To make an extensive evaluation about the effects of histopathological findings acquired from the resection materials of patients who underwent transurethral resection of prostate (TUR-P) due to benign prostate hyperplasia (BPH) on postoperative urethral stricture formation.
Materials and methodsAmong patients who had TUR-P due to BPH and were followed up for minimum 6 months, 51 patients detected to have urethral stricture based on endoscopic imaging were included in the urethral stricture group (Group 1) and 52 patients without urethral stricture were included in the control group (Group 2). The relation between histopathological findings of TURP materials and postoperative stricture occurrence was investigated.
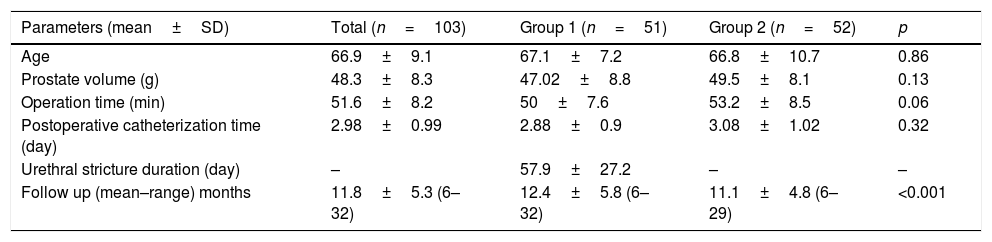
ResultsNo difference in age, prostate volume, operation time and postoperative catheterization time was detected among the groups (p=0.86, p=0.13, p=0.06, p=0.32, respectively). Average time until the urethral stricture diagnosis in the group with urethral stricture was measured as 57.9±27.2 days. In our study, inflammation intensity in peri-urethral, stromal and periglandular areas and intraglandular destruction ratios were higher in urethral stricture group (Group 1) (p=0.048, p=0.3, p=0.03, p=0.01, respectively). Again, it was detected that neutrophil, plasmocyte and eosinophil cell ratios were higher in peri-urethral, stromal and periglandular areas and lymphocyte values were lower compared to the control group.
ConclusionAcquired data has shown that acute inflammatory attacks may be related to urethral stricture with a mostly chronic inflammation background in the prostate. During histopathological examination of prostate tissue acquired through TURP, especially high peri-urethral neutrophil, plasmocyte and eosinophil cell ratios and intra-glandular destruction ratios are important for predicting postoperative urethral stricture occurrences.
Realizar una evaluación exhaustiva de los efectos de los hallazgos histopatológicos adquiridos de los materiales de resección de pacientes que tuvieron resección transuretral de próstata (RTU-P) debido a hiperplasia prostática benigna (HPB) en la formación de estenosis uretral postoperatoria.
Materiales y métodosEntre los pacientes que tenían RTU-P debido a BPH fueron seguidos durante un mínimo de 6 meses, 51 pacientes detectados con estenosis uretral basada en imágenes endoscópicas fueron tomados en el grupo de estenosis uretral (Grupo 1) y 52 pacientes sin estenosis uretral fueron tomados en el control grupo (Grupo 2). Se investigó la relación entre los hallazgos histopatológicos de los materiales de RTUP y la aparición de estenosis postoperatoria.
ResultadosNo se detectaron diferencias en la edad, el volumen de la próstata, el tiempo de operación y el tiempo de cateterismo postoperatorio entre los grupos (p=0,86; p=0,13; p=0,06; p=0,32, respectivamente). El tiempo promedio transcurrido hasta el diagnóstico de estenosis uretral en el grupo con estenosis uretral se midió como 57,9±27,2 días. En nuestro estudio, la intensidad de la inflamación en las áreas periuretral, estromal y periglandular y las relaciones de destrucción intraglandular se midieron más en el grupo de estenosis uretral (Grupo 1) (p=0,048; p=0,3; p=0,03; p=0,01, respectivamente). Nuevamente, se detectó que las relaciones de células de neutrófilos, plasmocitos y eosinófilos eran más altas en las áreas periuretral, estromal y periglandular y los valores de linfocitos eran más bajos en comparación con el grupo de control.
ConclusiónLos datos adquiridos han demostrado que los ataques inflamatorios agudos pueden estar relacionados con la estenosis uretral principalmente en el contexto de inflamación crónica en la próstata. En el examen histopatológico del tejido prostático adquirido a través de RTUP, especialmente las altas tasas de neutrófilos peri-uretrales, plasmocitos y células de eosinófilos y las tasas de destrucción intraglandular son importantes para predecir los casos de estenosis uretral postoperatoria.
Urethral strictures are a well-known complication following TURP procedures, affecting between 2.2 and 9.8% of patients (0.6% of all men) and causing low urinary flow, urinary tract infections, or acute urinary retention. In most cases, a second surgical procedure is required to correct the problem, often within 6 months.1–3
Urethral epithelium injury caused by strictures can result in epithelial or corpus spongiosum fibrosis. Location of the injury and the patient's age may play a role in the parameters and etiology of stricture. Anterior urethral strictures are usually, caused by inflammation, iatrogenic or trauma, while posterior urethral strictures mostly occur after surgery or pelvic fracture. A recent study based on histopathological examinations of samples taken during urethroplasty procedures demonstrated that urethral inflammation and inflammatory cells are significant factors in the development of strictures.3 Other previous studies based on histopathological examinations of the prostate evaluated the degree of inflammation based on the presence of inflammatory cells in the tissue. The results of these studies indicated that these cells may play an active role in the pathogenesis of prostate diseases.2–4
The objective of this study is to use histopathological examinations to evaluate levels of inflammation and inflammatory cells in patients who required a TURP procedure due to BPH, the relationship between these parameters, and the occurrence of postoperative urethral strictures.
Materials and methodsThe sample population for this study comprised 1745 patients who underwent TURP procedures to treat BPH in the urology clinic of a university hospital between January 2010 and June 2018. Only those patients who used 20 Fr three-way catheters after their operation were included in the study. Patients who were catheterized due to a preoperative condition (i.e. documented diabetes mellitus, chronic inflammatory disease, urinary infection or prostatitis, prostate cancer, a previous prostate or urethral operation, or urinary retention), who had urethral catheters larger than 20 Fr after TURP or monopolar TUR-P, and those who were not followed for at least 6 months, meatal and distal urethral strictures and bladder neck sclerosis were excluded. The patients who were included in the study were divided into two groups. Group 1, the stricture group, comprised 51 patients who developed BPH following a TURP procedure, were followed for a minimum of 6 months, were reported to have obstructive patterns based on their uroflowmetry results, and were found to have urethral stricture in bulbo-membranous urethra, as revealed by urethrocystoscopy. Group 2, the postoperative control group, was made up of 52 patients who reported post-TURP pathologies such as BPH, were followed for a minimum of 6 months, and had a minimum of two postoperative uroflowmetry examinations within a minimum of 3 months. These patients had minimum urination volume of 150mL, mean flow rate of 10mL/s, and maximum flow rate over 15mL/s.5 A flow diagram showing how the patients were selected is presented in Fig. 1.
Prostate size, operation time, catheterization time, and duration of hospitalization, all factors that affected the treatment of urethral stricture in both groups, were recorded for all patients; for Group 1, the time from their procedure to the diagnosis of stricture was also recorded. All patients received antimicrobial prophylaxis consisting of 1g cefazolin administered preoperatively and were operated using plasmakinetic bipolar energy (200/120W) and a 26F resectoscope under continuous isotonic irrigation (Gyrus Acmi, Olympus Medical, Japan).
Histopathological examinations of the TURP specimens from both groups were used to investigate the association between an elevated degree of inflammation and the development of stricture.
Histopathological examinationThe numbered glasses for the cases included in the study were located in the pathology laboratory slide archive. Five to 15 H&E-stained slides for each case (10 slides on average) were evaluated using a light microscope (Olympus BX51). All surfaces were examined for inflammation (periglandular, periurethral, stromal), glandular destruction, gland-to-stroma ratio, presence of corpora amylacea, and presence of lymphoid follicle. Periglandular, periurethral, and stromal inflammations were evaluated separately. Inflammation intensity (scored from 0 to 3), percentage of inflammatory cells (%), and presence of epithelial and intraluminal inflammatory cells in glandular areas (present/none) were recorded. As inflammation was not homogenously distributed on all slides, a dominance of intensity of more than 50% of all areas was assessed for each case.
The intensity of inflammation was scored as follows:
0: Few to no inflammatory cells present.
1: Low degree of inflammation (+), covering nearly 10% of the area, visible at 40× magnification.
2: Moderate degree of inflammation (++), covering nearly 30–40% of the area, visible at 40× magnification.
3: Very intense inflammation (+++), covering 70% or more of the area, visible at 40× magnification.
The types and percentages (%) of inflammatory cells present in the periglandular, urothelial, subepithelial, and stromal areas were recorded (i.e. lymphocytes, plasmocytes, histiocytes, neutrophils, eosinophils, and mast cells). Partially or completely fragmented glands with neutrophil-filled lumens were defined as destructed glands; destructed glands were recorded as present even if only one was identified. The percentages of glandular hyperplasia and stromal hyperplasia areas present in each slide were determined and recorded. The number of all glands with corpora amylacea in their lumens was determined as a percentage. The presence of lymphoid follicle structures (generally primary, rarely secondary) formed by lymphocytes were recorded as present or none. The presence and type (if identifiable) of inflammatory cells inside the gland, epithelium, or gland lumen were recorded.
Statistical analysisThe statistical analysis was performed using SPSS v. 23.0 statistical software (SPSS Inc., Armonk, NY, United States). χ2 was used to determine whether the distributions of categorical variables differed between groups. The categorical variables are described as frequencies and percentages. Continuous variables are presented as mean and standard deviations. The independent samples t-test was used to compare the continuous variables between groups. The Pearson's correlation coefficients of continuous variables were calculated and a p value <0.05 was considered statistically significant.
ResultsOf the 1745 patients who underwent a TURP procedure at a single hospital between January 2010 and June 2018, 103 were included in this study. The patients were separated into two groups: Group 1 comprised 51 patients who developed a urethral stricture after their TURP and Group 2 comprised 52 patients who were found not to develop a urethral stricture at the time of their 6-month follow-up.
No differences in average age, prostate volume, operation time, or postoperative catheterization time were detected between the groups (p=0.86, 0.13, 0.06, and 0.32, respectively). The average amount of time between the TURP procedure and the diagnosis of stricture in the patients who developed urethral stricture (Group 1) was measured as 57.9±27.2 days (see Table 1). Patients were followed up for mean 11.8±5.3 (6–32) months. The follow-up duration in the group with stricture was identified to be significantly longer (p<0.001) (Table 1).
Surgical parameters.
| Parameters (mean±SD) | Total (n=103) | Group 1 (n=51) | Group 2 (n=52) | p |
|---|---|---|---|---|
| Age | 66.9±9.1 | 67.1±7.2 | 66.8±10.7 | 0.86 |
| Prostate volume (g) | 48.3±8.3 | 47.02±8.8 | 49.5±8.1 | 0.13 |
| Operation time (min) | 51.6±8.2 | 50±7.6 | 53.2±8.5 | 0.06 |
| Postoperative catheterization time (day) | 2.98±0.99 | 2.88±0.9 | 3.08±1.02 | 0.32 |
| Urethral stricture duration (day) | – | 57.9±27.2 | – | – |
| Follow up (mean–range) months | 11.8±5.3 (6–32) | 12.4±5.8 (6–32) | 11.1±4.8 (6–29) | <0.001 |
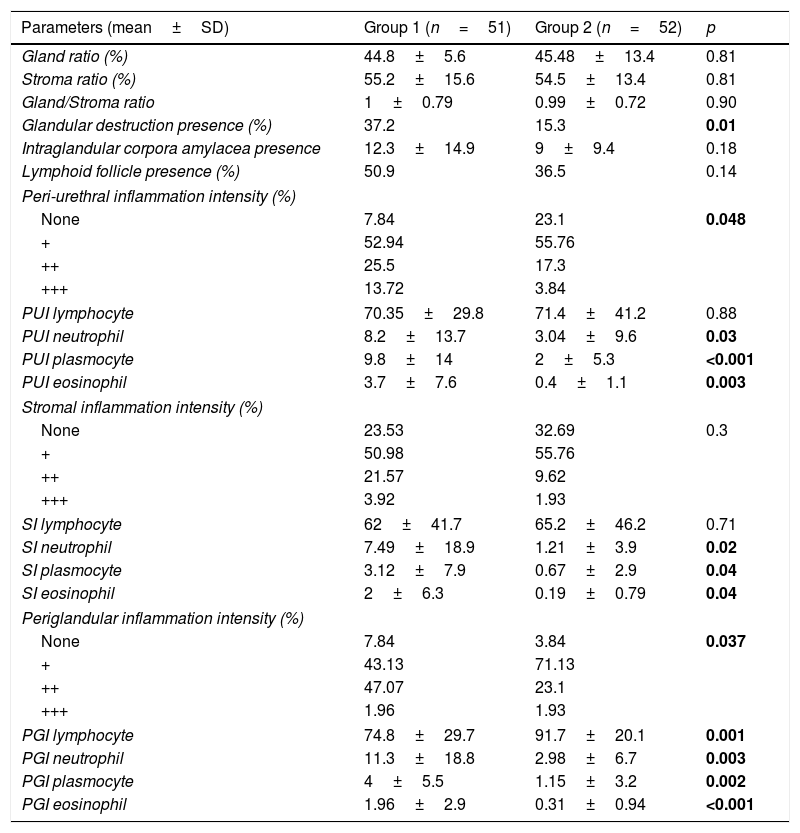
The evaluation of histopathological parameters revealed that there were no differences in gland ratio, stroma ratio, gland/stroma ratio, presence of intraglandular corpora amylacea, or presence of lymphoid follicles between the two groups (all parameters were p>0.05). However, the intraglandular destruction ratio was found to be significantly high in the urethral stricture group (Group 1) (p=0.01) (Fig. 2).
The periurethral inflammation intensity and the periurethral neutrophil, plasmocyte, and eosinophil percentages were significantly higher in Group 1; although the periurethral lymphocyte percentages were higher in Group 2, there was no significant difference between the groups in this regard (p=0.048, 0.03, <0.001, 0.003, and 0.88, respectively) (see Fig. 3). The stromal inflammation intensity and stromal neutrophil, plasmocyte, and eosinophil percentages were higher in Group 1; while there were statistically significant differences in these three numbers between the two groups, that of stromal inflammation intensity was not significantly high (p=0.02, 0.04, 0.04, and 0.3, respectively). Although the stromal lymphocyte percentage in Group 2 was high, the difference was not significant (p=0.71) (Fig. 4).
(A) 100×, H&E, mild inflammation involving only lymphocytes under the urethral epithelium in the non-stricture group. (B) ×200, H&E, inflammation in the stricture group with moderate eosinophils and plasmocytes under the urethral epithelium. (C) ×100, H&E, severe inflammation involving eosinophils and plasmocytes in sub-urethral epithelium in the stricture group, destroyed by the severity of epithelial inflammation (black arrow). (D) ×1000, H&E, severe inflammation involving eosinophils and plasmocytes in the stricture group. (E) ×200, H&E, periglandular, severe inflammation involving eosinophils and plasmocytes in the stricture group. (F) ×1000, H&E, eosinophils (red arrow) and plasmocytes (blue arrow) in the stricture group. (G) ×200, H&E, periglandular inflammation involving only lymphocytes in the non-stricture group. (H) ×1000, H&E, eosinophils and plasmocytes do not appear in the non-stricture group.
While the periglandular inflammation intensity and periglandular neutrophil, plasmocyte, and eosinophil percentages recorded for Group 1 were higher, the average periglandular lymphocyte percentage in these patients was lower than that of Group 2. The differences between the groups for all periglandular parameters were statistically significant (p=0.037, 0.003, 0.002, <0.001, and 0.001, respectively). The values for all histopathological parameters are presented in Table 2.
Parameters related to histopathological evaluation.
| Parameters (mean±SD) | Group 1 (n=51) | Group 2 (n=52) | p |
|---|---|---|---|
| Gland ratio (%) | 44.8±5.6 | 45.48±13.4 | 0.81 |
| Stroma ratio (%) | 55.2±15.6 | 54.5±13.4 | 0.81 |
| Gland/Stroma ratio | 1±0.79 | 0.99±0.72 | 0.90 |
| Glandular destruction presence (%) | 37.2 | 15.3 | 0.01 |
| Intraglandular corpora amylacea presence | 12.3±14.9 | 9±9.4 | 0.18 |
| Lymphoid follicle presence (%) | 50.9 | 36.5 | 0.14 |
| Peri-urethral inflammation intensity (%) | |||
| None | 7.84 | 23.1 | 0.048 |
| + | 52.94 | 55.76 | |
| ++ | 25.5 | 17.3 | |
| +++ | 13.72 | 3.84 | |
| PUI lymphocyte | 70.35±29.8 | 71.4±41.2 | 0.88 |
| PUI neutrophil | 8.2±13.7 | 3.04±9.6 | 0.03 |
| PUI plasmocyte | 9.8±14 | 2±5.3 | <0.001 |
| PUI eosinophil | 3.7±7.6 | 0.4±1.1 | 0.003 |
| Stromal inflammation intensity (%) | |||
| None | 23.53 | 32.69 | 0.3 |
| + | 50.98 | 55.76 | |
| ++ | 21.57 | 9.62 | |
| +++ | 3.92 | 1.93 | |
| SI lymphocyte | 62±41.7 | 65.2±46.2 | 0.71 |
| SI neutrophil | 7.49±18.9 | 1.21±3.9 | 0.02 |
| SI plasmocyte | 3.12±7.9 | 0.67±2.9 | 0.04 |
| SI eosinophil | 2±6.3 | 0.19±0.79 | 0.04 |
| Periglandular inflammation intensity (%) | |||
| None | 7.84 | 3.84 | 0.037 |
| + | 43.13 | 71.13 | |
| ++ | 47.07 | 23.1 | |
| +++ | 1.96 | 1.93 | |
| PGI lymphocyte | 74.8±29.7 | 91.7±20.1 | 0.001 |
| PGI neutrophil | 11.3±18.8 | 2.98±6.7 | 0.003 |
| PGI plasmocyte | 4±5.5 | 1.15±3.2 | 0.002 |
| PGI eosinophil | 1.96±2.9 | 0.31±0.94 | <0.001 |
Abbreviations: PUI: peri-urethral inflammation, SI: stromal inflammation, PGI: periglandular inflammation.
No significant correlation was detected with regard to age, prostate volume, operation time, or postoperative catheterization time, and the pathological parameters based on the correlation analysis were recorded (p>0.05 for all parameters).
DiscussionOne of the late postoperative period (>4–6 weeks) complications of prostatectomies required to treat BPH is the development of urethral stricture. The prevalence of urethral strictures following TURP is 2.2–9.8%.6,7 This rate has decreased in recent years (previous studies identified a rate of 4–29%) due to improvements in techniques and surgical instruments.8–10 The percentage of TURP patients who developed a stricture after their procedure was found to be 3.81% (51/1338) in this study.
High prostate volume and associated long operation time, the size and material of the catheter used, infected urine, thick shaft, high energy use, an excessive number of surgical incisions, and damage to the urethral mucosa caused by energy leaks in the shaft are among the causes of urethral stricture.9,10 No differences in age, prostate volume, operation time, or postoperative catheterization time were detected between the two groups in our study (p=0.86, 0.13, 0.06, and 0.32, respectively). For Group 1, the average amount of time that passed until stricture developed was 57.9±27.2 days (Table 1).
Apart from the causes discussed above, there are a number of other hypotheses regarding why urethral strictures develop. Immediately following a prostatectomy or TURP procedure, the wound sites on the bladder neck and membranous urethra form new mucosa that cover the operated prostatic urethra, and blood clots, fibrin, and necrotic cell groups are removed from the body through spontaneous miction. If there is no long-term miction during the postoperative period, blood clots and fibrin accumulate at these wound sites, causing fibrosis and the formation of strictures.11 Based on this theory—which has not yet been proven histopathologically or urodynamically—minimizing catheterization time may lower the occurrence of strictures. The average catheterization time was measured as 2.98 days in our study.
Another hypothesis posits that urethral trauma initiates the development of strictures and the resulting mucosal damage causes urinary extravasation into the subepithelial void, edema, and progressive inflammation, thereby causing urethral stenosis and strictures; the increased intramural urination pressure then exacerbates leakage in the mucosal barrier.8,9,12 It was reported that isolated erythematous areas can be seen on urethral mucosa during reinstrumentation a few days after a resection.8 Myofibroblasts are likely to be responsible for stricture formation and giant cells play a role in continuing collagen synthesis. Pansadoro and Emiliozzi claimed that prostatic urethral strictures occurred following delayed epithelialization combined with fibrotic tissue overgrowth.13
These complications may be prevented by avoiding over-resection of the bladder neck, using suitable generators, conducting periodic maintenance of the necessary devices, early detection of possible leakage points in the shaft used, and using an adequate amount of lubricant.14
Although BPH is most common in older men, very few predictive factors are known. Prostatic inflammation was recently identified as one such factor. Very few T and B lymphocytes, macrophages, and mast cells (all of which are inflammatory cells) are detected in the prostates of 12-week embryos. These cells proliferate gradually with age, and in most BPH tissue samples obtained via biopsy, surgery, and autopsy, prostate inflammation is commonly identified during histopathological examination, even if prostatitis anamnesis or clinical symptoms are not present.15–17 Histopathologically confirmed prostatic inflammation is a common finding in biopsy and surgical specimens from elderly male patients with BPH and is reportedly present in 43–77% of samples.18–20
Such inflammation is generally chronic, and the age of the patient and prostate size are directly proportional.21 In studies examining inflammatory cell populations in cases of BPH, it was shown that nearly 80% of inflammatory cells were T lymphocytes; B lymphocytes were usually found in areas with significant follicular formation and diffuse infiltration, and macrophages were especially common in areas with atrophic and cystic changes, regardless of inflammation.15 In their study of patients who did not have prostatitis anamnesis or other symptoms and who underwent TURP procedures, Nicket et al. graded inflammatory cell intensities and categorized the distribution patterns they identified as periglandular, stromal, and periurethral. Based on their comparative examination, they also detected that increases in inflammatory cell intensity and prevalence of these cells in tissues were concurrent.22 In this study, inflammatory intensity and cellular distribution patterns of TURP specimens were evaluated separately in the periglandular, stromal, and periurethral areas.
Two hypotheses were presented to explain the development of chronic inflammation in BPH. The first hypothesis, which focuses on infection and its results, asserts that nonspecific chronic inflammation occurs due to the infiltration of prostatic secretions into the stroma due to the rupture of acini.23 The second hypothesis posits that the development of strictures is an autoimmune response, which is supported by the frequent detection of bacteria and viruses in BPH tissue samples and the co-occurrence of these factors in chronic prostatitis.24 While inflammation was not observed in 16.4% of specimens following histopathological examinations of the prostate tissues from the 103 patients included in this study, low, medium, and very intense (+, ++, +++) degrees of inflammation were observed in 83.6% of the specimens.
As far as is known, a high number of neutrophils present in inflammation indicates acute inflammation and high numbers of plasmocytes and eosinophils indicate chronic inflammation. These assumptions are supported by studies of prostatitis and eosinophilic cystitis. Increases in neutrophil intensity in periglandular areas is an especially important indicator for acute prostatitis.25 In this study, it was observed that neutrophil intensity reflecting acute inflammation in the periurethral, stromal, and periglandular areas of the prostate and plasmocyte and eosinophil cell ratios reflecting chronic inflammation were significantly higher in Group 1 (patients with urethral strictures). Distinctive intra-glandular destruction is the most important histopathological indicator of acute prostate inflammation and was found to be significantly high in Group 1 (p=0.01).
Acute and chronic inflammation, as indicated by high neutrophil, plasmocyte, and eosinophil cell ratios, especially in periurethral areas, may be significant contributing factors to the occurrence of urethral strictures. Such high ratios also exacerbate fibrosis and were found to be higher in Group 1 patients.
Another interesting finding of this study was that lymphocyte cell percentages were significant in the periglandular area (p=0.001) but insignificant in the stromal and periurethral areas, and were lower in Group 1 patients than the control group. This may also be a result of the dominance of those cells involved in chronic inflammation.
All these findings indicate that inflammation may promote the occurrence of urethral stricture after TURP, particularly the presence of chronic inflammation in prostate tissue. We think that assessing inflammation intensity and inflammatory cells while evaluating TURP specimens may be of value in predicting the occurrence of urethral strictures.
The limitations of this study are the number of patients evaluated, the TURP procedures reviewed being conducted by more than one surgeon, and the evaluation of inflammation only at the cellular level.
ConclusionThis study examined the role of prostate inflammation in the pathogenesis of urethral strictures. The data we reviewed revealed that the presence of acute inflammation may lead to the development of urethral strictures, particularly when chronic inflammation is present in the prostate. Histopathological examinations of prostate tissue revealed that periurethral neutrophil, plasmocyte, and eosinophil cell percentages and rates of intra-glandular destruction are especially important in predicting the development of postoperative urethral strictures. We think that recording inflammation intensity and inflammatory cell levels in pathologist reports while evaluating tissue specimens after TURP procedures would help urologists predict the occurrence of urethral strictures. We also expect that our findings will be confirmed by further studies that include higher numbers of patients and different parameters for inflammation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Ethics Committee: Ethics Committee Approval: Authors declared that the research was conducted according to the principles of the World Medical Association Declaration of Helsinki “Ethical Principles for Medical Research Involving Human Subjects” (amended in October 2013).
FundingThe authors received no financial support for the research, authorship, and/or publication of this article.
Author's contributionA Aydın: project development, manuscript writing.
P Oltulu: data collection, data analysis.
M Balasar: project development, manuscript writing, data management, manuscript editing.
MG Sönmez: data analysis, manuscript editing.
HH Taşkapu: data collection, manuscript editing.
MS Özkent: data collection.
F Kilinç: data analysis.
Conflict of interestNone of the authors have any potential conflict of interest.
The authors state that they have no proprietary interest in the products named in this article.
Author hereby declares that he has no financial disclosures and acknowledging any financial support.