There is a considerable literature supporting the role of lipids in fertility. However, little is known about their impact on male and female gametes. Our study aimed to investigate the relationships between lipids levels in serum, follicular fluid and seminal plasma with ovarian response and sperm concentration regardless of age and body mass index (BMI).
Methods51 follicular fluid and serum samples of IVF-ICSI cycles and 52 seminal plasma and serum samples of males in the infertility study were analyzed for cholesterol, triglycerides, and non-esterified fatty acids. The parameters used to assess gonadal response were number of mature oocytes in metaphase II and total motile sperm. Differences between groups were studied by means Principal Component Analysis, Kolmogorov–Smirnov test, Pearson correlation, Student's T, and multivariate linear regression.
ResultsUsing a multivariate linear regression model to exclude the effect of the age and BMI, we found that the lipid profile in follicular fluid and plasma influence inversely and significantly on ovarian response and the number of matured oocytes recovered. Moreover, we found that seminal lipid levels are predictors of seminal quality independent of plasma lipid values.
ConclusionOur current analysis demonstrates the association of low ovarian response and low number of motile sperms with abnormal lipids levels.
Existen muchas publicaciones que apoyan el papel de los lípidos en la fertilidad. Sin embargo, se sabe poco sobre su impacto en los gametos masculinos y femeninos. Nuestro estudio tuvo como objetivo investigar las relaciones entre los niveles de lípidos en plasma sanguíneo, líquido folicular y plasma seminal con la respuesta ovárica y concentración espermática, independientemente de la edad y el índice de masa corporal (IMC).
MétodosSe analizaron 51 muestras de plasma sanguíneo y líquido folicular de ciclos de FIV-ICSI y 52 muestras de plasma sanguíneo y plasma seminal de varones en estudio de infertilidad para analizar el nivel de colesterol, triglicéridos y ácidos grasos no esterificados. Los parámetros utilizados para evaluar la respuesta gonadal fueron el número de ovocitos maduros y el número total de espermatozoides móviles. Las diferencias entre los grupos se estudiaron mediante la prueba de análisis de componentes principales, la prueba de Kolmogorov-Smirnov, la correlación de Pearson, la T de Student y regresión lineal multivariante.
ResultadosUtilizando un modelo de regresión lineal multivariante para excluir el efecto de la edad y el IMC, se encontró que el perfil de lípidos en el líquido folicular y el plasma influyen inversa y significativamente en la respuesta ovárica y el número de ovocitos maduros recuperados. Además, los niveles de lípidos seminales son predictores de calidad seminal independientemente de los valores de lípidos en plasma.
ConclusiónLos resultados de este estudio demuestran la asociación de la baja respuesta ovárica y bajo número de espermatozoides móviles con niveles anormales de lípidos.
Lipids comprise a wide-range class of molecules that play a crucial role in the structure and function of cells in mammals. They serve as storage compounds, cellular metabolism, signaling molecules, and are involved in various membrane-related functions such as transferring, regulation of proteins, and creating membrane sub compartments.
It is becoming increasingly clear that deregulated lipid metabolism plays an important role in many human diseases as lipid homeostasis is fundamental to health maintenance.1
Progress in the lipidomic field has shed light in the knowledge and understanding of lipids. Especially concerning is their role in cell signaling pathways which can indicate changes in the cellular microenvironment, such as steroidogenesis processes, cell proliferation and Apoptosis.2
There is a considerable literature supporting the role of lipids in male and female fecundity.3 For example, some studies reported a correlation between the fatty acid profile, oocyte maturity and embryonic development.4 Higher high-density lipoprotein (HDL) concentrations have been associated with better oocyte and embryo outcomes5 as well as its effects on spermatogenesis.6 In the pre-ovulatory follicle, mammalian oocytes are surrounded by cumulus granulosa cells and are immersed in follicular fluid (FF) containing various proteins, lipids, sugars, hormones, and metabolites. The relative composition of the FF plays a critical role in supporting oocyte development and competence.7 However, it is difficult to compare the different studies investigating the influence of serum FF fatty acids on fertility and oocyte quality due to the diverse experimental designs.
On the other hand, lipid metabolism disorders (dyslipidemia) are causes of male infertility in mice,8 but little is known about their impact on male gametes, given that studies mostly focus on endocrine dysfunctions and testicular consequences.9
Our group has recently showed associations between serum, seminal plasma, and follicular fluid concentrations of lipids with altered sperm parameters and low ovarian reserve in humans.10 Nevertheless, it is important to consider the confounding factor of age and body mass index (BMI), both related to the lipid profile and fertility.
The primary endpoint of this study was to assess the relationships between lipids levels (cholesterol, triglycerides, and non-esterified fatty acids), in plasma, follicular fluid and seminal plasma with ovarian response and sperm concentration regardless of age and body mass index (BMI). A lipid profile in plasma common for men and women, and specific lipid profiles in seminal plasma and follicular fluid were evaluate as secondary outcomes.
Material and methodsThis prospective clinical study was conducted as a part of a research in infertility at Tambre Foundation, from February 2018 to June 2018 and it was approved by the Ethical Review Board of the Hospital de la Princesa (Madrid, Spain). All subjects gave their written consent.
Female patientsFollicular fluid (FF) and blood samples were collected on the day of oocyte retrieval from 51 women (aged in between 24 and 41 years old) undergoing treatment for infertility.
Were included 27 healthy fertile oocyte donors and 24 women with low response in at least one previous controlled ovarian stimulation cycle.
Patients were recruited according to the following criteria: (i) absence of any apparent abnormality in the reproductive system, revealed by their medical history, clinical examinations and common hormonal tests; (ii) absence of any metabolic or endocrine system-associated diseases, such as hyperprolactinemia, thyroid dysfunction or polycystic ovary syndrome defined by the Rotterdam criteria11; (iii) absence of any surgical history regarding the reproductive system; (iv) normal ovulatory cycle, with cycle lengths in between 25 and 35 days.
All study participants were previously screening for basic infertility test: basal Follicular Stimulant Hormone (FSH,) Luteinizing Hormone (LH), 17 Beta Estradiol, Antimullerian Hormone (AMH) and sexually transmitted diseases (STD).
Patients gave written informed consent and did not receive any monetary compensation for participating in the study. This study was part of routine IVF treatment in our center; thus, it was funded internally.
Women were included as donors after being thoroughly informed about oocyte donation and later fully evaluated to assess fulfilment of the criteria required to be admitted into a donation program. In short, oocyte donors were in between 18 and 35 years old. Complete medical history examination was performed, which had to include absence of current or past exposure to radiation or hazardous chemical substances, drug abuse and past reproductive history. All donors must have had, because of the exhaustive evaluation, a normal physical and gynecological examination, no family history of hereditary or chromosomal diseases, normal karyotype, and negative screening for sexually transmitted diseases (STD).
Ovarian stimulation protocolsOvarian stimulation in patients was initiated with 225–300UI/day rec-FSH (Gonal-F®; Merck, Madrid, Spain) from day 2 of the menstrual cycle. The GnRH antagonist (Cetrotide; Merck, Madrid, Spain) was introduced according to a multiple-dose protocol (0.25mg/day) when a leading follicle of 14mm and/or oestradiol concentrations of 400pg/ml was reached. Recombinant human chorionic gonadotrophin (HCG) (Ovitrelle®, Merck, Madrid, Spain) was applied when ≥2 follicles reached ≥17mm and oocyte retrieval was performed under sedation at the 36th hour following HCG.
The ovarian stimulation in donors began with 125–225 IU of recombinant FSH (Gonal-F®; Merck Serono, Madrid, Spain) from day 2 of the menstrual cycle. The GnRH antagonist (Cetrotide; Merck, Madrid, Spain) was introduced according to a multiple-dose protocol (0.25mg/day) when a leading follicle of 14mm and/or oestradiol concentration of 400pg/ml was reached. Triggering was performed when at least three follicles >17mm was present with 0.2mg of triptorelin (SC Decapeptyl, Ipsen Pharma, Barcelona, Spain) and oocyte retrieval was performed under sedation at the 36th hour following GnRHa. In all groups, the first control (ultrasonography and serum oestradiol) was performed after 5 days of stimulation, and the daily dose of FSH was adjusted individually according to the ovarian response.
Male patientsSemen samples were obtained from 52 patients (32 patients and 20 donors) (aged in between 24 and 46 years old) who attended the Andrology Laboratory.
Patients included in the study had to meet the following inclusion criteria: patients with a normal 46 XY karyotype evaluated by conventional cytogenetic analysis, normal hormone profile, no history of radiotherapy, chemotherapy, chronic illness or medication, and patients without sperm defects genetically originated. Physical examination and scrotal Eco-color Doppler were performed on all patients to detect the presence of varicocele. To identify the presence of clinically asymptomatic genitourinary infections, a bacteriological analysis was performed on all semen samples. Exclusion criteria were employed to exclude regular alcohol drinkers, heavy smokers, azoospermic men and the men with 100% immotile sperm.
All infertile patients were individuals who did not obtain pregnancy after 2 years of unprotected sexual intercourse.
Semen analysisSemen samples were collected by masturbation after a period of 3–5 days of sexual abstinence. After liquefaction (37°C, 30min), smears of neat semen were prepared for sperm morphology assessment and sperm concentration and motility were evaluated according to the WHO criteria.12
Seminal, follicular and blood plasma sample preparationSeminal plasma was obtained by centrifuging the semen samples at 300×g for 7min at room temperature. Samples were stored in liquid nitrogen. At the time of semen retrieval, 5ml of fasting blood from each male was placed in an EDTA-coated tube and centrifuged at 600×g for 15min at 4°C. For each analysis, 100μl of blood plasma was added to 0.9ml of butylated hydroxy-anisole (BHA solution) and stored in liquid nitrogen.
Oocytes of each patient were separated and placed into culture media, whereas follicular fluid was collected in Eppendorf tubes. Only uncontaminated follicular fluid minimally stained with blood were kept for further determinations. At oocyte retrieval, and after removing the oocytes, follicular aspirates of each patient were individually centrifuged at 600×g for 10min and the supernatant stored at −70°C for a maximum of 2 weeks.
Venous blood samples were placed in an EDTA-coated tube and centrifuged at 600×g for 15min to isolate serum for detection of lipid level.
Total cholesterol, triglyceride (TG) and non-esterified fatty acids (NEFA) acid analysisLipids were separated according to the standard Folch method.13 Briefly, 100μl of sample (blood, follicular fluid, or seminal plasma in BHA) was mixed with 1.9ml of chloroform/methanol (2:1 v/v) and 1ml of cold water. The mixture was vortexed for 90min at 48°C. From the resulting organic phase, aliquots were then used for phospholipid analysis by thin-layer chromatography (TLC), acid hydrolysis and methylation as described by Schlenk and Gellerman.14
Commercially available kits for the determinations of TC, TG, NEFA and calibration and quality control products were purchased from Wako Chemicals GmbH. The determinations were carried out using Hitachi 7600–210 (Hitachi High-Technologies, Tokyo, Japan), automatic biochemical analyzer.
The reference range for non-esterified fatty acids was in women: 0.1–0.45mmol/L and men: 0.1–0.60mmol/L (limit of detection: 0.02mmol/L; coefficient of variation (CV): 1.90%); reference range for cholesterol was: 140–400mg/dL (women and men) (limit of detection: 25mg (dL; CV: 4%) and the reference range for triglycerides was 25–400mg/dL (limit of detection: 4mg/dL; CV: 2.3%).
The sample with higher lipid level exceeding the linear range of the kit should be diluted with normal saline and the diluted volume was calculated.
Biochemical analyses of TG and total cholesterol were performed as previously described.14
NEFA were measured by an enzymatic colorimetric method (Wako Chemicals, Neuss, Germany).
Statistical analysisNormality was examined by the Kolmogorov–Smirnov Test. Variables presented with normal distribution are presented as mean±Standard Deviation (SD).
The Pearson's correlation coefficient was used to examine any possible correlations between serum, seminal plasma and FF lipids and age, number of MII oocytes and sperm concentration between groups.
T-Student test was used to compare means between male and female groups.
Principal Component Analysis (PCA) was used to clarify the relationship between the plasmatic and gonadal lipid profile with the reproductive efficacy. The analysis was performed without the confounding effect of age and obesity. PCA is a dimension-reduction tool that can be used to reduce a large set of variables into a small set that still contains most of the information in the large set. Using PCA we can extract a common factor to women and men which could explain the lipid profile in plasma and describe the lipid profiles in follicular and seminal fluids, respectively.
p-Values of <0.05 were considered statistically significant. Statistical analysis was performed using the SPSS 22.0 edition (Chicago, IL, USA).
ResultsGeneral dataThe clinical patients’ characteristics of the female study population (patients and oocyte donors) are represented in Table 1 by age, Müllerian hormone (AMH), amount of basal FSH, amount of administered FSH, the estradiol value on the day of hCG/GnRH injection, number and maturity of oocytes retrieved.
Female characteristics in the patients (n=24), and control groups (oocyte donor, n=27) in terms of age, BMI (kg/m2) anti-Müllerian hormone (AMH), amount of basal FSH, amount of administered FSH, the estradiol value on the day of hCG/GnRH injection, number and maturity of oocytes retrieved.
| Female patients (n=24) | Oocyte donors (n=27) | p-value | |
|---|---|---|---|
| Age (years) | 33.4±7.1a | 23.4±4.1a | 0.01 |
| BMI (kg/m2) | 23.62±2.1 | 22.4±2.3 | NS |
| AMH (ng/ml) | 2.66±1.2 | 3.74±1.3 | NS |
| Basal FSH (IU/l) | 5.2±1.2 | 5.7±3.2 | NS |
| Total FSH administered (IU) | 2877±158 | 1962±146 | 0.01 |
| E2 value of hCG/GnRH day (pg/ml) | 1094±1098b | 2473±1901b | 0.01 |
| Total oocytes retrieved | 4.2±1.3c | 15.5±12.8c | 0.001 |
| Total MII oocytes | 3.3±1.3d | 14.4±10.8c | 0.001 |
Values are mean±SD.
BMI=body mass index; AMH=anti-Mullerian hormone.
The value of age,a Estradiol,b number of retrieved oocytes,c and number of mature oocytes,d are significantly different among the patients and donors.
The value of age (p<0.01), estradiol (p<0.01), amount of administered FSH (p<0.01), number of retrieved oocytes (p<0.001) and number of mature oocytes (p<0.001) are significantly different among the patients and donors.
The clinical characteristics of the male study population are represented in Table 2, in terms of age, BMI (kg/m2), semen volume (ml), total sperm count (106), sperm concentration (106), motility (%), morphology (%) and number of total motile sperm recovered. There were significant differences between male patients and controls, for total sperm count (p<0.001), sperm concentration (p<0.001), motility (p<0.01), morphology (p<0.01), and total number of motile sperm recovered (p<0.001).
Characteristics of male patients (n=32) and fertile volunteers (n=20) in terms of age, BMI (kg/m2), semen volume (ml), total sperm count (106), sperm concentration (106), motility (%), morphology (%) and number of motile sperms recovered. There were no significant differences between male study population and controls, except for total sperm count (p<0.001), sperm concentration (p<0.001), motility (p<0.01), morphology (p<0.01), and total number of motile sperms recovered.
| Male patients (n=32) | Sperm donors (n=20) | p value | |
|---|---|---|---|
| Age (years) | 38.5±10 | 22.0±12 | 0.01 |
| BMI (kg/m2) | 22.1±2.2 | 23.1±2.1 | NS |
| Semen volume (ml) | 3.2±1.8 | 2.85±1.5 | NS |
| Total sperm count (×106) | 34±3.5 | 106±19 | 0.001 |
| Sperm concentration/ml (×106) | 10.2±4.1 | 87±15 | 0.001 |
| Motility (%) | 24.4±6 | 58.6±10 | 0.01 |
| Normal morphology (%) | 3.2±1.6 | 16.5±5 | 0.01 |
| Total motile sperm recovered (×106) | 5.7±1.4 | 88.6±24 | 0.001 |
Differences between categories were assessed by independent-samples t-test. p-value 0.05 was considered statistically significant.
Relationships between lipids levels in blood plasma, follicular fluid and seminal plasma with ovarian response, sperm concentration, BMI, and age.51 IVF cycles were analyzed to assess the influence of the blood plasma and intraovarian lipid profile of women on the number of mature oocytes. 52 sperm samples were studied to assess the influence of the blood plasma and seminal plasma lipid profile of men on the number of motile spermatozoa.
The blood plasma values of cholesterol (μmol/l), triglycerides (μmol/l) and non-esterified fatty acids (NEFA) (μmol/l) were classified as independent variables, as well as the concentration of these same lipids in follicular fluid at the time of ovarian puncture or seminal plasma in the sperm samples in males.
The total number of metaphase II oocytes obtained at the puncture and the total count of spermatozoa with progressive motility in the ejaculate were considered as dependent variables.
Age and body mass index (BMI) were considered as the main confounding variables as they can be related to lipid profile, ovarian response, or sperm quality.
A correlation test of Pearson was performed to elucidate the association of lipid levels, age, and body mass index (BMI) with the number of metaphase II oocytes in women or the number of motile spermatozoa in men.
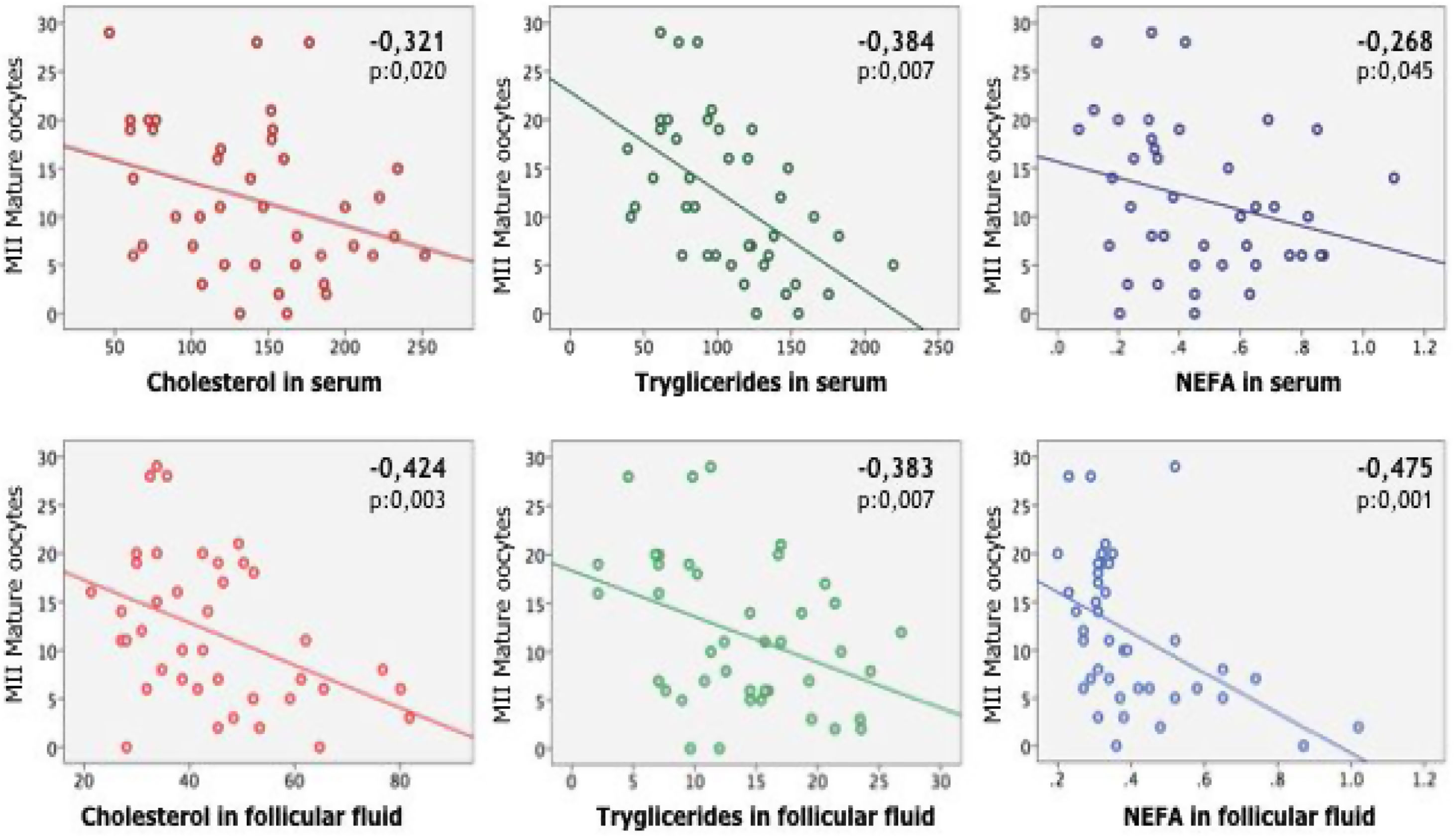
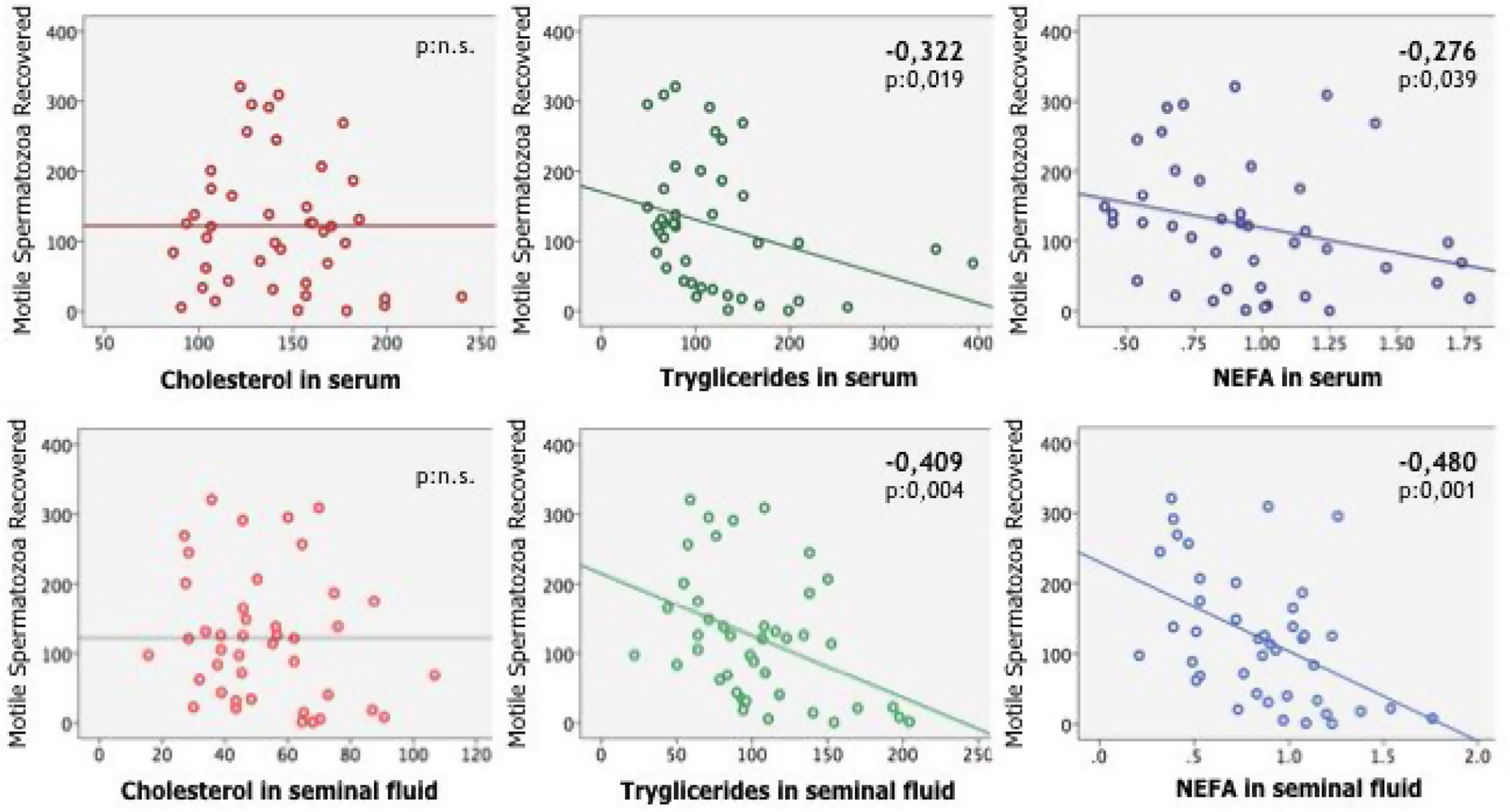
In women, was found a statistically significant correlation between the number of recovered mature oocytes on the one hand and age and all the lipid parameters analyzed on the other (Table 3). In males the number of motile sperm was significantly correlated with age and all lipid values, especially in seminal fluid, except cholesterol (Table 4).
Pearson correlation Test between number of recovered Metafase II oocytes, body mass index (BMI) and age with the independent variables analyzed in serum and follicular fluid: cholesterol, triglycerides, and non-esterified fatty acids (NEFA).
| Correlation | MII oocytes | BMI | Age |
|---|---|---|---|
| Cholesterol in serum | |||
| Pearson correlation | −.321 | .117 | .212 |
| Sig. (unilateral) | .020 | .234 | .092 |
| Cholesterol in follicular fluid | |||
| Pearson correlation | −.424 | .249 | .443 |
| Sig. (unilateral) | .003 | .058 | .002 |
| Triglycerides in serum | |||
| Pearson correlation | −.384 | .091 | −.007 |
| Sig. (unilateral) | .007 | .286 | .482 |
| Triglycerides in follicular fluid | |||
| Pearson correlation | −.383 | .226 | .147 |
| Sig. (unilateral) | .007 | .078 | .179 |
| NEFA in serum | |||
| Pearson correlation | −.268 | −.093 | .528 |
| Sig. (unilateral) | .045 | .281 | .000 |
| NEFA in follicular fluid | |||
| Pearson correlation | −.475 | .169 | .495 |
| Sig. (unilateral) | .001 | .145 | .001 |
Pearson's correlation test shows the existence of a significant negative correlation between the number of metaphase II oocytes obtained in women and the lipids analyzed. There was also found a statistically significant correlation between age and cholesterol in follicular fluid and NEFA (in serum and follicular fluid).
Significant difference: p<0.05. Correlations with p, 0.05 in bold typeface.
Pearson correlation test between number of motile spermatozoa count, BMI and age with the independent variables analyzed in serum and seminal fluid: cholesterol, triglycerides, and non-esterified fatty acids (NEFA).
| Correlation | Motile spermatozoacount (mill) | BMI | age |
|---|---|---|---|
| Cholesterol in serum | |||
| Pearson correlation | −.135 | −.092 | .269 |
| Sig. (unilateral) | .197 | .282 | .043 |
| Cholesterol in seminal fluid | |||
| Pearson correlation | −.193 | −.114 | .128 |
| Sig. (unilateral) | .110 | .237 | .209 |
| Triglycerides in serum | |||
| Pearson correlation | −.322 | .317 | .279 |
| Sig. (unilateral) | .019 | .021 | .037 |
| Triglycerides in seminal fluid | |||
| Pearson correlation | −.409 | −.154 | .499 |
| Sig. (unilateral) | .004 | .165 | .000 |
| NEFA in serum | |||
| Pearson correlation | −.276 | .180 | .286 |
| Sig. (unilateral) | .039 | .126 | .033 |
| NEFA in seminal fluid | |||
| Pearson correlation | −.480 | −.080 | .232 |
| Sig. (unilateral) | .001 | .308 | .070 |
The number of motile sperm was significantly correlated with age and all lipid values, especially in seminal fluid, except cholesterol.
Significant difference: p<0.05. Correlations with p, 0.05 in bold typeface.
Cholesterol, triglycerides and non-esterified fatty acid concentration levels in blood plasma and follicular fluid are statistically significantly correlated with the number of mature oocyte (MII) recovered after puncture (Fig. 1). Triglycerides and non-esterified fatty acid concentration levels in blood plasma and seminal plasma (excluding cholesterol) are statistically significantly correlated with the number of motile spermatozoa (Fig. 2).
To clarify the relationship between blood plasma and gonadal lipid profile and reproductive efficacy without the confounding effect of age and obesity, we used the method of principal component analysis (PCA). By using PCA we can extract a common factor to women and men which could explain the lipid profile in blood plasma and describe the lipid profiles in follicular and seminal fluids, respectively.
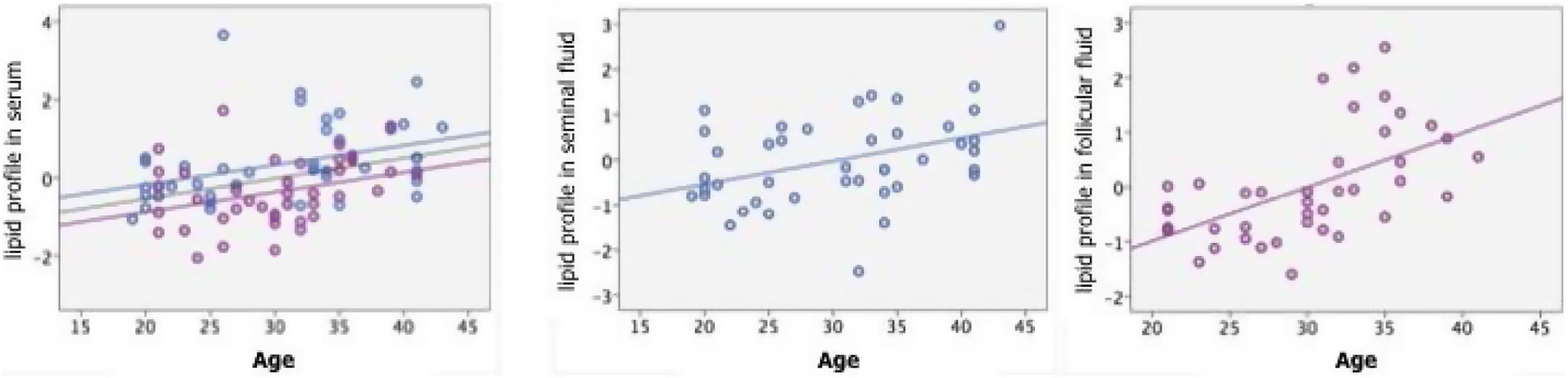
In a univariate way, significant correlations were found between the value of blood plasma lipids with age (Pearson: 0.346, p: 0.001) (Fig. 3), BMI (Pearson: 0.289, p: 0.008) and with gender, being higher in males than in females (T Student F: 0.401, p: 0.001). In follicular fluid, there was positive and significant correlation between lipids and age (Pearson: 0.551, p<0.001) (Fig. 3), although not with BMI (Pearson: 0.269, p: 0.089). This influence of age is maintained in a multivariate analysis independently of the BMI and the plasma lipid profile (t: 3.361, p: 0.002).
In seminal plasma, there was also a positive and significant correlation between lipids and age (Pearson: 0.385, p: 0.012) (Fig. 3) although not with BMI (Pearson: 0.152, p: 0.335).
This influence is maintained in a multivariate model independently of BMI and plasma lipid profile (t: 2.717, p: 0.010).
Effects of lipid profiles on the gonadal responseAccording to univariate analysis of variance, the number of MII oocytes and the number of motile spermatozoa were statistically significant correlated with age and lipid profile, both in blood plasma and gonadal fluid. However, no relationship was found with BMI (Table 5).
Values of the univariate and multivariate correlation between age, BMI and plasma lipid profiles with the oocyte response or seminal quality. For the two groups of women (left) and men (right) the first column indicates the value of the Pearson univariate correlation coefficient, and the second the correlation coefficient of a multivariate linear regression model with constant.
| Women | Men | |||
|---|---|---|---|---|
| Mature oocytes recovered | Motile spermatozoa count (mill/ml) | |||
| Univariate Correlation | Multivariate Linear Regression | Univariate Correlation | Multivariate Linear Regression | |
| BMI | –0.220 | –0.592 | –0.075 | 0.134 |
| Age | –0.330 | 0.288 | –0.515 | –2.194 |
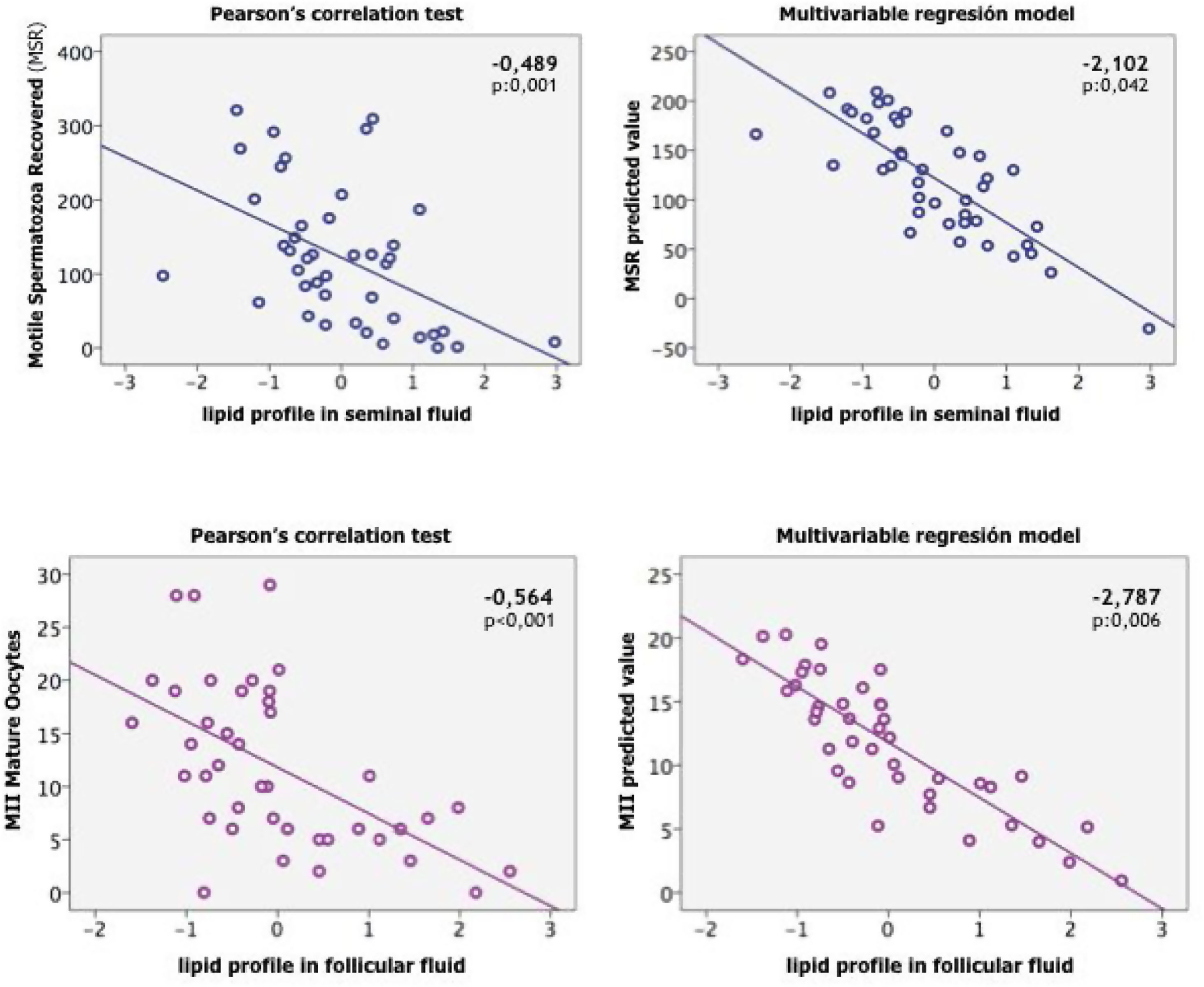
| Gonadal lipids | –0.564 | –2.787 | –0.489 | –2.102 |
| Plasma lipids | –0.532 | –2.709 | –0.350 | –1.013 |
| p BMI | 0.167 | 0.557 | 0.638 | 0.895 |
| p age | 0.035 | 0.775 | <0.001 | 0.035 |
| p gonadal lipids | <0.001 | 0.008 | 0.001 | 0.042 |
| p plasma lipids | 0.001 | 0.010 | 0.023 | 0.318 |
Using a multivariate linear regression model to exclude the effect of the age and BMI we found that in women, the lipid profile in follicular fluid impacts the number of mature oocytes retrieved, inversely, significantly, and independently of the age (Fig. 4).
In the male we also found that lipid profile in seminal plasma was independently associated with age and the number of motile spermatozoa (Fig. 4).
DiscussionLipids are involved in many important biological functions such as cellular signaling and in the development of different important pathologies including infertility.15 Lipid lipoprotein alterations are common in reproductive-age PCOS women.16 It can be found esterified FAs [triglycerides (TG), cholesterol esters (CHE) and phospholipids (PL)] or non-esterified FAs (NEFAs) in the follicular fluid (FF), mainly bound to albumin.17 Studies have associated elevated no esterified fatty acid and impaired oocyte development and even relationships have been found between serum fatty acids and pregnancy of women undergoing IVF.18
On the other hand, regarding the male population, other studies have showed significant correlation in between lipid composition in the sperm membrane and seminal plasma as well as increased phospholipid levels in seminal plasma in oligospermic and azoospermic patients.19 Studies on the lipid composition of human testis in patients with bilateral varicocele as cause of infertility have showed abundant lipids in the testis tissue. The total amount of lipids was 1.90 percent of the total wet weight of the human testis tissue. Testicular cholesterol, triglycerides and phospholipids were 26.50 percent, 28.50 percent, and 45 percent, respectively, of the total percentage of lipids.20 Also, abundant cholesterol and phospholipids were found in sperm membranes, especially polyunsaturated fatty acids (PUFA) closely related to sperm function.21
We have recently analyzed the correlation in between lipid levels in blood plasma and seminal plasma and semen parameters in infertile men compared with fertile sperm donors. We have found a correlation in between the levels of triglycerides both in serum and sperm, and sperm quality. A relationship was also found in between non-esterified fatty acids in seminal plasma and sperm quality, suggesting that male infertility could be influenced by lipid metabolism.10
Furthermore, we investigated the correlation of lipid levels in blood plasma and follicular fluid with number of oocyte and response to ovarian stimulation. The results showed that the level of NEFA in blood plasma as well as in follicular fluid and triglycerides in follicular fluid were slightly higher in patients compared with oocyte donors. Our study revealed that lipid levels in follicular fluid vary remarkably between women.10 Additionally, differing levels of these lipids, and presumably many additional cofactors in follicular fluid, can exert dramatically different effects, as previously showed by Yang et al.22 In agreement with our results, the study by Tehrani et al.,23 documented a positive association between lipid profiles and low ovarian reserve. These findings provide plausible mechanisms for our observed independent associations between female and male lipid concentrations and gonadal response.
Nevertheless, to this we add the confounding factors of age and BMI, which are related both to the lipid profile and to fertility. To the best of our knowledge, there is no other study evaluating the lipid profile in blood plasma, seminal plasma, and follicular fluid to assess the effect of lipid metabolism on the gonadal response regardless of age and BMI.
The findings presented here show the correlations between levels of Cholesterol, TG and NEFA in blood plasma, seminal plasma, and follicular fluid, with BMI, age, total number of metaphase II oocytes obtained at the puncture and total count of spermatozoa with progressive motility (Tables 3 and 4). Cholesterol, TG and NEFA levels in follicular fluid were correlated with ovarian response and age. We also found that levels of TG and NEFA but not cholesterol in seminal plasma were significantly correlated with sperm quality and age (Table 5).
Cholesterol, triglycerides and non-esterified fatty acid concentration levels in blood plasma and follicular fluid are statistically significantly correlated with the number of mature oocyte (MII) recovered after puncture (Fig. 1). Triglycerides and non-esterified fatty acid concentration levels in blood plasma and seminal plasma (excluding cholesterol) are statistically significantly correlated with the number of motile spermatozoa (Fig. 2).
In blood plasma, the levels of lipids were significantly correlated with age, (Pearson: 0.346, p: 0.001), BMI (Pearson: 0.289, p: 0.008) and gender, being higher in males than in females (T Student F: 0.401, p: 0.001) (Fig. 3). In follicular fluid, there was positive and significant correlation between lipids and age (Pearson: 0.551, p<0.001) although not with BMI (Pearson: 0.269, p: 0.089) (Fig. 3). In seminal plasma, there was also a positive and significant correlation between lipids and age (Pearson: 0.385, p: 0.012) although not with BMI (Pearson: 0.152, p: 0.335) (Fig. 3). In summary, we found that lipid profiles, both blood plasma and gonadal, are directly influenced by age and gender, with no direct relationship to BMI.
We further used a multivariate linear regression model between age, BMI and blood plasma lipid profiles with the oocyte response or seminal quality to exclude the effect of age and BMI. From the above-mentioned analysis, we found that that in women, the lipid profile in follicular fluid impacts the number of mature oocytes retrieved, inversely, significantly, and independently of the age (Fig. 4). In the male we also found that lipid profile in seminal plasma was independently associated with age and the number of motile spermatozoa (Fig. 4).
Valckx et al.24 studied BMI-related metabolic composition of the follicular fluid of women undergoing assisted reproductive treatments and the consequences for oocyte and embryo quality. In agreement with our results, they showed that metabolic anomalies in blood plasma are manifested in the follicular fluid, affecting oocyte quality, regardless of BMI. Similarly, Robker et al.25 demonstrated that the concentrations of follicular fatty acids had no significant relationships with BMI. Mirabi et al.,26 analyzed the effects of obesity-related follicular fluid (FF) fatty acids (FAs) on the number and quality of oocytes, good embryo quality rate, and pregnancy rate. They found that differences in BMI are not associated with the fatty acid composition of the FF.
Regarding the male factor, although there are controversial results in the relationships between obesity and male fertility. Chun et al.27 showed that serum lipid levels almost had no correlation with semen parameters, while lipid levels in seminal plasma could influence on semen volume, sperm motility, progressive motility, sperm concentration and total sperm count to a certain extent.
The observation that both gonadal response in men and women are not related with lipid profile in plasma, is supported by other studies. It has been postulated that reactive oxygen species levels may be higher in target tissues than in blood plasma and Conquer et al.,28 examined fatty acid composition simultaneously in spermatozoa, seminal plasma, and blood plasma in normozoospermic and asthenozoospermic males. They found decreased weight percentage of DHA both in spermatozoa and seminal plasma of asthenozoospermic patients compared with normozoospermic males.
ConclusionOur study highlights a significant influence of the lipid profile in follicular fluid and seminal plasma on the gonadal response. The effect can be observed on seminal production in the case of men and follicular response to hormonal stimulation in the case of women. The gonadal lipid profile is the only variable analyzed that maintains a statistical significance independent to the rest of the covariates, something that does not happen with the plasma lipid profile. Our current analysis further reinforces the association of both low ovarian response and low number of motile sperm with abnormal lipids levels. Our findings are the first to demonstrate that lipids in follicular and seminal fluid are associated with reduced gonadal response as measured by a PCA method.
The exact mechanisms by which both male and female lipid concentrations are shown to be independent predictors of gonadal response remain elusive. In the same way, it is yet not certain the origin of lipids in seminal plasma and the mechanism by which lipids affect sperm quality and ovarian reserve. However, as this is an ongoing study, we continue our research with follow-up which will allow us to arrive to further conclusions.
To conclude, we believe that these findings can lead to improvements in fertility assessment by the addition of lipid screening. After identifying the patients with abnormal metabolic profiles, women and men with abnormal lipid levels, several guidelines could be recommended such as increased medical controls and lifestyle changes.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document
Ethics approval and consent to participateThis work was approved by the Ethical Review Board of the Hospital de la Princesa (Madrid, Spain) (Register number: PI-827), and all subjects gave their written consent.
Availability of data and materialThe authors declare that data supporting the findings of this study are available within the article. The patient's files of this study are available from the corresponding author upon reasonable request.
FundingRocío Núñez Calonge declares that the study was funded by the special research fund, Fundacion Tambre.
Authors’ contributionsRN and JAG contributed to the conception and design of the study, data analysis, interpretation of the data and drafting of the manuscript. CA helped to draft the manuscript and English revision. SC was responsible for the collection of follicular fluid samples and aided to draft the manuscript. MS was responsible for the collection of semen samples. PC was responsible for patient recruitment, LMG and RK carried out the gas chromatographic analyses.
Conflict of interestThe authors declare that they have no conflict of interests.
We acknowledge the expert assistance and motivation of the gynecologists, embryologists and nurses of the Clinica Tambre for the recruitment of patients and medical procedures of oocyte retrieval and follicular fluid sampling. We also thank our colleagues of Andrology Laboratory for their assistance in processing sperm samples.