria, Ibadan.
Preparation of Carpolobia lutea root extractCLRE was prepared using methanol. Carpolobia lutea root was washed, cut into pieces and oven dried at 40°C to a relatively constant dry weight. 4kg of the oven dried, pulverized sample was soaked in 15L of absolute methanol in a glass bowl at room temperature for 72h. It was then filtered with Wattmann filter paper. The filtrate was concentrated in a rotary evaporator at 40°C15 to yield 105.2g of methanol extract (brown oily substance)m(2.63% yield) which was stored in a refrigerator at −4°C.
Preparation of animalsFour healthy adult male (4–6 months) New Zealand rabbits weighing 2.5–3.0kg were used. The animals were acclimatized for two weeks and maintained under conventional laboratory condition of temperature (20°C) humidity (70%) and 12h light/dark period. Free access was allowed for food and water ad libitum. The study was conducted in compliance with the tenets of the National Institute of Health (NIH) guide on the use of animals in research.
Drugs and chemicals usedN-nitro-l-arginine methyl ester (l-NAME), phenylephrine, 1H-[1,2,4] Oxalodiazolo[4,3-a]quinoxalin-1-one (ODQ), methylene blue, glucose, Potassium chloride, Calcium chloride, were purchased from Tocris online scientific store, United Kingdom. All chemicals were of highest analytical grade commercially available.
Preparation of the corpus cavernosum tissue stripsThe male rabbits were anesthetized with an intravenous injection of pentobarbitone (30mg/kg) before they were sacrificed by exsanguinations.16 The penis was surgically removed en bloc, with care being taken to keep the tunica albunigea intact and the harvested tissue was placed in a petri dish containing physiological salt solution (PSS). Then, the corpus cavernosal tissue was isolated and carefully dissected from the surrounding tunica albuginea. Two cavernosal strips were taken from each animal. Each corpus cavernosum was suspended in a 50ml chamber of the organ bath containing Kreb's buffer solution with the following composition (m/mol): NaCl (119.0), KCl (4.7), KHPO4 (1.2), MgSO4 (1.2), NaHCO3 (15.0), CaCl2 (1.6) and glucose (11.5). The temperature of the organ tissue bath was maintained at 37°C, and the solution was bubbled with a 95% O2+5% CO2 gas mixtures (pH 7.35–7.40). The strips were suspended with silk tied to a force transducer (model 7004; Ugo Basile, Varese, Italy) connected to a data capsule acquisition system model 17400 which was connected to a computer for rapid data recording and display of simultaneous contractile and relaxation responses. An initial tension of 2g was applied to all corpus cavernosum strips. This level of initial tension produces maximal active contractions in corpus cavernosum smooth muscle stimulated with phenylephrine (PE) or a depolarizing solution.17 An equilibrium period of 60–90min was allowed before the start of the experiments. During this time, it was stimulated three times with 10−7M PE for 5min at 30-min intervals.16
Dose responses of corpus cavernosum strips to CLRE in PE or KClThe corpus cavernosum strips were exposed to cumulative concentrations of PE (10−9–10−5M) after incubation with the vehicle (0.2ml distilled water) for 15min. The tissues were then washed by flushing with Krebs-Henseleit physiological solution 3 times at 15min interval, until the tension level came to the initial resting level. After adequate rest the tissues were incubated with CLRE (0.8mg/ml) for 15min, and the concentration–response to PE was repeated in the presence of the extract. The same procedure was repeated for the concentration–response to KCl (10–50mM) with and without CLRE by using different sets of corpus cavernosum strips.
Determination of relaxant effect of CLRE on Nitric Oxide (NO) soluble guanylate cyclase (sGC) system in PE-induced contractionsThe relative responses of CLRE to l-NAME, ODQ and Methyl blue were carried out. The corpus cavernosum strips were incubated for 15min in l-NAME (10−4M) to block nitric oxide (NO) or ODQ (10μM, 20μM and 30μM) and Methylene blue (10μM and 30μM) to block soluble guanylate cyclase (sGC)-mediated component of the response to CLRE. After 15min incubation period, the strips were precontracted with 10−7M PE, after which the extract 0.1–1.2mg/ml were added cumulatively.
Determination of K+ channel inhibitors role in the relaxant effect of CLREIn these experiments, the relaxative effect of CLRE in the presence of TEA, apamin and glibenclamide were investigated. The corpus cavernosum strips were incubated with TEA (100μM), Apamin (100ηm) and glibenclamide (10μM and 20μM) to block potassium channels for 15min. After the 15-min incubation period, the strips were pre-contracted with 50mM KCl, after which cumulative doses of extract (0.1–1.2mg/ml) were added.
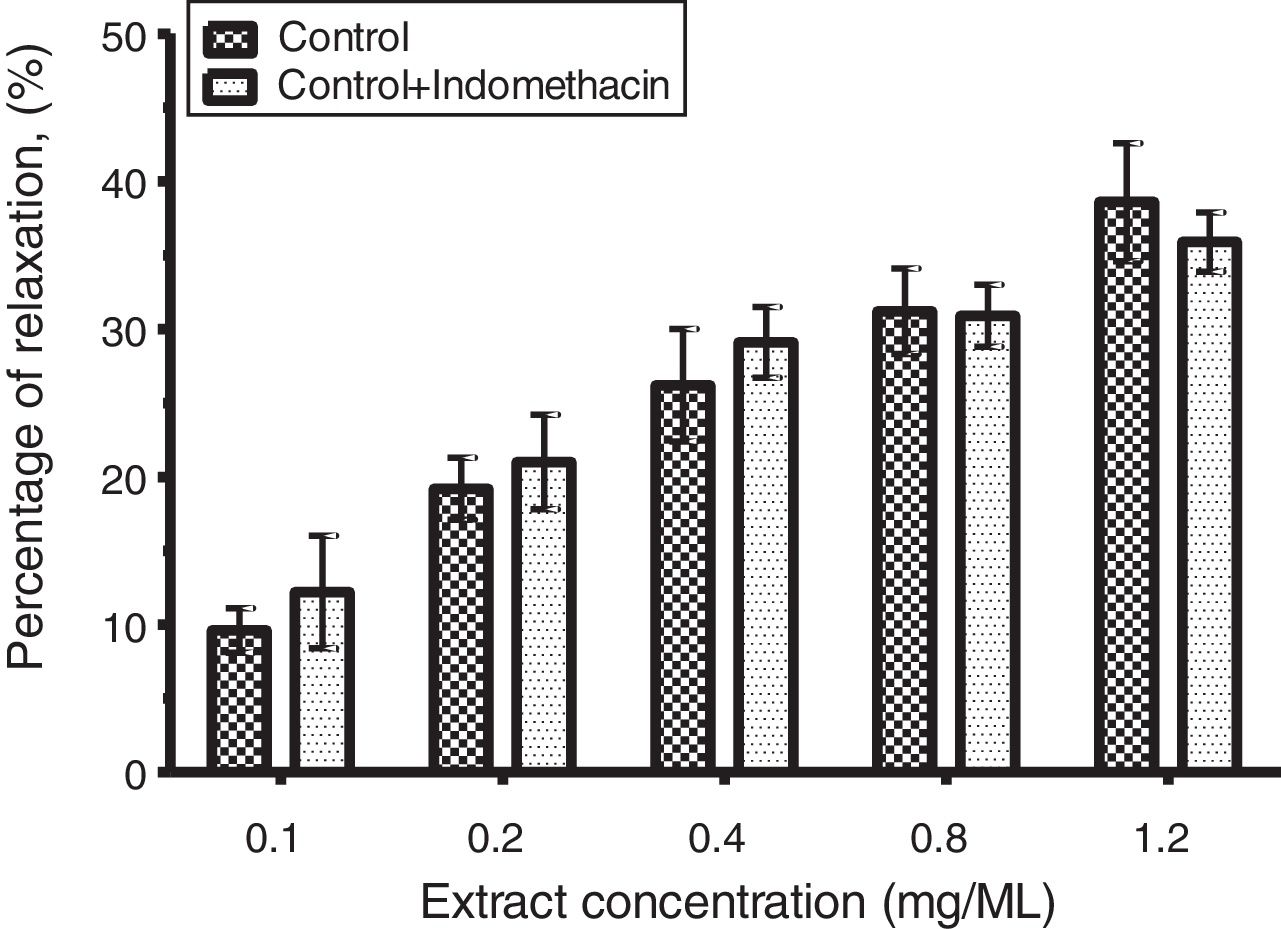
Determination of prostacyclin role in CLRE-induced corpus cavernosum strips relaxationThe relaxative response of CLRE was investigated by incubating the corpus cavernosum strip with indomethacin (10−4 M); a nonspecific cyclooxygenase inhibitor to verify the involvement of prostacyclin on CLRE-induced corpus cavernosum smooth muscle strip relaxation.
Determination of Ca2+ channel activity in CLRE-induced corpus cavernosum relaxationTo verify the involvement of Ca2+ channels on CLRE-induced corpus cavernosum strip relaxation, corpus cavernosum strip was treated with 10−4M nifedipine, a voltage-gated Ca2+ channel blocker before precontracting with 10−7M of phenylephrine.
Statistical analysisThe results were analyzed using one-way analysis of variance followed by Student–Newman–Keuls post hoc test at α0.05. Values were expressed as Mean±SEM. In all cases results with a value of p<0.05 was considered to indicate statistical difference.
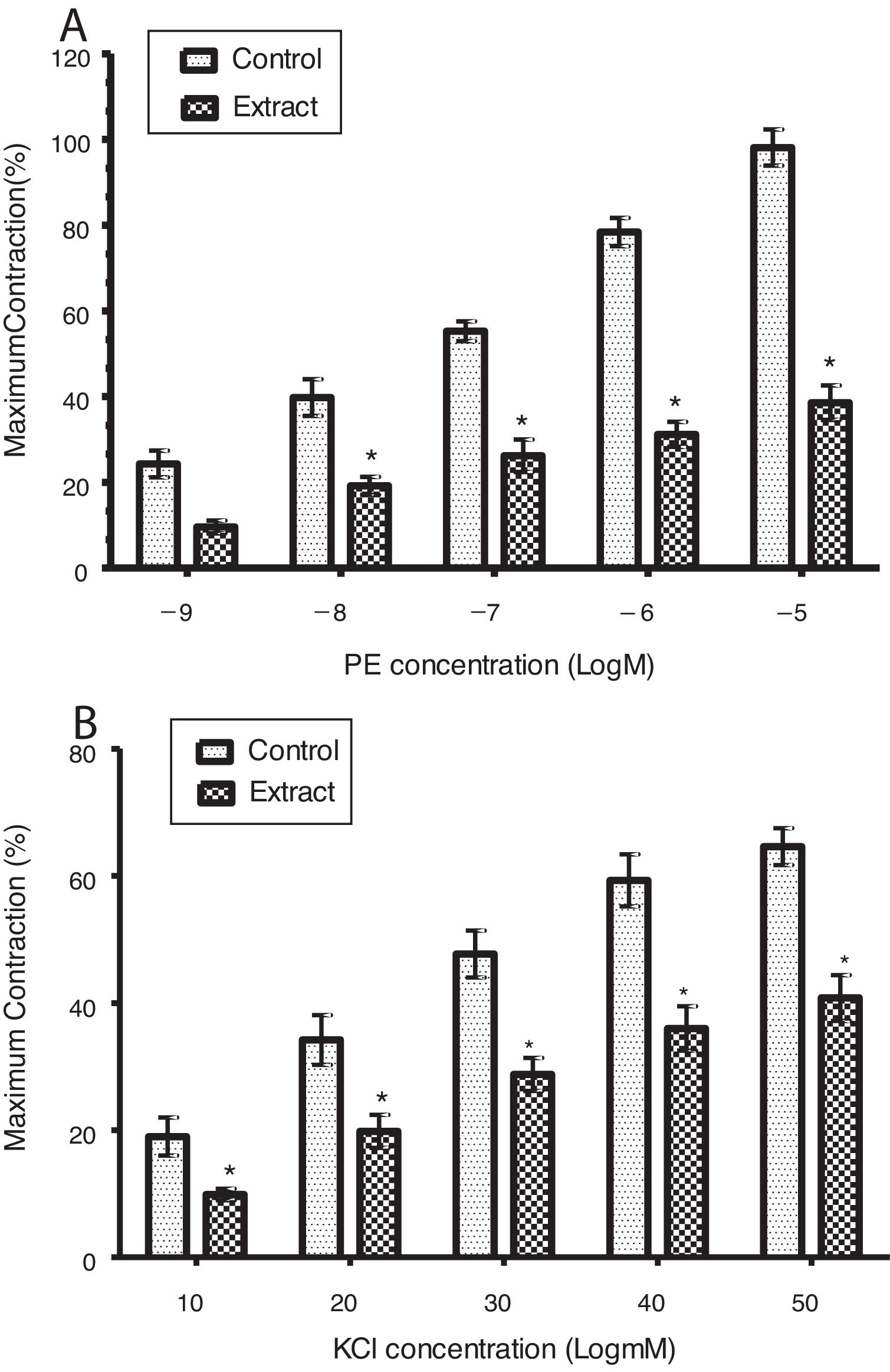
ResultsEffect of CLRE on contractile responses to PE and KClIn PE contracted corpus cavernosum strips, a significant and concentration-dependent inhibition of contraction was induced by CLRE (0.8mg/ml) as shown in Fig. 1A (p<0.05). Similarly, CLRE also produced a significant and concentration-dependent reduction in KCl induced contraction (Fig. 1B). Maximal contractions of KCl and PE contracted strips were significantly reduced to 40.8±3.6% and 38.6±4.0% from 64.6±2.9% and 98.1±4.2% respectively.
(A) Concentration–response for corpus cavernosum strips contracted with PE (10−9–10−5M) in the presence and absence of CLRE (0.8mg/ml). Each column represents mean±SEM. n=8, (*p<0.05). (B) Concentration response to KCl (10–50mM) in corpus cavernosum strips in the presence and absences of CLRE (0.8mg/ml). Results are expressed as mean±SEM, n=8, (*p<0.05).
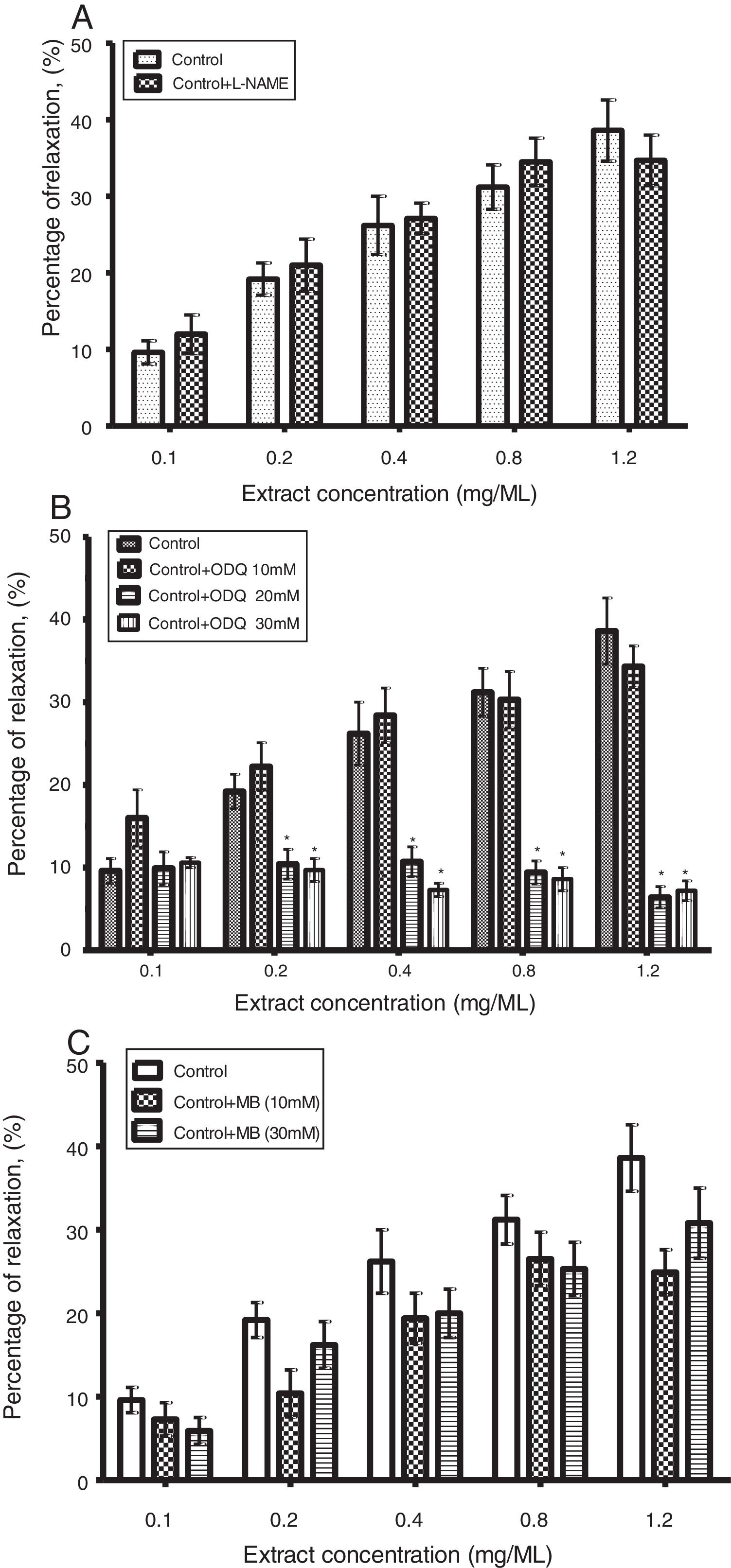
As shown in Fig. 2B, the relaxant effect of CLRE (0.1–1.2mg/ml) on PE pre-contracted (10−7M) strips was significantly (p<0.05) reduced by ODQ (20 and 30μM) from 38.6±4.0% to 6.4±1.3% and 38.6±4.0% to 7.2±1.2%. However, the response of corpus cavernosum strips to CLRE (0.1–1.2mg/ml) was not significantly reduced by pretreatment of l-NAME (10−4M) (Fig. 2A) and methylene blue (10μM and 30μM) (Fig. 2C).
(A) Effect of l-NAME (10−4M) on concentration response to CLRE (0.1–1.2mg/ml) in corpus cavernosum strip pre-contracted with Phenylephrine (10−7M). Each column represents mean±SEM, n=8. (B) Effect of ODQ (10μM, 20μM, and 30μM) on cumulative concentration–response to CLRE (0.1–1.2mg/ml) in corpus cavernosum strip pre-contracted with PE (10−7M). n=8, (*p<0.05). (C) Effect of methylene blue (10μM and 30μM) on cumulative concentration–response of CLRE (0.1–1.2mg/ml) in corpus cavernosum strip precontracted with Phenylephrine (10−7M). Each column represents mean±SEM, n=8.
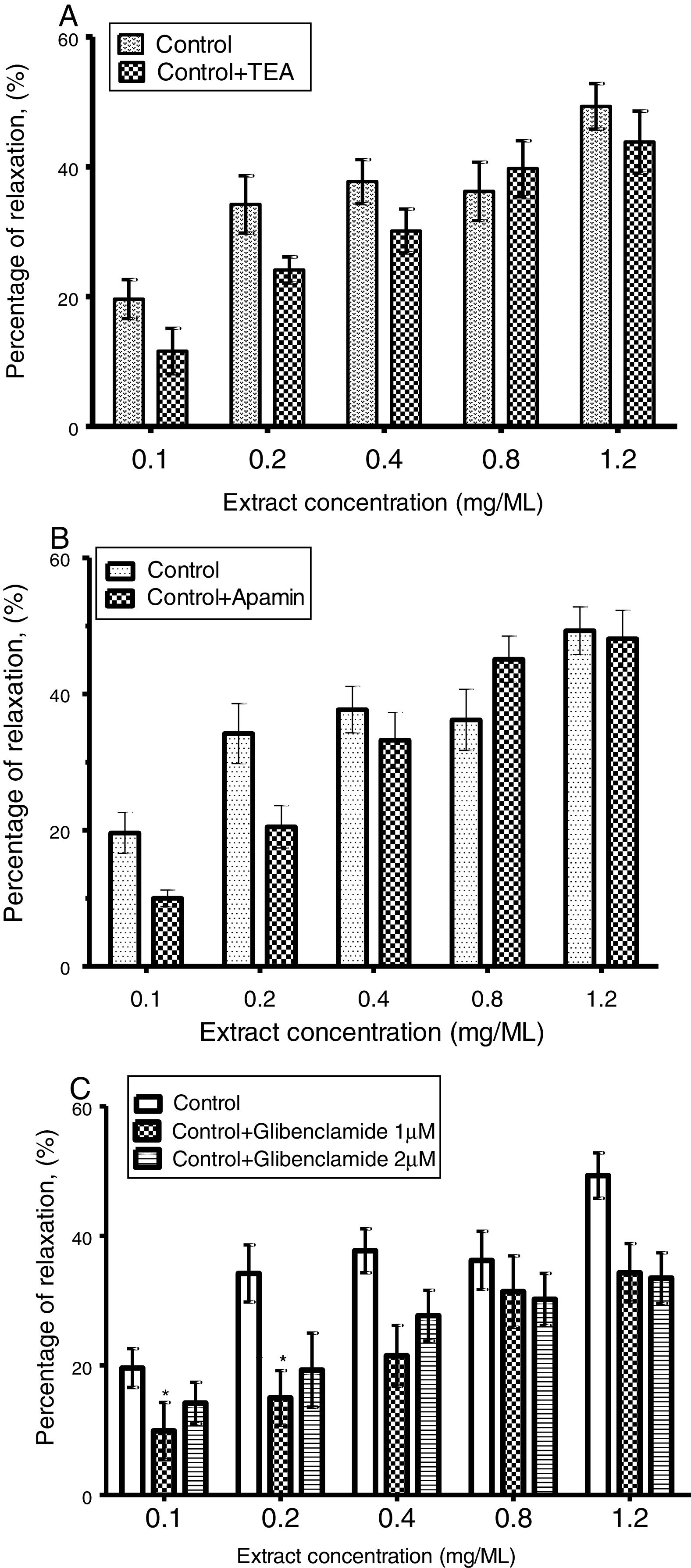
CLRE induced cavernous smooth muscle strip relaxation was significantly attenuated by 10μM glibenclamide from 40.8±3.6% to 31.5±3.3% (Fig. 3C). However, 100μM TEA (Fig. 3A) and 100ηM apamin (Fig. 3B) had no such effect.
(A) Cumulative concentration response of corpus cavernosum strip treated with TEA (100μM) and precontracted with KCl (50mM) in the absence and presence of CLRE (0.1–1.2mg/ml). Each point represents mean±SEM (n=8). (B) Effects of Apamin (100nM) on cumulative concentration–response of CLRE (0.1–1.2mg/ml) in corpus cavernosum strip precontracted with KCl (50mM). Each point represents mean±SEM, n=8. (C) Effects of glibenclamide (10μM and 20μM) on cumulative concentration–response of CLRE (0.1–1.2mg/ml) in corpus cavernosum strip pre-contracted with KCl (50mM). Each point represents mean±SEM. n=8, (*p<0.05).
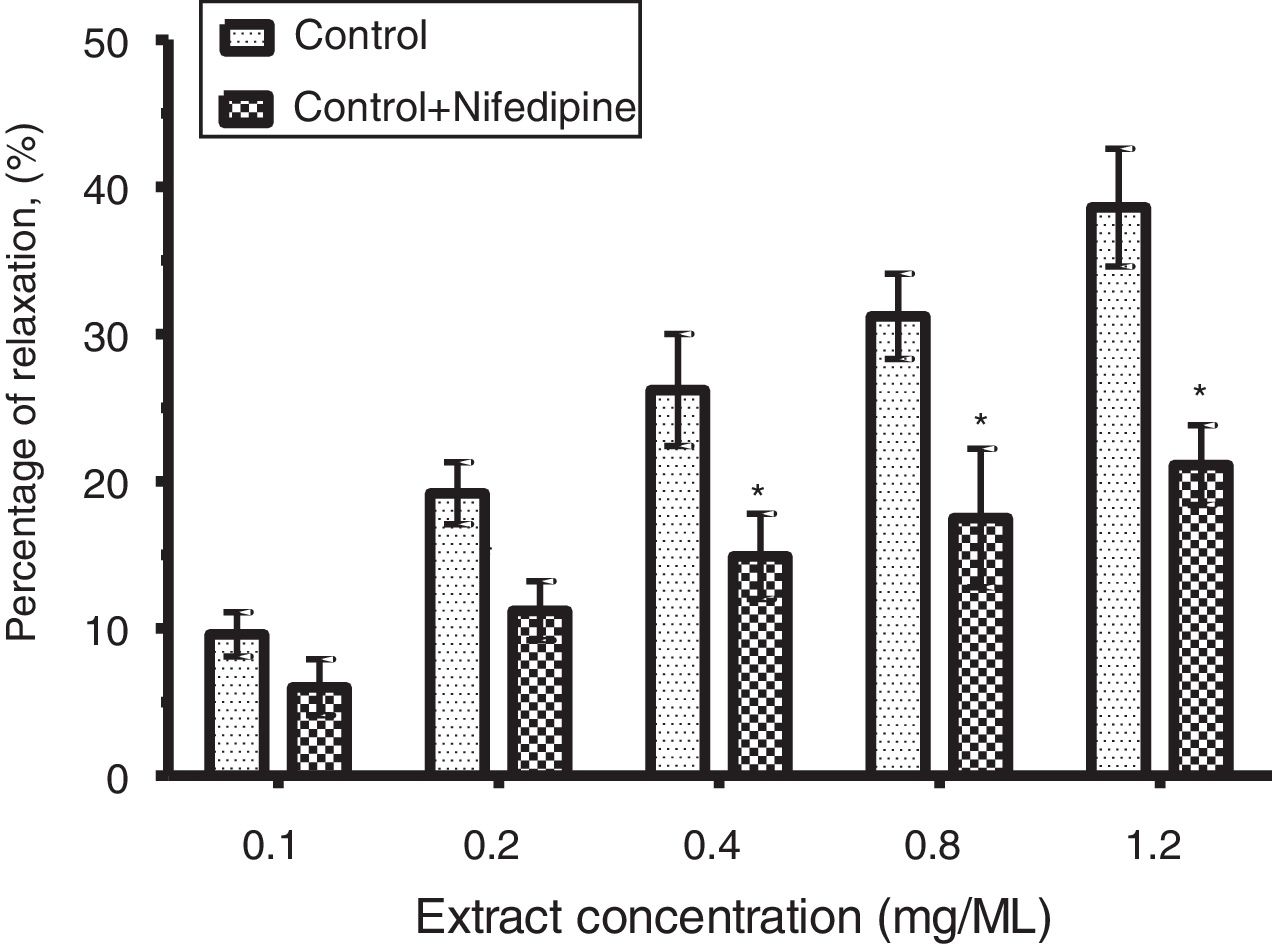
The relaxant effect of CLRE on PE-precontracted (10−7M) corpus cavernosum strip was significantly (p<0.05) reduced by nifedipine from 38.6±4.0% to 21.1±2.7% (Fig. 4).
Effects of prostacyclin on CLRE induced corpus cavernosum strip relaxationAs shown in Fig. 5, the relaxant effect of CLRE on corpus cavernosum strip precontracted with PE (10−7M) was not significantly increased by 10−4M indomethacin.
DiscussionThis study represents the first attempt to investigate the mechanisms of the relaxant effects of CLRE on the corpus cavernosum tissue. The methanolic extract of C. lutea showed potent relaxant effect on rabbit corpus cavernosum.
The results showed that CLRE induced relaxation of rabbit cavernosum smooth muscle strips following precontraction induced by either phenylephrine or KCl in a concentration-dependent manner.
The mechanisms of contraction involving the response of cavernosal smooth muscle to phenylephrine and K+ solution are different. Phenylephrine is a α-adrenoceptor agonist which causes vasoconstriction by activating phospholipase C and primarily triggering Ca2+ release from the sarcoplasmic reticulum followed by sustained Ca2+ entry.18 K+ induces contraction mainly by Ca2+ influx upon depolarization of the cell membrane, which activates voltage-dependent L-type Ca2+ channel.19
The observed inhibiting effect of CLRE on the contraction of cavernosal smooth muscle strips produced by these two mechanisms is similar, suggesting that the blockade of voltage-gated Ca2+ channels may be an important factor in the relaxation induced by CLRE. That is, inhibitory actions of CLRE may occur in a voltage-independent manner in smooth muscle cells of the rabbit cavernosal muscle strips.
Many studies have demonstrated that nitric oxide (NO) is the most important factor for immediate relaxation of penile vessels and corpus cavernosum. It is not NO itself, but the second messenger cGMP that promotes erection. Erectile dysfunction is often due to an imbalance between the NO-guanylate cyclase- cGMP and cyclic AMP pathway resulting in abnormal corporal smooth muscle contraction and relaxation.20 Nitric oxide (NO) is released from non-adrenergic non-cholinergic nerve endings and the endothelium of the erectile tissue following sexual stimulation. Nerve stimulation activates the release of NO from neuronal nitric oxide synthase (nNOS)21 and endothelial NO synthase (eNOS). NO activates guanylyl cyclase, resulting in an increased conversion of guanosine triphosphate to cyclic guanosine monophosphate (cGMP), which in turn activates protein kinase C which phosphorylates certain proteins and ion channels. This causes the smooth muscle to relax, resulting in erection.22,20 CLRE has been reported to enhance erectile function but the mechanism through which it works is still unclear. Yakubu and Jimoh23 reported that the aqueous extract of Carpolobia lutea root restored erectile function in the case of paroxetine-induced erectile dysfunction in sexually active male rats. Dare et al.14 also reported that the methanol extract of Carpolobia lutea enhances sexual activity in male rabbits by increasing nitric oxide concentration in corporal cavernosa. Despite these findings, we explored further on the mechanism through which CLRE works. To investigate whether relaxation induced by CLRE was attributable to an interaction with NO-cGMP pathway, corpus cavernosum tissues were pretreated with l-NAME (a nitric oxide synthase inhibitor) and ODQ, Methylene blue (guanylyl cyclase inhibitors) respectively. The treatment of corpus cavernosum tissues with l-NAME and Methylene blue did not significantly affect the relaxant activity of CLRE. However, treatment of corpus cavernosum strip with ODQ, a cGMP inhibitor significantly attenuated CLRE-induced cavernosum smooth muscle relaxation. The present study demonstrating ODQ inhibition of CLRE-induced relaxation indicates that soluble guanylate cyclase activation plays an important role in the relaxation effects of CLRE in PE precontracted cavernosal smooth muscle. These findings suggest that CLRE induces relaxation via sGC-cGMP signaling.
Prostaglandin (especially PG12 and PGE1) is derived from arachidonic acid by the action of cyclooxygenases. Prostaglandin inhibits the release of noradrenaline from penile adrenergic nerves and increases intracellular cAMP levels in corpus cavernosum smooth muscle.24,25 The process promotes muscle relaxation through intracellular reduction of calcium concentration, resulting in erection. Therefore, the possibility that relaxation induced by CLRE was a result of an interaction with prostaglandins was further investigated. However, the treatment of corpus cavernosum tissues with cyclooxygenase inhibitor (indomethacin) did not affect the relaxant activity of CLRE. Therefore, the corpus cavernosum tissues relaxation effect of CLRE was not related to prostaglandins.
Recent studies both in vitro and in vivo have documented the potential physiological and pathophysiological importance of potassium channels to the modulation of corpus cavernosal smooth muscle tone.26,27 Potassium channels particularly Ca2+-activated K+ channel, voltage dependent K+ channel and ATP sensitive K+ channel play important roles in the modulation of corpus cavernosum smooth muscle cell. Potassium channel opening is expected to cause hyperpolarization, inhibit Ca2+ channel activity, lower intracellular Ca2+ concentration and result in relaxation.28,29 Because corporal endothelium and smooth muscle contains several K+ channels, it is expected that drugs interfering with these channels may affect relaxation in corporeal tissue. In the present experiment, we found that the treatment of corpus cavernosum tissues with TEA (a voltage operated K+ channel blocker) and apamin (small conductance Ca2+-activated K+ channel blocker) did not change the relaxant activity of CLRE. However, treatment of corpus cavernosum tissues with glibenclamide (10μM) (ATP-sensitive K+ channel blocker) significantly reduced the relaxant effects of CLRE. These findings suggest that the relaxant effect of CLRE is unrelated to Ca2+-activated and voltage dependent K+ channels but may be due to opening of ATP-sensitive K+ channels.
Calcium channels play an essential role in NO synthesis and release in endothelial cells. NO is known to activate K+ channels, either through the activity of cGMP-dependent protein kinase 25 or by direct opening of Ca2+-activated K+ channels without the requirement for cGMP.20 cGMP is thought to cause the relaxation of smooth muscle by lowering the intracellular Ca2+ concentration, either by stimulating Ca2+-ATPase activity or through opening K+ channels, leading to hyperpolarization and subsequent reduction of Ca2+ influx through voltage-operated Ca2+ channels.30,31 This study showed that CLRE-induced relaxation was inhibited by nifedipine (extracellular calcium channel blocker) in cavernous smooth muscle.
Currently in this study, we have not been able to identify the active principle for the dilatory/relaxant activities of CLRE. However, we have previously14 implicated the saponin and flavonoids constituents of CLRE. Future studies will try to ascertain the active component of CLRE responsible for its dilatory/relaxant role.
In conclusion, the present study suggests that Carpolobia lutea root extract possesses a relaxant effect on rabbit corpus cavernosum tissues. Our findings suggest that the major mechanism underlying the relaxation of penile tissue by Carpolobia lutea root extract involves the sGC/cGMP system, activation of ATP-dependent K+ channels and inhibition of release of extracellular Ca2+ influx in the corpus cavernosum.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare that there was no conflict of interest.