To investigate the role of suprapubic bladder aspiration (SBA) in the diagnosis of retrograde ejaculation (RE) which is diagnosed with the observation of sperm in post-ejaculatory urine (PEU). However, sperm is also observed in PEU after the wash out of the retained ejaculate in the urethra with the expulsion of urine in several subjects. Therefore, detection of sperm in PEU in the diagnosis of RE is problematic and a better method is needed to overcome the ambiguity of positive PEU and to identify which patient experience true RE.
Material and methodsA cohort of patients underwent an examination for RE over a two-year period at a single specialist centre. All patients underwent SBA and semen analysis. Sperm was investigated in urine aspirated from the bladder and in PEU.
ResultsThirty-two patients (age range 18–62 years) underwent SBA and PEU for investigation of RE. Sperm was detected both in SBA and PEU in 19 patients, while 5 patients revealed sperm only in PEU. The mean number of sperm found in SBA was less than the mean number of sperm observed in PEU in all 19 patients.
ConclusionSBA is a reliable and feasible method in the diagnosis of RE and can distinguish the true RE in which sperm flows backward into the bladder from the retained ejaculate in the urethra. The whole ejaculate does not likely flow retrogradely and RE could be a partial leakage of the ejaculate into the bladder.
Investigar el papel de la aspiración vesical suprapúbica (AVS) para el diagnóstico de la eyaculación retrógrada (ER), que es diagnosticada con la observación de esperma en la orina posteyaculatoria (OPE). Sin embargo, el esperma también se observa en la OPE después del lavado del eyaculado retenido en la uretra con la expulsión de la orina en algunos sujetos. Por tanto, la detección de esperma en la OPE es puede ser problemático para el diagnóstico de la ER y es necesario un método mejor para superar la ambigüedad de OPE positiva e identificar qué pacientes experimentan verdadera ER.
Material y métodosSe examinó una cohorte de pacientes para ER durante un periodo de dos años en un único centro. A todos los pacientes se les realizó una AVS y un análisis del semen. Se investigó la presencia de esperma en la orina aspirada de la vejiga y en la OPE.
ResultadosSe incluyeron treinta y dos pacientes (rango de edad 18-62 años) a los que se les realizó AVS y análisis de OPE para investigar la ER. Se detectó esperma tanto en la AVS como en la OPE en 19 pacientes, mientras que en 5 pacientes sólo se detectó esperma en la OPE. El número medio de esperma encontrado en la AVS fue inferior al observado en la OPE en los 19 pacientes.
ConclusiónLa AVS es un método fiable y factible para el diagnóstico de la ER y puede distinguir entre verdadera ER en la que el esperma fluye marcha atrás hacia la vejiga, del eyaculado retenido en la uretra. Es probable que no todo el eyaculado fluya retrógradamente y que la ER pueda ser una fuga parcial del eyaculado hacia la vejiga.
Ejaculation which is the propulsion of semen out of the body, consists of two phases in succession, emission and expulsion.1,2 During expulsion, semen is ejected throughout the urethra and the bladder neck which is under sympathetic control, necessarily remains closed to prevent retrograde flow of semen into the bladder.1–3 Retrograde ejaculation (RE), the backward passage of semen into the bladder, occurs as a consequence of inadequate closure of the bladder neck2,3 and is associated with low volume or null ejaculate with low sperm count which may result in infertility.3 Etiological factors of RE include diabetes mellitus, bladder neck injury, transurethral prostatectomy (TURP), congenital abnormality, medications (i.e. alpha blockers, antipsychotics, ganglion blockers), neurological disorders, loss of sympathetic innervation at the bladder neck (i.e. retroperiteonal lymph node dissection, spinal trauma, abdominopelvic resection) or idiopathic.3,4
The diagnosis of RE is traditionally based on the detection of sperm in post-ejaculatory urine (PEU) in patients with semen volume under 1.5 or 2ml.5–7 However, according to previous studies, sperm was observed in PEU of 60 to 88% of fertile men5,6,8 and in 65 to 98% of infertile men5,6,9,10 most probably due to the wash out of the urethra with the expulsion of urine.6,8,10 It was reported that a significant number of sperm (10–15 sperm per high power field) should be demonstrated in urine to confirm the diagnosis of RE.7 Nevertheless, neither the volume of semen nor the number of sperm in urine is enough to distinguish RE from the retained ejaculate in the urethra frequently seen in healthy (both in fertile and infertile) men.5,9 Therefore, detection of sperm in PEU in the diagnosis of RE is problematic and a better method is needed to overcome the ambiguity of positive PEU.
Until now, retrograde ejaculation of the semen into the bladder has not evidently been proven yet. Whether the presence of sperm in the PEU is the result of the wash out of the sperm retained in the urethra or retrograde flow of the sperm into the bladder is still unknown.9 We intended to overcome this problem by suprapubic bladder aspiration (SBA) and search for sperm directly in the bladder. SBA is a well-defined procedure easy to be performed and is used in several diagnostic and therapeutic medical applications with minimal complications.11 To the best of our knowledge SBA for the diagnosis of RE has never been experienced. The main objective of the present study is to investigate SBA in the diagnosis of RE and to develop a new method to identify which patient has a true RE. Detection of sperm in SBA would mean that the ejaculate passes backward into the bladder and the patient has a true RE. No observation of sperm in the SBA but presence in the PEU would be approved as retained ejaculate.
Material and methodsThis prospective study was designed to investigate the reliability of a new method, SBA, in the diagnosis of RE. The study was compliant with ethical principles laid down in the Declaration of Helsinki, following approval of the protocol by a properly constituted Institutional Independent Ethics Committee. Written informed consent was obtained from each participant of the study.
Study groupMale patients presumed to have RE due to risk factors such as history of transurethral prostatectomy and rectopexy, low semen volume in the first semen analysis (<1.5ml), myotonic dystrophy, type 1 diabetes mellitus and feeling of incomplete ejaculation underwent SBA and PEU analysis and were recruited into the study. All patients included in the study were subjected to a diagnostic work-up including medical history, physical examination, determination of circulating sex steroidal hormones, scrotal Doppler ultrasound, semen analysis and searching sperm both in the bladder aspirate and PEU. Patients with urinary tract infection, urethral stenosis, obstructive azoospermia and ejaculatory duct obstruction were not included in the study.
After three days of sexual abstinence, semen samples were collected by masturbation in a sterile special container for semen analysis. The patients were asked to drink fluids and not to void after ejaculation. As the patient had sensation of bladder fullness, SBA was performed and 10ml of urine was collected in a second container free of culture medium. Then the patient was asked to void in a third container to collect the post-ejaculatory urine for PEU analysis.
SBA methodUltrasound-guided SBA was performed by experienced physicians in the urology department. Bedside ultrasound was used to determine whether the urinary bladder is adequately filled for aspiration, to identify anatomic abnormalities or variants, to reduce the risk of complications, and to guide the needle aspiration. Patients were asked to lay on supine position and their bladder was visualised. The transducer was placed in a transverse fashion just superior to the pubis symphysis. Then the bladder volume was calculated.11 Total bladder volume was used to determine whether bladder volume was appropriate for aspiration. The amount of bladder fullness required for aspiration was varied according to patient's functional bladder capacity and was clinically decided individually under ultrasound visualisation. The time elapsed for bladder aspiration differed according to fluid intake and sensivity to bladder fullness. When the bladder volume was not sufficient for aspiration the scan was repeated later. Once there was enough urine noted in the bladder, the needle (10ml, 0.8×38mm, 21G×1 ½) was inserted under direct ultrasound visualisation approximately 1cm cranial to the pubic symphysis. As soon as the anterior wall of the bladder was successfully punctured, 10ml of urine was aspirated.
Semen analysisSemen analysis was performed by experienced biologists in our Laboratory of Andrology Unit according to 2010 WHO (World Health Organisation) 5th criteria.12 Semen samples were analysed for semen volume, sperm concentration, sperm motility and sperm morphology. When the semen sample was delivered to the andrology laboratory, the sample was kept in 37°C incubator during liquefaction. Subsequently, semen volume was estimated from the weight of the semen by assuming that sperm density is 1g/ml. Semen volume was calculated by gravimetric method. Improved Neubauer haemocytometer was used to count the number of sperm. Semen volume and semen sperm count (106/ml) were recorded.
Assessment of sperm in the urine10ml of urine was aspirated through SBA in each patient. Then the voided urine after SBA was collected and the volume was calculated gravimetrically. Ten μl of urine derived from aspirated and voided urine was separately examined under microscope (Olympus BX43). If sperm was not observed, 10ml of urine was centrifuged (3000×g, 15min) for each specimen. If no sperm was detected in pellet in both SBA and PEU analysis, the result was negative. Sperm count in the urine (106/ml) was calculated with an improved Neubauer haemocytometer. Patients with sperm detection both in SBA and PEU were diagnosed as RE. Sperm observation only in PEU was considered retained ejaculate.
Statistical analysisA Wilcoxon signed-rank test was conducted to determine the difference between the number of sperm in SBA and in PEU in the subgroup of 19 patients whose sperm was found both in SBA and PEU.
ResultsA total of 32 subjects were involved in the study. The median age at presentation was 37.5 (18–62). All patients underwent both SBA and PEU. 19 patients were diagnosed with RE, that is, sperm was detected both in SBA and PEU. However, no sperm was found neither in SBA nor in PEU in 8 patients, while 5 patients had sperm only in PEU but not in SBA (Table 1).
In the subgroup analyses of 19 patients whose sperm was found both in SBA and PEU, eleven patients had aspermia and eight patients had low semen volume, four of whom reported incomplete feeling of ejaculation. Additional findings were left side grade 3 varicocele in two patients and familial hypercolesterolemia in one patient. Two patients reported that they have obstructive urinary complaints and have a habit of void postponement due to social conditions and were treated with alfuzosine. Only one out of 19 patients had a semen volume of 2.16cc (Table 2).
Etiology and sperm concentration in semen and urine in patients with RE.
| Etiology (n) | Sperm concentration (ml) (SBA) | Sperm concentration (ml) (PEU) | Sperm concentration (ml) (semen) | Semen volume (ml) | |
|---|---|---|---|---|---|
| 1 | Low semen volume | 1100000 | 2300000 | 5000000 | 0.77 |
| 2 | Low semen volume | 200000 | 400000 | 1300000 | 1.39 |
| 3 | Low semen volume | 300000 | 500000 | 120000000 | 1.24 |
| 4 | Low semen volume | 200000 | 400000 | 21000000 | 2.16 |
| 5 | Low semen volume | 200000 | 500000 | 48000000 | 1.00 |
| 6 | Low semen volume | 100000 | 500000 | 28000000 | 0.3 |
| 7 | Low semen volume | 200000 | 500000 | 42000000 | 1.1 |
| 8 | Low semen volume | 500000 | 1000000 | 55000000 | 1.01 |
| 9 | Myotonic dystrophy | 14100000 | 22000000 | 0 | 0 |
| 10 | Type 1 DM | 4200000 | 18400000 | 0 | 0 |
| 11 | Turp | 300000 | 500000 | 0 | 0 |
| 12 | Turp | 200000 | 400000 | 0 | 0 |
| 13 | Turp | 1300000 | 5000000 | 0 | 0 |
| 14 | Turp | 300000 | 2100000 | 0 | 0 |
| 15 | Turp | 2200000 | 4300000 | 0 | 0 |
| 16 | Turp | 200000 | 400000 | 0 | 0 |
| 17 | Turp | 4000000 | 5200000 | 0 | 0 |
| 18 | Turp | 200000 | 500000 | 0 | 0 |
| 19 | Laparoscopic rectopexy for Rectal prolapse | 300000 | 700000 | 0 | 0 |
RE: retrograde ejaculation, PEU: post-ejaculatory urine, SBA: suprapubic bladder aspiration, Turp: transurethral prostatectomy.
We observed that in all of 19 patients whose sperm was detected both in SBA and PEU, the mean number of sperm in SBA (1144×103±3286.7) was less than the mean number of sperm in PEU (3252×103±6157.7), z=3.51, p<.0005, even in patients with aspermia (Table 2).
Eight of 32 patients investigated for RE had no sperm neither in SBA nor in PEU. Semen volume was measured below 2 (0.9–2)ml. Four and 3 patients were concluded as non-obstructive azoospermia and severe oligozoo-asteno-teratospermia, respectively. One patient with type 2 diabetes mellitus was considered to have unejaculation since any ejaculate was not obtained (aspermia).
On the other hand 5 of 32 patients had sperm only in PEU. Low semen volume was the main factor to investigate RE in three of five patients. One patient with the feeling of incomplete ejaculation underwent TRUS and no pathology was observed. One patient revealed no sperm in 0.7ml of semen (Table 3).
Patients with negative SBA and positive PEU.
| Etiology (n) | Sperm concentration (ml) (SBA) | Sperm concentration (ml) (PEU) | Sperm concentration (ml) (semen) | Semen volume (ml) | |
|---|---|---|---|---|---|
| 1 | Low semen volume | 0 | 400000 | 28000000 | 2.09 |
| 2 | Low semen volume | 0 | 500000 | 35000000 | 1.02 |
| 3 | Low semen volume | 0 | 1000000 | 14700000 | 1.32 |
| 4 | Low semen volume | 0 | 5200000 | 70000000 | 0.76 |
| 5 | Low semen volume | 0 | 3000000 | 0 | 0.7 |
PEU: post-ejaculatory urine, SBA: suprapubic bladder aspiration.
There was no patient whose sperm was detected in SBA without sperm in PEU. No complication occurred due to SBA and no patient gave up due to discomfort caused by fullness of bladder.
DiscussionWe observed sperm both in suprapubic bladder aspirate and PEU analysis in 19 patients whereas 5 patients revealed sperm only in PEU with no sperm in the bladder. Four of these five patients had a semen volume of ≤1.5ml, thus it is obvious that patients with low semen volume or sperm existence in PEU do not literally indicate retrograde ejaculation. SBA successfully distinguishes true RE from the retained ejaculate in the urethra and is an effective method to overcome the ambiguity of PEU results. In addition, as the bladder volume which SBA is done is supposed to be equal to the voided volume which PEU analysis was performed; and the concentration of sperm in SBA was found lower than the concentration of sperm in PEU in all patients, means that, the whole ejaculate does not flow backward into the bladder in RE.
Although there is no specific symptom that points out RE, the most common complaint is the sense of incomplete ejaculation. Traditionally, the diagnosis of RE is confirmed with the presence of sperm in PEU. However, the only presence of sperm in PEU is non-informative with regard to RE since at least one sperm was found in PEU of fertile or infertile men, ranging from 60 to 98%.5,6,8–10 The majority of the sperm was particularly demonstrated in the first fractions of the PEU6,13 and this was explained with the remaining ejaculate located in the urethra is washed out during voiding.6,8,10 Some researchers investigated men with low (≤1.5 or 2ml) semen volume, and diagnose RE in patients demonstrating 10–15 sperm per high powered field in urine,7 but still no one can claim that a fertile or infertile man within these criteria has a true RE or retained ejaculate in the urethra. For this reason, researchers made efforts to overcome the ambiguity of PEU results by forming a retro-ejaculation index (R-ratio) which expresses the percentage of sperm in PEU to the total number of sperm found in both semen and PEU.5,6,9 However, the results of these studies are conflicting and it is not possible to develop a specific threshold to disclose the true RE.14 Thus, we intended to overcome this problem by SBA of the bladder and distinguish which patient suffers from true RE or retained ejaculate. If we detect sperm in SBA and PEU, that will mean that the ejaculate flows retrograde into the bladder. But if we do not observe sperm in the urine aspirated through the bladder but detect sperm in PEU, we will agree that the ejaculate does not flow backward into the bladder and retains in the prostatic urethra. Accordingly, 19 patients in our study were regarded as RE and the other 5 subjects who had sperm only in PEU were accepted as patients with retained ejaculate.
SBA is a well-known technique and used frequently for several diagnostic and therapeutic purposes. In children under 2 years of age it is the gold standard for urine culture because of very low (1%) contamination rates.11 SBA can also be used to decompress the bladder in cases of urinary retention and to place a catheter into the bladder where transurethral catheterisation is not possible in medical conditions such as urethral stricture and trauma.11 Although hematuria and accidental puncture of adjacent structures are reported in the literature, we did not experience any complication since SBA under ultrasound provided a more safe intervention.
RE is usually complete and rarely partial.15 In complete RE, the whole ejaculate travels up into the bladder and sperm is detected only in PEU.7 However, in partial RE, a portion of ejaculate flows antegrade through the urethra and the rest of the ejaculate flows backward into the bladder and thereby, sperm is observed both in the antegrade ejaculate and in the PEU.7 Ejaculate was not assumed to be retained in prostatic urethra partially in patients with RE. However, we found higher number of sperm in PEU compared to number of sperm in SBA analysis in all of our 19 patients with RE regardless of the etiological factor. These results showed us that the whole ejaculate did not pass into the bladder in any patient with or without an antegrade ejaculate and the remaining part always retained in the prostatic urethra. Human ejaculation was observed in a patient with RE using transrectal colour Doppler ultrasonography, and it is demonstrated that once the expulsion phase is concluded the remaining ejaculate in the prostatic urethra began passing into the bladder slowly.16 We think the slow pattern of retrograde flow of a portion of the whole ejaculate could be regarded as leakage.
In the case of idiopathic RE, no abnormal findings of the bladder neck was detected by ultrasonography. However, flattening of the bladder neck was not as marked as in the healthy man without RE and the prostatic urethra distended to a globular-shape sac which may be responsible for the flow of ejaculate into the bladder.16 The urethral pressure increases during ejaculation.2 We suppose that the bladder neck is a barrier not only to prevent the ejaculate backflow into the bladder, but also to maintain the pressure in the prostatic urethra needed for the expulsion of semen through the urethra. We think that when the integrity of the bladder neck impairs, the pressure needed for the expulsion could not be provided, and the ejaculate retains in the prostatic urethra, then a leakage develops due to the disabled bladder neck integrity.
RE may exist in case of an intact bladder neck without a neurological or anatomical etiologic risk factor. In patients with idiopathic RE or in spinal cord injured men with detrusor sphincter dyssynergia, it is postulated that the increased tone of the external urethral sphincter or the lack of coordination between the external sphincter and bladder neck results in RE.16,17 A non-neurogenic ejaculatory dysfunction could also cause RE by an inability to complete the ejaculation due to contracting the external urethral sphincter involuntarily. Ejaculation has similarities with the bladder drainage. Emission can be regarded as the counterpart of the storage phase of the bladder as both are under the control of sympathetic system.18 Many men have experienced postponing ejaculation by contracting their external urethral sphincter due to undesirable social conditions, religious beliefs or anxiety. In similar, many children and adults postpone their voiding by contracting their external urethral sphincter due to several reasons such as play, bad toilet conditions. This undesirable habit is known as non-neurogenic dysfunctional voiding.19 Men having a habit of postponing their ejaculation could develop a similar kind of dysfunctional ejaculation. Like residual urine as a result of dysfunctional voiding, non-neurogenic ejaculation may lead to residual semen. This may occur separately or may accompany to the leakage of sperm backward into the bladder. These men may or may not have an antegrade ejaculate. In our study patients with only PEU positivity and 8 of 19 patients with low semen volume may manifest a non-neurogenic ejaculatory dysfunction.
We suggest four different consequences could be related to this ejaculatory dysfunction which may sometimes be temporary. In the first condition, all ejaculate can be retained in the urethra. In these patients a very small part of ejaculate can expulse out of the body and may not contain sperm. This is thought as incomplete semen collection.20 One of our patients with only PEU positivity has 3 million sperm in PEU, however no sperm was observed in 0.7ml of semen (Table 3). In the second presumption, an amount of ejaculate can be retained in the urethra and the remaining portion is expelled out of the urethra. Patients in this group have many sperm both in PEU and semen analysis (Table 3). We think that men without ejaculatory dysfunction and feeling of incomplete ejaculation could also have a considerable semen volume and sperm in PEU. We prefer to define this condition as ‘retained ejaculate’. The third group consists of patients with an amount of ejaculate leaks into the bladder and the remaining part retains in the urethra (complete form of RE). In the fourth group an amount of ejaculate leaks into the bladder, retains in the prostatic urethra and is also expulsed out the urethra (partial form of RE).
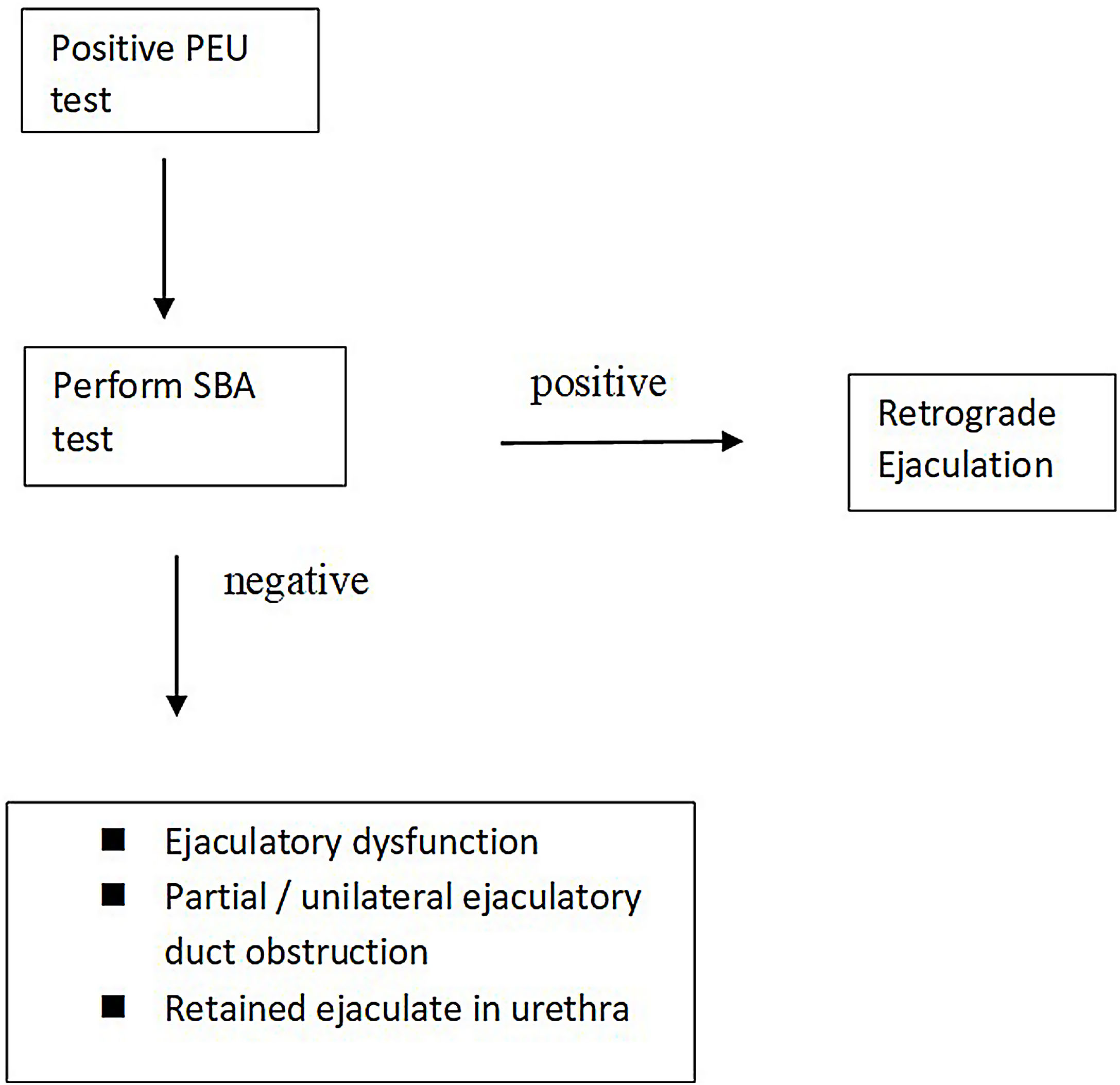
A PEU test is routinely advised in patients with low semen volume to search RE in most of the andrology labs and treatment is advised in positive results. However, a positive PEU test does not imply a real RE in practice. According to our new findings a simple algorithm can be advised (Fig. 1). A PEU test could be performed in patients with low semen volume. If the test is negative, RE is excluded. If the test is positive, the likely diagnoses are RE, retained ejaculate, ejaculatory dysfunction and partial/unilateral ejaculatory duct obstruction which is controversial in its own right.20 For the definitive diagnosis a SBA is recommended. If the test is positive, RE is diagnosed. If it is negative the other choices should be considered.
The major limitation of our study is that we did not screen all the patients with low semen volume. However, the major aim of our study is to search the role of SBA in the diagnosis of RE. Although 1.5 or 2ml is traditionally accepted as a threshold for PEU to search RE, semen volume of one patient with RE was measured as 2.16ml in our study. We believe that a definitive threshold has to be established by SBA and more future studies are needed.
ConclusionSBA is a reliable and feasible method in the diagnosis of RE and can distinguish the true RE which sperm flows backward into the bladder from the retained ejaculate in the urethra. According to our results, the whole ejaculate did not likely flow retrogradely and RE could be a partial leakage of the ejaculate into the bladder.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors’ contributionsResearch design and data acquisition: HU, NA. Data analysis and interpretation, drafting of the manuscript: HU, NA, MH, BS, AOY, YOO. All authors critically reviewed the manuscript and approved the final version.
Conflict of interestNone of the authors have anything to disclose.
We gratefully thank to Dr. Feyzahan Uzun for reading drafts of this paper.