We present a case of an 83-year-old-male with painless penile nodules several months after he was diagnosed with pure prostatic small cell carcinoma. Penile doppler ultrasound and magnetic resonance imaging demonstrated solid nodules in both corpora cavernosa. Fine-needle aspiration of the nodules with immunohistochemical examination confirmed prostatic small cell carcinoma origin of metastases.
Small cell carcinoma of the prostate is a rare disorder accounting for less than 1% of all prostate cancers, the penis being an uncommon site for metastasis. An extremely low number of cases of penile metastases from prostatic small cell carcinoma has been reported to date in the literature.
Presentamos un caso de un varón de 83 años con nódulos peneanos indoloros, tras ser diagnosticado meses atrás de carcinoma prostático de células pequeñas puro. La eco-doppler e imagen de resonancia magnética de pene reflejaron nódulos sólidos en ambos cuerpos cavernosos. La aspiración con aguja fina de los nódulos con prueba inmunohistoquímica confirmó el origen metastásico del carcinoma de células pequeñas.
El carcinoma prostático de células pequeñas es un trastorno inusual que representa menos del 1% de todos los cánceres prostáticos, siendo el pene un sitio infrecuente de metástasis. Hasta la fecha se ha reportado en la literatura un número de casos extremadamente bajo de metástasis peneanas en el carcinoma de próstata de células pequeñas.
Neuroendocrine tumors of the prostate represent a heterogeneous group that includes carcinoid tumors, large-cell neuroendocrine carcinomas (LCNECs) and small cell carcinomas (SCCs).1 Prostatic SCC was first reported by Wenk et al.,2 about 10% of extrapulmonary SCCs occur in the prostate. Prostatic SCC is associated with conventional prostatic adenocarcinoma in most cases, but pure SCC of the prostate is a rare disorder accounting for less than 1% of all prostate cancers.
Metastatic involvement of the penis is relatively infrequent. The vast majority of the primary lesions are in the genitourinary organs, with the rectosigmoid region contributing to the bulk of the remainder.3 The penis is an uncommon site for metastasis originated from prostate cancer despite their proximity (0.3%).4 Metastatic spread of prostate cancer to the penis occurs by several routes. Retrograde venous or direct lymphatic/vascular invasion and direct extension through the lumen of the vas deferens are the most common mechanisms.4 Despite its abundant vascularization and extensive circulatory communication with neighboring organs, metastases from other cancers to the penis are rare and consequently lack an efficient treatment. These lesions are often associated with disseminated malignancy and have a poor prognosis.5
An extremely low number of cases of penile metastases from other than prostatic adenocarcinoma (epidermoid or squamous carcinoma, sarcoma, SCC)6,7 has been reported until now in the literature, but to the best of our knowledge, this is only the second case of pure SCC of the prostate with metastases to the penis.8
Case reportAn 83-year-old-male presented to our department with the complaint of painless penile nodules. The patient was diagnosed with pure prostatic small cell carcinoma (SCC) after Millin's adenomectomy for severe LUTS six months ago. He denied radical prostatectomy and was treated by 6 cycles of Cisplatin+Etoposide chemotherapy, with adjuvant external radiotherapy (50Gy). Physical examination revealed bilateral nodules in the penile palpation. Both on the dorsal aspect of the corpora cavernosa, and the ventral aspect of the corpus spongiosum. Blood tests were unremarkable except for elevated (PSA) prostate-specific antigen (12, 2ng/mL).
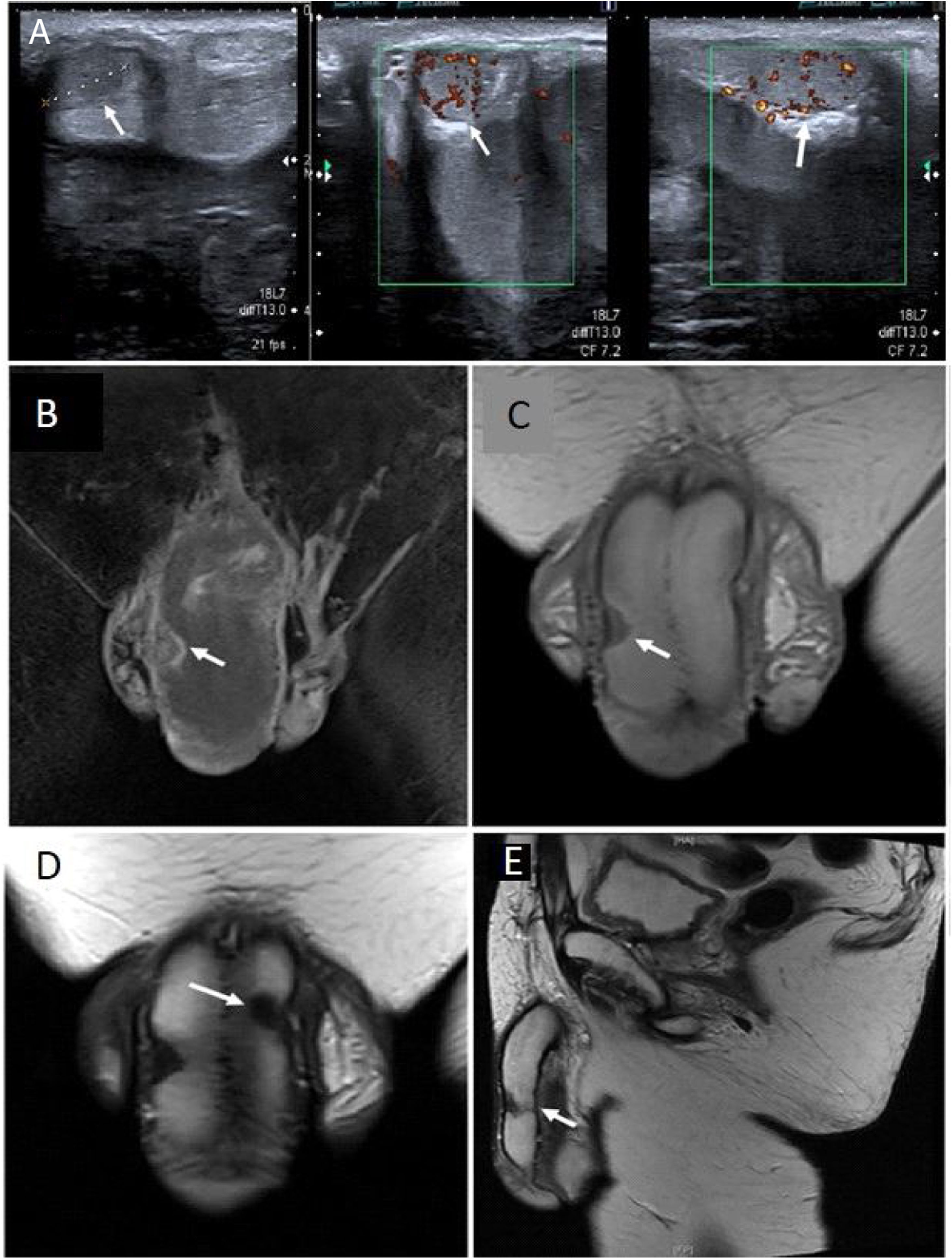
Doppler ultrasound images revealed the presence of bilateral hypoechoic solid nodules in both cavernous bodies, with intra-lesional vascularization (Fig. 1A). T2-weighted magnetic resonance imaging (MRI), performed with an 1.5 T system (GE Signa Excite HD, GE Medical Systems, Milwaukee, WI, USA) showed a 20×11×7mm hypointense solid nodule in right corpora cavernosa, an 11×13×9mm solid nodule in left cavernous body, and a 15×7×7mm in corpus spongiosum (Fig. 1B–E).
(A) Ultrasound images revealed the presence of hypoechoic solid nodules, with intralesional vascularization in the echo-color doppler examination (white arrows). The MRI images show (B) T1 coronal (C) T2-weighted coronal with nodule in right cavernous body (white arrows). (D) T2-weighted coronal right cavernous body nodule (white arrows). T2-weighted sagittal (E) images with nodules in both right and left cavernous bodies (white arrows).
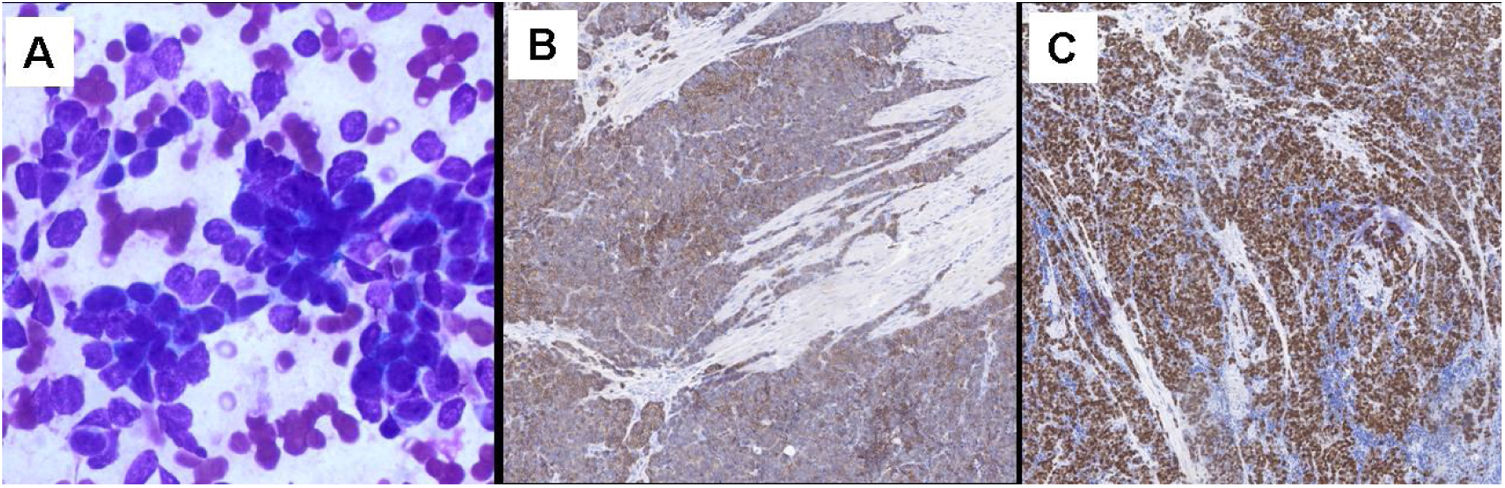
Fine-needle aspiration (FNA) of the nodules was performed, and histopathologic examination confirmed the prostatic origin of metastases, with small-sized cells with scant cytoplasm, finely granular nuclear chromatin, nuclear molding, and high mitotic activity. Immunohistochemical study of the tumor was carried out and lesional cells were immunoreactive for CD56 and negative for chromogranin and synaptophysin, with a positive Ki-67 rate of 40% (Fig. 2).
DiscussionFrom a review of the literature we could find more than 400 cases of secondary penile metastasis. Among them, only 33% had originated from prostate adenocarcinoma. Primary tumor generally involved the genitourinary tract: bladder, prostate, kidney, ureter, testis and urethra (69%) followed by gastrointestinal cancer (19%), and respiratory system (7.2%).9 The patients with metastases from non-urological tumors generally seemed to have a poorer prognosis than those with tumors of urological origin.10
The first confirmed case of a metastasis to the penis from prostate cancer was reported in 1907 by Motz et al.11 Since then, 182 cases were reported,5,8,9,12–14 but almost all cases were from prostate adenocarcinomas. Penile metastases from prostate cancer typically occur in the central portion of the corpus cavernosum or spongiosum, and this distinguishes them from primary penile lesions that are mainly located at glans.9 They usually present as hard skin nodules, more frequently located at the root (38.8%), shaft (38.8%), being less common in glans (22.2%).10 Other modes of presentation are priapism, severe penile pain, and urethral ulceration. Obstructive voiding symptoms and hematuria are very rarely reported.3
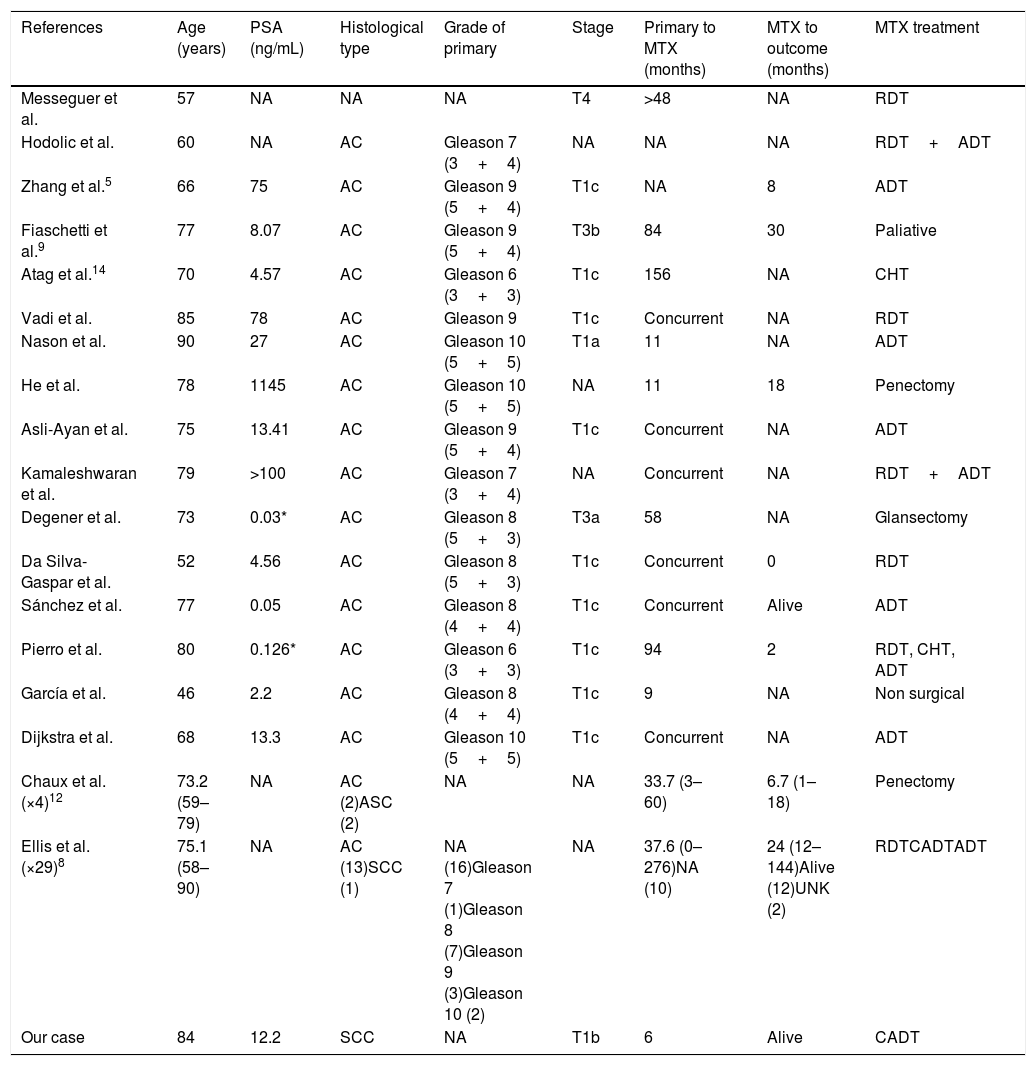
According to our review with all the cases published since the last review in 2011 (Table 1), the mean age and blood PSA level of penile metastases presentation was 71.9 years (46–90), and 105.9ng/mL respectively. Adenocarcinoma was the most frequent histological type (88.2%), with a much smaller presence of adenosquamous carcinoma (5.9%)12 and SCC (5.9%).8 We found a mean interval of 32.2 (0–276) months after the first diagnosis of prostate cancer, with a median cancer-specific survival time of 11.6 (1–144) months, with most of the patients (more than 80%) died one year after the detection of penile metastasis.9,12
Total of reported cases of penile metastasis from prostate cancer (including the present case) from 2011 till date.
| References | Age (years) | PSA (ng/mL) | Histological type | Grade of primary | Stage | Primary to MTX (months) | MTX to outcome (months) | MTX treatment |
|---|---|---|---|---|---|---|---|---|
| Messeguer et al. | 57 | NA | NA | NA | T4 | >48 | NA | RDT |
| Hodolic et al. | 60 | NA | AC | Gleason 7 (3+4) | NA | NA | NA | RDT+ADT |
| Zhang et al.5 | 66 | 75 | AC | Gleason 9 (5+4) | T1c | NA | 8 | ADT |
| Fiaschetti et al.9 | 77 | 8.07 | AC | Gleason 9 (5+4) | T3b | 84 | 30 | Paliative |
| Atag et al.14 | 70 | 4.57 | AC | Gleason 6 (3+3) | T1c | 156 | NA | CHT |
| Vadi et al. | 85 | 78 | AC | Gleason 9 | T1c | Concurrent | NA | RDT |
| Nason et al. | 90 | 27 | AC | Gleason 10 (5+5) | T1a | 11 | NA | ADT |
| He et al. | 78 | 1145 | AC | Gleason 10 (5+5) | NA | 11 | 18 | Penectomy |
| Asli-Ayan et al. | 75 | 13.41 | AC | Gleason 9 (5+4) | T1c | Concurrent | NA | ADT |
| Kamaleshwaran et al. | 79 | >100 | AC | Gleason 7 (3+4) | NA | Concurrent | NA | RDT+ADT |
| Degener et al. | 73 | 0.03* | AC | Gleason 8 (5+3) | T3a | 58 | NA | Glansectomy |
| Da Silva-Gaspar et al. | 52 | 4.56 | AC | Gleason 8 (5+3) | T1c | Concurrent | 0 | RDT |
| Sánchez et al. | 77 | 0.05 | AC | Gleason 8 (4+4) | T1c | Concurrent | Alive | ADT |
| Pierro et al. | 80 | 0.126* | AC | Gleason 6 (3+3) | T1c | 94 | 2 | RDT, CHT, ADT |
| García et al. | 46 | 2.2 | AC | Gleason 8 (4+4) | T1c | 9 | NA | Non surgical |
| Dijkstra et al. | 68 | 13.3 | AC | Gleason 10 (5+5) | T1c | Concurrent | NA | ADT |
| Chaux et al. (×4)12 | 73.2 (59–79) | NA | AC (2)ASC (2) | NA | NA | 33.7 (3–60) | 6.7 (1–18) | Penectomy |
| Ellis et al. (×29)8 | 75.1 (58–90) | NA | AC (13)SCC (1) | NA (16)Gleason 7 (1)Gleason 8 (7)Gleason 9 (3)Gleason 10 (2) | NA | 37.6 (0–276)NA (10) | 24 (12–144)Alive (12)UNK (2) | RDTCADTADT |
| Our case | 84 | 12.2 | SCC | NA | T1b | 6 | Alive | CADT |
In our case, it is a patient with penile metastases derived from a prostatic SCC, which is extremely rare, with only one case published in the literature so far.8 It has a very aggressive behavior and is associated with a poor prognosis and reduced survival. Our patient had a low PSA level, more common than in adenocarcinomas and the interval time from prostate cancer diagnosis to metastasis presentation was very short. Thus typical features of SCC include rapid progression, unresponsiveness to hormonal therapy, increased risk of lytic bone lesions, visceral metastases, and low PSA relative to disease burden.15 Radiologic diagnosis involves CT scan (with an important role for the detection of secondary lesions and follow-up), and MRI (for differentiation of penile lesions and for staging).9 Management of the patients with penile metastases from carcinoma of the prostate should be focused on improving the quality of life in view of the poor prognosis. Role of surgery (local excision, penectomy) is limited to relieving severe intractable pain and ulceration.4
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.