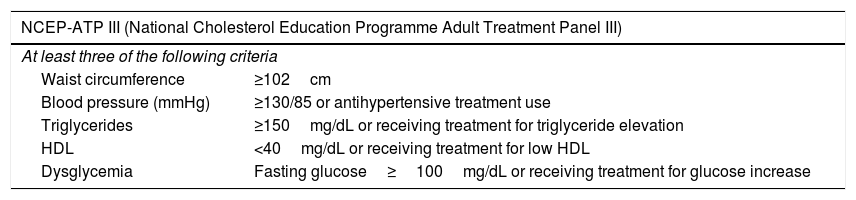Prostate cancer (PCa) is the most widespread malignancy within men. Androgen deprivation therapy (ADT), which is the central component of advanced PCa treatment, causes side effects. The goal of this study was to examine the metabolic changes and bioelectrical impedance analysis differences in PCa patients who received ADT.
Materials and methodsAfter age-related match-pair analysis, a total of 519 patients with PCa and control group who had benign disease were enrolled in the study. Biochemical blood parameters and TANITA measurements were recorded for all patients. Patients were categorized into three groups, ADT group (Group 1, n=124) and non-ADT group (Group 2, n=248), control group (Group 3, n=147).
ResultsThe mean age of groups was similar. Body mass index, waist circumference, body fat mass and fat ratio, which were among the TANITA parameters, were higher in group 1 (p<0.05). Total cholesterol, high density lipoprotein, non- high density lipoprotein, triglycerids and fasting blood glucose values were also higher in group 1 (p<0.05). Myocardial infarction and metabolic syndrome rates were also higher in this group.
ConclusionsWhile the use of ADT is manifested by an increase in fat mass and fat ratio in body composition, it negatively affects waist circumference measurements. It is associated with metabolically unfit body composition changes that predispose to diabetes mellitus and may increase cardio-vascular disease. For this reason, it is necessary to be careful about metabolic and endocrinological diseases in long-term therapy.
El cáncer de próstata (CaP) es la neoplasia maligna más extendida en los hombres. La terapia de privación de andrógenos (ADT), que es el componente central de su tratamiento avanzado, causa efectos secundarios. El objetivo de este estudio fue examinar los cambios metabólicos y las diferencias de análisis de impedancia bioeléctrica en pacientes con CaP que recibieron ADT.
Materiales y métodosDespués del análisis de pares de parejas relacionados con la edad, un total de 519 pacientes con CaP y un grupo de control con enfermedad benigna se inscribieron en el estudio. Se registraron parámetros sanguíneos bioquímicos y mediciones de TANITA para todos los pacientes. Los pacientes se clasificaron en 3 grupos: grupo ADT (grupo 1; n=124), grupo no ADT (grupo 2; n=248) y grupo control (grupo 3; n=147).
ResultadosLa edad media de los grupos fue similar. El índice de masa corporal, la circunferencia de la cintura, la masa de grasa corporal y la proporción de grasa, que se encontraban entre los parámetros de TANITA, fueron mayores en el grupo 1 (p<0,05). El colesterol total, las lipoproteínas de alta densidad, las lipoproteínas de baja densidad, los triglicéridos y los valores de glucosa en sangre en ayunas también fueron más altos en el grupo 1 (p<0,05). Las tasas de infarto de miocardio y síndrome metabólico también fueron más altas en este grupo.
ConclusionesSi bien el uso de ADT se manifiesta por un aumento en la masa grasa y la proporción de grasa en la composición corporal, en cambio, afecta negativamente las mediciones de circunferencia de la cintura. Se asocia con cambios en la composición corporal metabólicamente inadecuados que predisponen a la diabetes mellitus y pueden aumentar la enfermedad cardiovascular. Por esta razón, es necesario tener cuidado con las enfermedades metabólicas y endocrinológicas en la terapia a largo plazo.
Prostate cancer (PCa) was proven to be dependent on androgens in 1941 by Huggins and Hodges.1 After these developments, PCa was tried to be treated by eliminating androgenic stimulation. Discontinuation of androgen production or desensitization of androgen receptors has become the options of this treatment. Gonadotropin releasing hormone (GnRH) agonists or performing bilateral orchiectomy provide this effect.2 Androgen deprivation therapy (ADT) is a milestone in the cure of locally advanced or metastatic PCa. However, this treatment has very substantial disadvantages. Some metabolic side effects of ADT, which are not known much, have gained importance in recent years and the studies on this subject have increased gradually. It is important for physicians to know the effects during this treatment due to both the occurrence of metabolic side effects and the increase in cardio-vascular morbidity in patients with ADT. In this study, we aimed to compare the body structure, biochemical values and metabolic parameters of PCa patients with and without ADT together with the control group.
Materials and methodsAfter the local ethics committee approval of our hospital (decision number is 2020/0093), patients with PCa and benign prostate hyperplasia diagnosis in our clinic between May 2018 and February 2020 were retrospectively reviewed. After age-related match-pair analysis, a total of 519 patients who had lipid profile values, bioelectrical impedance analysis and metabolic parameters were included in the study. Patients were categorized into three groups; ADT group (Group 1), non-ADT group (Group 2) and control group (Group 3). The patients in Group 1 consisted of men who received ADT treatment for at least 12 months. Patients in groups 2 and 3 consisted of age-matched PCa who received a treatment other than ADT and non-PCa men, respectively. Body mass index (BMI), measurement of waist circumference (WC) and biochemical blood parameters were recorded for all patients. Body composition of all patients was measured using a bioelectrical impedance analyzer (Tanita MC-780MA). BMI was calculated as weight/height square (kg/m2). WC was measured at the midpoint between the bottom of the rib cage and the uppermost boundary of the iliac crests at the end of expiration in standing positions with a measuring tape. Patients carried out the laboratory measurements after an 8-h fast in the mornings. Blood pressure was measured three times in a row with the standard sphygmomanometer manual blood pressure cuff monitor kit on patient's visit. The metabolic syndrome (MetS) was determined considering the National Cholesterol Education Programme Adult Treatment Panel III criteria (NCEP-ATP III).3 Visceral adiposity index (VAI) score was calculated using the male-specific equation ((WC/(39.68+(1.88×BMI)))×(Triglycerides (Tg)/1.03)×(1.31/High Density Lipoprotein (HDL)).4
Statistical analysisThe examination of data was obtained using the Statistical Package for the Social Sciences version 22.0 for Windows (SPSS Inc., IBM, NY, USA). One-Sample Kolmogorov–Smirnov test was applied to evaluate the normality of the distribution for quantitative data. The one-way ANOVA analysis was used for the variables of quantitative data that had a normal distribution and the Kruskal–Wallis test was utilized for the others. The Pearson chi-square or Fisher's exact tests were used for the comparison of independent categorical variables. The level of statistical significance was defined as p<0.05.
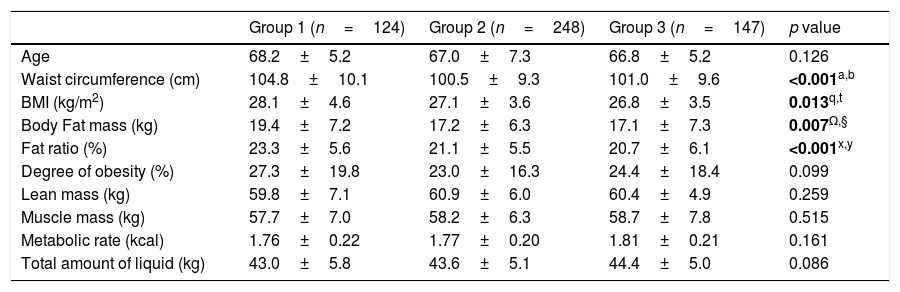
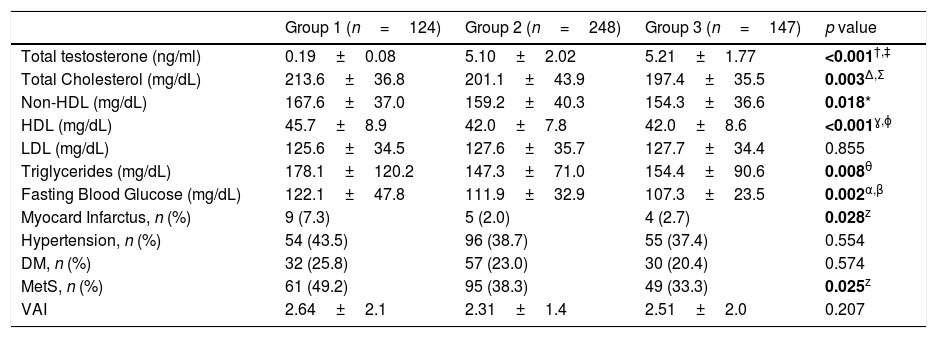
ResultsPatient characteristics and body composition measurements of all 3 groups are summarized in Table 1. In our comparison, the patients in group 1 had significantly higher BMI (p=0.013). WC measurements of patients in group 1 were also higher (p<0.001). PCa patients who received ADT had significantly higher body fat mass, fat ratio and lean mass values in body composition measurements compared with the other two groups (p<0.05). Serum total testosterone levels in the ADT group were remarkably lower than the other two eugonadal groups (p<0.001). Serum lipid values other than low density lipoprotein (LDL) cholesterol were also higher in the ADT group (p<0.05). Although the groups were similar in terms of patients with Diabetes Mellitus (DM), fasting glucose levels were higher in group 1 (p=0.002). It was found that the incidence of having a heart attack was higher in the ADT group (p=0.028). Almost half of the men in the ADT group (49.2%) met the criteria for MetS compared with 38.3% and 33.3% of men in the non-ADT and control groups, respectively (p=0.025). Although the VAI score, which was stated to predict cardiometabolic risk, was higher in the ADT group, no significant difference was found (p=0.207) (Table 2).
Clinical characteristics of patients and Tanita components.
| Group 1 (n=124) | Group 2 (n=248) | Group 3 (n=147) | p value | |
|---|---|---|---|---|
| Age | 68.2±5.2 | 67.0±7.3 | 66.8±5.2 | 0.126 |
| Waist circumference (cm) | 104.8±10.1 | 100.5±9.3 | 101.0±9.6 | <0.001a,b |
| BMI (kg/m2) | 28.1±4.6 | 27.1±3.6 | 26.8±3.5 | 0.013q,t |
| Body Fat mass (kg) | 19.4±7.2 | 17.2±6.3 | 17.1±7.3 | 0.007Ω,§ |
| Fat ratio (%) | 23.3±5.6 | 21.1±5.5 | 20.7±6.1 | <0.001x,y |
| Degree of obesity (%) | 27.3±19.8 | 23.0±16.3 | 24.4±18.4 | 0.099 |
| Lean mass (kg) | 59.8±7.1 | 60.9±6.0 | 60.4±4.9 | 0.259 |
| Muscle mass (kg) | 57.7±7.0 | 58.2±6.3 | 58.7±7.8 | 0.515 |
| Metabolic rate (kcal) | 1.76±0.22 | 1.77±0.20 | 1.81±0.21 | 0.161 |
| Total amount of liquid (kg) | 43.0±5.8 | 43.6±5.1 | 44.4±5.0 | 0.086 |
PCa: prostate cancer, ADT: androgen deprivation therapy, BMI: body mass index.
Biochemical values and curriculum vitae of patients.
| Group 1 (n=124) | Group 2 (n=248) | Group 3 (n=147) | p value | |
|---|---|---|---|---|
| Total testosterone (ng/ml) | 0.19±0.08 | 5.10±2.02 | 5.21±1.77 | <0.001†,‡ |
| Total Cholesterol (mg/dL) | 213.6±36.8 | 201.1±43.9 | 197.4±35.5 | 0.003Δ,Σ |
| Non-HDL (mg/dL) | 167.6±37.0 | 159.2±40.3 | 154.3±36.6 | 0.018* |
| HDL (mg/dL) | 45.7±8.9 | 42.0±7.8 | 42.0±8.6 | <0.001ɣ,ɸ |
| LDL (mg/dL) | 125.6±34.5 | 127.6±35.7 | 127.7±34.4 | 0.855 |
| Triglycerides (mg/dL) | 178.1±120.2 | 147.3±71.0 | 154.4±90.6 | 0.008θ |
| Fasting Blood Glucose (mg/dL) | 122.1±47.8 | 111.9±32.9 | 107.3±23.5 | 0.002α,β |
| Myocard Infarctus, n (%) | 9 (7.3) | 5 (2.0) | 4 (2.7) | 0.028z |
| Hypertension, n (%) | 54 (43.5) | 96 (38.7) | 55 (37.4) | 0.554 |
| DM, n (%) | 32 (25.8) | 57 (23.0) | 30 (20.4) | 0.574 |
| MetS, n (%) | 61 (49.2) | 95 (38.3) | 49 (33.3) | 0.025z |
| VAI | 2.64±2.1 | 2.31±1.4 | 2.51±2.0 | 0.207 |
Non-HDL: non-high density lipoprotein; HDL: high density lipoprotein; LDL: low density lipoprotein; DM: diabetes mellitus; MetS: metabolic syndrome; VAI: visceral adiposity index.
Prostate cancer is the most widespread malignancy within men. ADT, which is the central component of advanced PCa treatment, causes side effects such as chronic fatigue, osteoporosis, diabetic predisposition, cardiac side effects, fever pressures and decreased libido.1,2 Androgens, especially testosterone, are hormones necessary for muscle development, bone mineralization, hemopoetic system, sexual functions and reproduction, and have an important place in men's health. It has been reported that there is a positive relationship between serum testosterone level and muscle mass and a negative relationship between fat mass.5 It is known that ADT, which is used as adjuvant in locally advanced PCa, increases overall survival and improves cancer-related morbidity such as impaired quality of life and bone pain in metastatic disease. The goal of ADT is to reduce serum testosterone levels to castration levels. We can achieve this in two ways. Surgical excision provides castration level within 24h. Medical treatment (GnRH agonists) primarily increases serum testosterone levels, followed by a decrease in the receptors in gonadotropin cells in the pituitary and pulsative stimulation disappears. Testosterone levels decrease to castration levels within approximately 3 weeks. In the use of luteinizing hormone releasing hormone (LHRH) antagonists, which do not yet have long-term results, serum testosterone levels decrease to castration level without transient increase.6
In a study conducted by Braga-Basaria et al.,7 20 prostate cancer cases receiving ADT for 12 months, 18 cases with PCa without ADT treatment, and 20 healthy cases were compared. In the first group, the BMI was found to be higher than the other groups.7 Similarly, in our current study, BMI was higher in the group with ADT. Androgens are hormones that act a significant role in determining body structure by causing an increase in lean body mass. The reduction of endogenous testosterone production by GnRH agonists is closely associated with a decrease in body lean mass, known as sarcopenia, and an increase in MetS.7,8 Sarcopenia is frequently accompanied by decreases in strength, physical function capacity and quality of life and also increases in fat mass.9 It has been reported that lean body mass decreased by 2–4% in the first year of treatment in patients receiving ADT therapy.10–13 In such patients, the sarcopenic obesity term, which has the characteristics of higher WC, higher fat mass and fat ratio, is used. In the current study, although lean mass was lower in the ADT group, no statistical difference was found. The metabolic rate, which is one of the parameters examined in Tanita measurement, is not affected by ADT treatment or prostate cancer. Similarly, total amount of liquid was not different in all 3 groups.
The definition of MetS includes biochemical and clinical variables such as body composition disorder, dyslipidemia, hypertension and DM (Table 3).14 Interestingly, patients treated with ADT usually have higher HDL and, unlike standard MetS, subcutaneous fat has increased. Although its effect is vague, considering the relationship between MetS and cardio-vascular disease, cautious approach with close follow-up seems reasonable. In addition, loss of libido, hot flashes, fatigue, osteoporosis, anemia, and gynecomastia are other significant side effects observed due to ADT.15 These side effects are caused by impaired body composition, lipid profile and glucose-insulin metabolism. In patients receiving ADT due to PCa, some body composition changes and serum glucose and lipid parameters are deteriorated.16 A consistent guide has not been proposed to treat the metabolic outcomes of ADT. These metabolic changes also cause an increase in cardio-vascular morbidity. Therefore, it is important to carry out a risk assessment including BMI, WC, blood pressure, fasting lipid profile and blood glucose level while determining the treatment plan.12
Metabolic syndrome criteria.
| NCEP-ATP III (National Cholesterol Education Programme Adult Treatment Panel III) | |
|---|---|
| At least three of the following criteria | |
| Waist circumference | ≥102cm |
| Blood pressure (mmHg) | ≥130/85 or antihypertensive treatment use |
| Triglycerides | ≥150mg/dL or receiving treatment for triglyceride elevation |
| HDL | <40mg/dL or receiving treatment for low HDL |
| Dysglycemia | Fasting glucose≥100mg/dL or receiving treatment for glucose increase |
HDL: high density lipoprotein.
Men receiving ADT have a higher prevalence of abdominal type obesity and hyperglycemia, which are among the criteria for MetS. In a prospective study in which 26 PCa cases receiving ADT were followed up for a year, it was shown that the increase in fat tissue was 11.2% and the lean body mass decreased by 3.6%. Also in this study, it has been shown that the increase in abdominal adipose tissue is particularly concentrated in the subcutaneous tissue and there is no significant increase in visceral adipose tissue.13 The increased total body fat mass accumulates significantly in the subcutaneous fat tissue, especially near the waist. The outcomes of ADT on body fat structure are seen in the early period, usually in the 3rd month of treatment.17 Although the effect is limited, the initial step to restrain these changes in body composition was regular exercise.18
The implications of ADT on fasting glucose levels were examined and it was determined that there was a considerable increase in insulin levels in the 3rd month of treatment. However, there was no significant difference in fasting glucose levels in the early period.19 In another study, in patients with metastatic PCa who received ADT, fasting glucose level was higher at the end of the first year compared to other patients with PCa without ADT and healthy group.7 In addition, ADT plays a role in the improvement of insulin resistance regardless of age and BMI. Various reports have shown that fasting insulin levels increase by 13–65% compared to the pre-treatment period since the third month of treatment.17,19 In addition, it was found that fasting glucose and HbA1c levels increased and insulin requirement increased within 24 months after ADT was started.20 In the light of these reports, it is seen that ADT decreases insulin sensitivity in the early period and causes an increase in insulin levels, and creates a tendency to hyperglycemia by making changes in glucose metabolism in the later periods of treatment.
The relationship between hypogonadism and dyslipidemia has been previously reported. ADT also has various effects on serum lipid metabolism. In a study of 40 patients receiving ADT treatment, a significant rise in total cholesterol, HDL, LDL and Tg levels was shown at the end of the first year.21 HDL cholesterol and total cholesterol values were differently higher in the ADT group than in the other two groups in the current study. Dockery et al.19 reported that this increase in total cholesterol and HDL levels was seen in the 3rd month of treatment. Considering the relationship between the increase in serum cholesterol levels and cardio-vascular morbidity and mortality, the profit-loss balance should be considered in the treatment plan. Keating et al.11 stated the connection between ADT and cardio-vascular morbidity in 73,196 patients in the Medicare and SEER database and noticed that ADT group had an increased hazard of myocardial infarction (HR:1.11; p=0.03), onset of coronary heart disease (HR:1.16; p<0.001), and sudden cardiac death (HR:1.16; p=0.004). In the patient population in our study, the group receiving ADT had a higher myocard infarctus rate. Since abdominal obesity and hyperglycemia were liable for higher cardio-vascular risk, healthy nutrition, regular exercise and medical treatments should be recommended in order to achieve target serum lipid values, especially in cases at risk.
A high level of evidence-related study on blood pressure values, which are criteria of MetS and are considerable risk factors for cardio-vascular diseases, is not available in the literature. In the cross-sectional study of Braga-Basaria et al.7 reported that the mean blood pressure values of the patients who received ADT for 12 months, patients with prostate cancer without ADT, and the healthy group were not different. We did not study out any difference between the groups in terms of the presence of hypertension and DM. Similarly, although the VAI score which is associated with cardiometabolic risk was higher in the ADT group, there was no significant difference between the groups. Studies show that this index based on anthropometric measurement generally has a stronger relationship with DM and insulin resistance.
ConclusionThe adverse effects of ADT treatment on adipose tissue and glucose and insulin metabolism reveal that we need to evaluate our patients in this direction. It should be noted that negative effects on lipid metabolism other than HDL increase should be taken into consideration and the close relationship of these effects with cardio-vascular diseases. First of all, patients should be carefully evaluated in terms of profit and loss before treatment, and they should be informed about the metabolic complications that may develop and their results. Patients who are already at high risk for MetS should be evaluated more carefully than the standard PCa follow-up protocol. Performing this assessment every 3 months in patients with ADT may be beneficial in terms of the prognosis of the treatment. In addition, patients with deteriorated body composition and serum biochemical values should be offered healthy nutrition, regular physical exercise and, if necessary, medical treatment recommendations.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Financial disclosureThe authors declared that this study has received no financial support.
Conflict of interestThe authors declare that they have no conflict of interest.