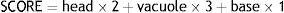Hormonal changes alter the physiological level of ROS and cause oxidative stress in the cell. As estimated, hormonal deficiencies, environmental and ideological factors make up about 25% of male infertility. Pathogenic reactive oxygen species (ROS) is a chief cause of unexplained infertility. Limited studies exist on the effects of testosterone on human sperm culture. Therefore, in the current study, the effect of different doses of testosterone on sperm parameters and chromatin quality was investigated.
Materials and methodsSemen samples from 15 normospermic and 15 asthenospermic patients were prepared by swim up method, and then were divided into four groups by exposing to different concentrations of testosterone (1, 10, and 100nM) for 45min. Samples without any intervention were considered as control group. All samples were washed twice. Sperm parameters and chromatin protamination were assessed in each group and the remains were frozen. After two weeks, all tests were repeated for sperm thawed. Also, the MSOM technique was used to determine the sperm morphology of class 1.
ResultsAlthough sperm parameters were not show any significant differences in normospermic and asthenospermic samples exposed to different concentrations of testosterone before and after freezing, chromatin protamination was significantly decreased in the normospermic samples exposed to 10nM of testosterone before freezing (p<0.006), as well as 1 and 10nM of testosterone after freezing compared to control samples (p=0.001 and p=0.0009, respectively). Similarly, chromatin protamination in the asthenospermic samples was significantly decreased at concentration of 1nM of testosterone before and after freezing (p=0.0014 and p=0.0004, respectively), and at concentration of 10nM of testosterone before and after freezing (p=0.0009, p=0.0007) compared to control samples.
ConclusionUsing a low dose of testosterone in the sperm culture medium, has positive effects on chromatin quality.
Los cambios hormonales alteran el nivel fisiológico de las especies reactivas de oxígeno (reactive oxygen species [ROS]) patógenas y provocan estrés oxidativo en la célula. Según estimaciones, las deficiencias hormonales, los factores ambientales y los ideológicos constituyen alrededor del 25% de la infertilidad masculina. Las ROS son una causa principal de infertilidad inexplicable. Existen estudios limitados sobre los efectos de la testosterona en el cultivo de esperma humano. Por lo tanto, en el estudio actual se ha investigado el efecto de diferentes dosis de testosterona sobre los parámetros del esperma y la calidad de la cromatina.
Materiales y métodosSe prepararon muestras de semen de 15 pacientes normospérmicos y 15 astenospérmicos mediante el método swim up, y luego se dividieron en cuatro grupos exponiéndolos a diferentes concentraciones de testosterona (1, 10 y 100nM) durante 45min. Las muestras sin ninguna intervención se consideraron como grupo control. Todas las muestras se lavaron dos veces. En cada grupo se evaluaron los parámetros espermáticos y la protaminación de la cromatina, y los restos se congelaron. Dos semanas después se repitieron todas las pruebas de esperma descongelado. Asimismo, se utilizó la técnica MSOM para determinar la morfología espermática de clase 1.
ResultadosAunque los parámetros espermáticos no mostraron diferencias significativas en las muestras normospérmicas y astenospérmicas expuestas a diferentes concentraciones de testosterona antes y después de la congelación, la protaminación de la cromatina disminuyó significativamente en las muestras normospérmicas expuestas a 10nM de testosterona antes de la congelación (p<0,006), así como a 1 y 10nM de testosterona después de la congelación, en comparación con las muestras de control (p=0,001 y p=0,0009, respectivamente). De manera similar, la protaminación de la cromatina en las muestras astenospérmicas disminuyó significativamente a una concentración de 1nM de testosterona antes y después de la congelación (p=0,0014 y p=0,0004, respectivamente) y a una concentración de 10nM de testosterona antes y después de la congelación (p=0,0009 y p=0,0007), en comparación con las muestras de control.
ConclusiónEl uso de dosis bajas de testosterona en el medio de cultivo de semen tiene efectos positivos sobre la calidad de la cromatina.
Reactive oxygen species is an essential factor in male infertility.1 Oxidative, antioxidant, and hormone imbalances in semen cause pathogenic ROS.2–4 So, during this event, the function of enzymes, protein kinases, in the sperm membrane are compromised. In addition to cascade events such as apoptosis and lipid peroxidation sperm membrane are activated.5–7 During lipid peroxidation, the ionic balance between the sperm membrane and its surroundings is disturbed. As a result, the integrity of the membrane and necessary energy is lost for sperm motility and viability.1 In addition, membrane changes following lipid peroxidation are associated with abnormal sperm morphology. Also, the results show a correlation between motility, viability, and sperm morphology.4,8
Steroid hormones play an important role on expression of intracellular antioxidant enzymes.9,10 It can play an important role in balancing oxidants and antioxidants and improve the quality of sperm function as well. Estrogen and androgen receptors have been proven in the sperm structure, especially the middle pieces (mitochondrial position). Estrogen and androgen receptors have been proven in the sperm structure, especially the middle pieces (mitochondrial position). Researchers believe that there is a linkage between this receptor and the regulation of sperm energy and viability.11–13 For example, presence of testosterone and estrogen in semen are associated with decreased intracellular calcium ion penetration, decreased plasma membrane fluidity, and intact sperm acrosome membrane.14 It has also been reported that testosterone and dihydrotestosterone could significantly increase the enzymes secretion, in a concentration-dependent manner. Moreover, favorable effects of transdermal testosterone have been showed on sperm parameters in hypogonadal patients.15,16 Due to antioxidant properties, the presence of this hormone during sperm freezing may protect the membrane structure from temperature and osmotic changes.17–19
According to the literature, serum levels of FSH and LH are higher in oligospermia and asthenospermia than in normospermia individuals. Also, the level of testosterone decreases in unexplained infertility of normospermia subjects.20–22 Despite the important role of this hormone in semen, it is removed during sperm preparation,14 as a result, sperm may be exposed to oxidative stress. The aim of this study was to use different concentrations of testosterone in sperm culture medium, and to investigate the effect of this hormone on sperm parameters and chromatin quality before and after freezing in normospermic and asthenospermic samples.
Material and methodsIn this study, 15 normospermia patients with unexplained infertility and 15 asthenospermia samples referred to Yazd infertility center (Yazd, Iran) were enrolled. The study (before–after freezing) was approved by the Research Ethics Committee of Yazd Reproductive Sciences Institute (IR.SSU.DRUG.REC.1395.241), and all participants signed the written consent form. The inclusion criteria were high semen volume (4–5ml) and a sperm count between 50 and 70million/ml. Those with varicocele, leukocytospermia, low sperm count, and low semen volume (<1ml) were excluded. Each semen sample was divided to control and interventional (treatment) groups. Semen analysis was performed according to the WHO criteria.23 The semen of each group was divided into four equal parts and washed separately by the swim up method. The sperms prepared for each division were exposed to equal amounts (0.5ml) of different testosterone concentrations for 45min. The testosterone was added to the sperm culture medium at different concentrations as follows: Group 1 (control, without any intervention), Group 2 (100nM of testosterone), Group 3 (10nM of testosterone), and Group 4 (1nM of testosterone). After 1h, samples were washed twice with distilled water, and then sperm sampled were aliquoted for freezing, evaluating sperm parameters and chromatin protamination. After two weeks, all tests were repeated in sperm thawed as well the MSOM test was used to determine class 1 sperm morphology.
Sperm motilityThe treated sperm (10μL) were placed on the slide and then the movements of 100 sperms were counted based on three distinct types of movement defined in the WHO criteria (progressive, non-progressive, and immotile movement).23
Sperm viabilityEosin–nigrosine staining (10μL) was inserting into a microtubule. Then, 10μL of treated sperm were added into the microtubule and mixed. After 30s, 10μL of this mixture was poured on the slide and a smear was prepared. After drying, the slides were observed by an optical microscope at 1000× magnification. Sperm with red or pink heads were considered as dead sperm and sperm with white heads were evaluated as live sperm.23
Sperm morphologySperm morphology was evaluated using the Diff Quick kit (Diane Biomedicine Company) and based on the WHO criteria.23 A hundred sperm with normal or abnormal sperm morphology were observed with optical microscope lenses. Normal range for sperm morphology was 4%.
Sperm protaminationChromatin protamination was evaluated by CMA3 (Sigma-Aldrich) staining.24 First, the CMA3 powder was dissolved in 1cm3 distilled water, then 50μL of solution was diluted with 450μL of Mcilvaine buffer. Smears of the treated sperm were prepared and fixed with carnoy solution at 4°C for 10min. Then 30μL of CMA3 solution was spread on the slides and the sperms were counted with a fluorescent microscope with a 400× magnification. Sperm with protamine deficiency displayed a bright yellow color and colorless sperm indicated natural protamination.
Rapid sperm freezingAn equal amount of treated sperm was transferred to the cryovial. Then, 1:1 ratio of the sperm freezing medium (Vitrolife Company, Sweden) was slowly added. Next, the cryovials were placed at room temperature for 10min, and then placed horizontally above the nitrogen liquid for 30min. After that, the cryovials were rapidly released into the liquid nitrogen and then transferred to the nitrogen tank. After two weeks, the cryovials were removed from the nitrogen tank and placed in a ben marie at 35±2 for 10min. The sperm samples were transferred into a test tube. Hamsf10 was added to the sperm samples with the same volume.25 After washing the sperm, all tests were performed in addition to the MSOM technique to assess class 1 sperm morphology.
High magnification morphology (MSOME)The treated sperm morphology and the control group were evaluated by an invert microscope equipped with lens and MSOME software. 5μL of treated sperm were mixed with 5μL of 7% PVP medium (Irvine Scientific Company, Japan). Then, 100 sperms based on morphology described by Cassuto scoring.26 This scoring is based on the number and presence of vacuoles, the shape of the head and core, and the basic shape of the sperm. The summarized formula is as follows:
Statistical analysisFor the data analysis, SPSS software (version 20, USA) was used. Before and after freezing, all sperm parameters and sperm chromatin were evaluated based on the analysis of variance (ANOVA) test, followed by Tukey's post-hoc test for comparison of specific pairs of groups. p-Value of <0.05 was set to be significant.
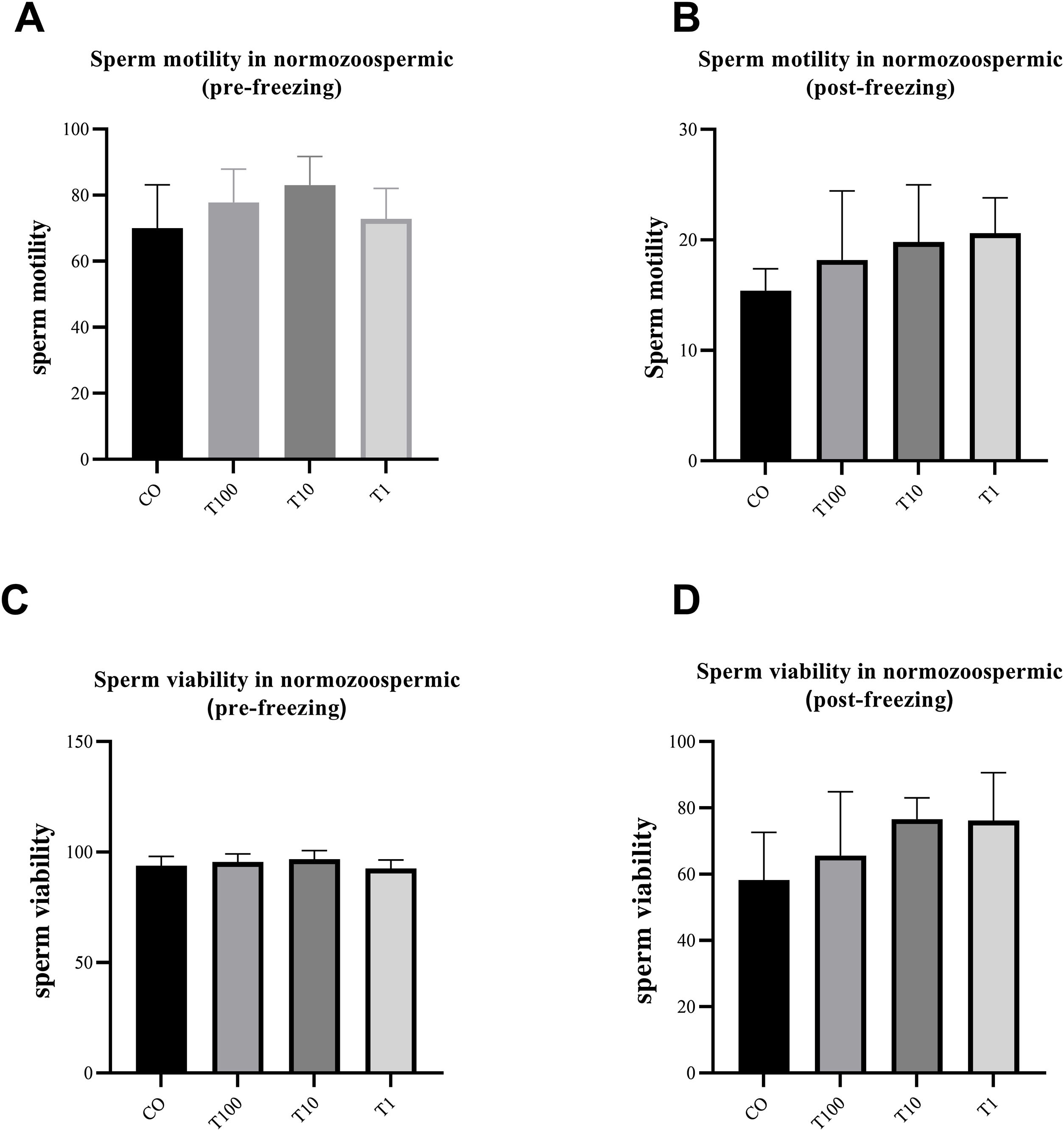
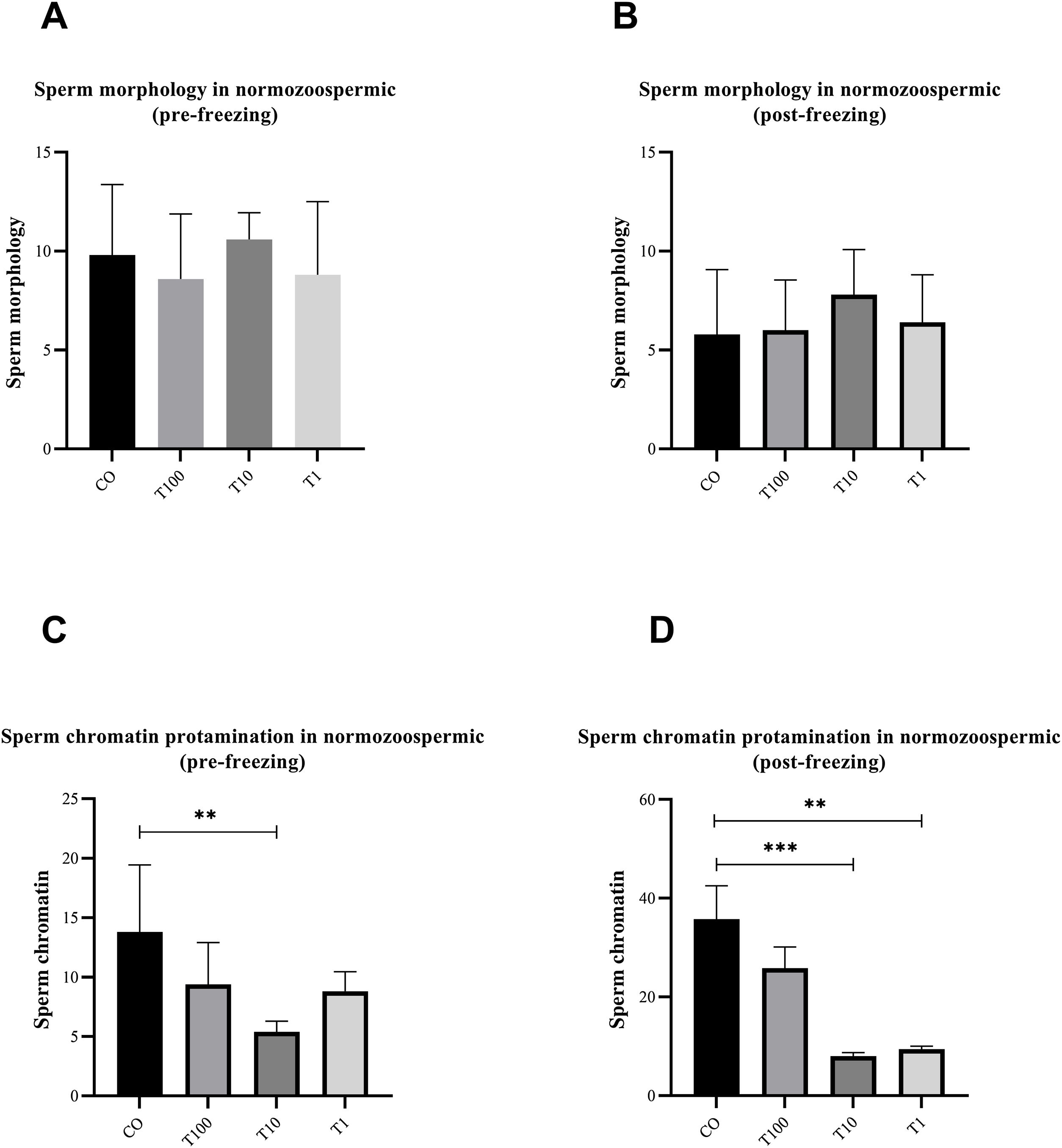
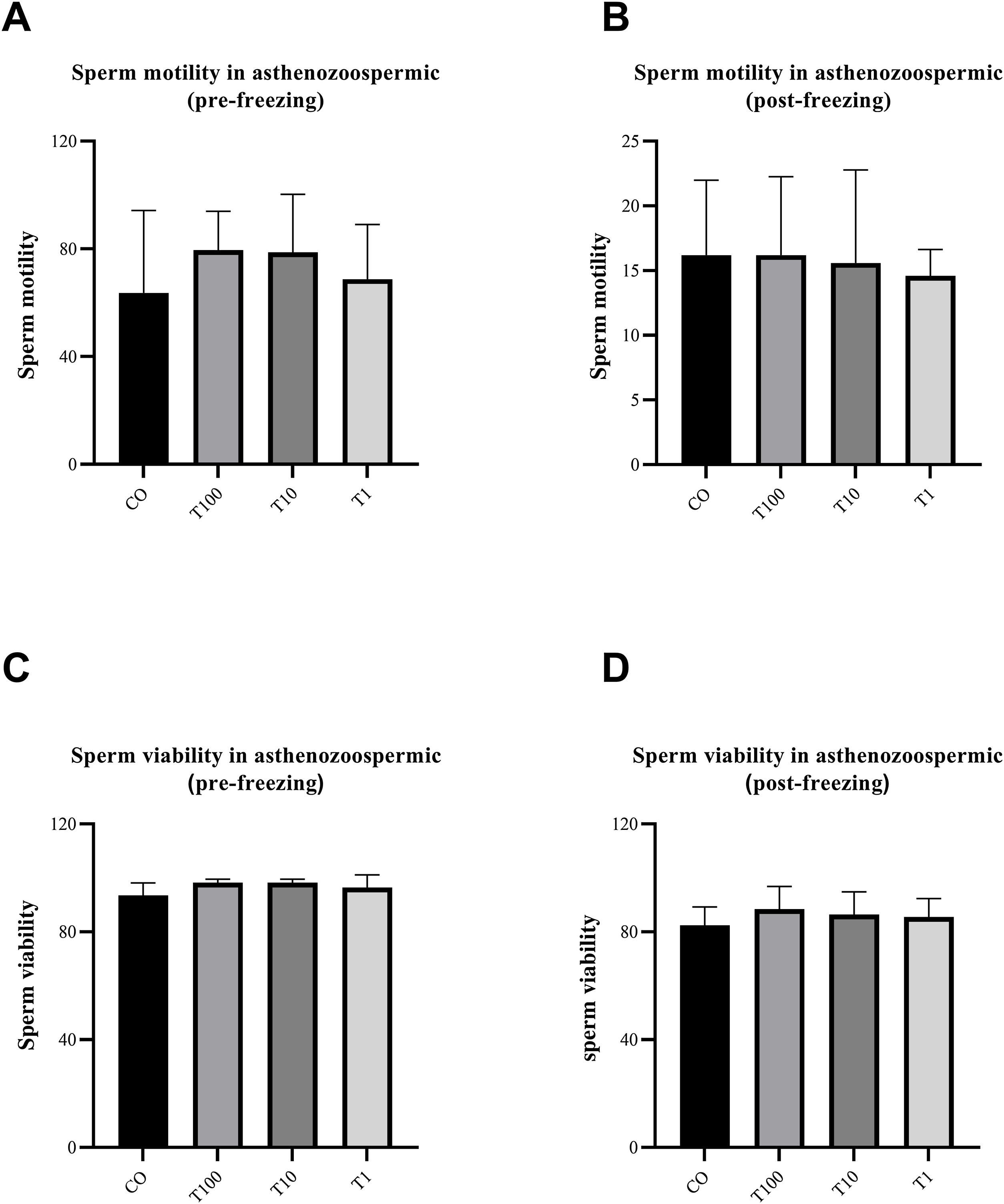
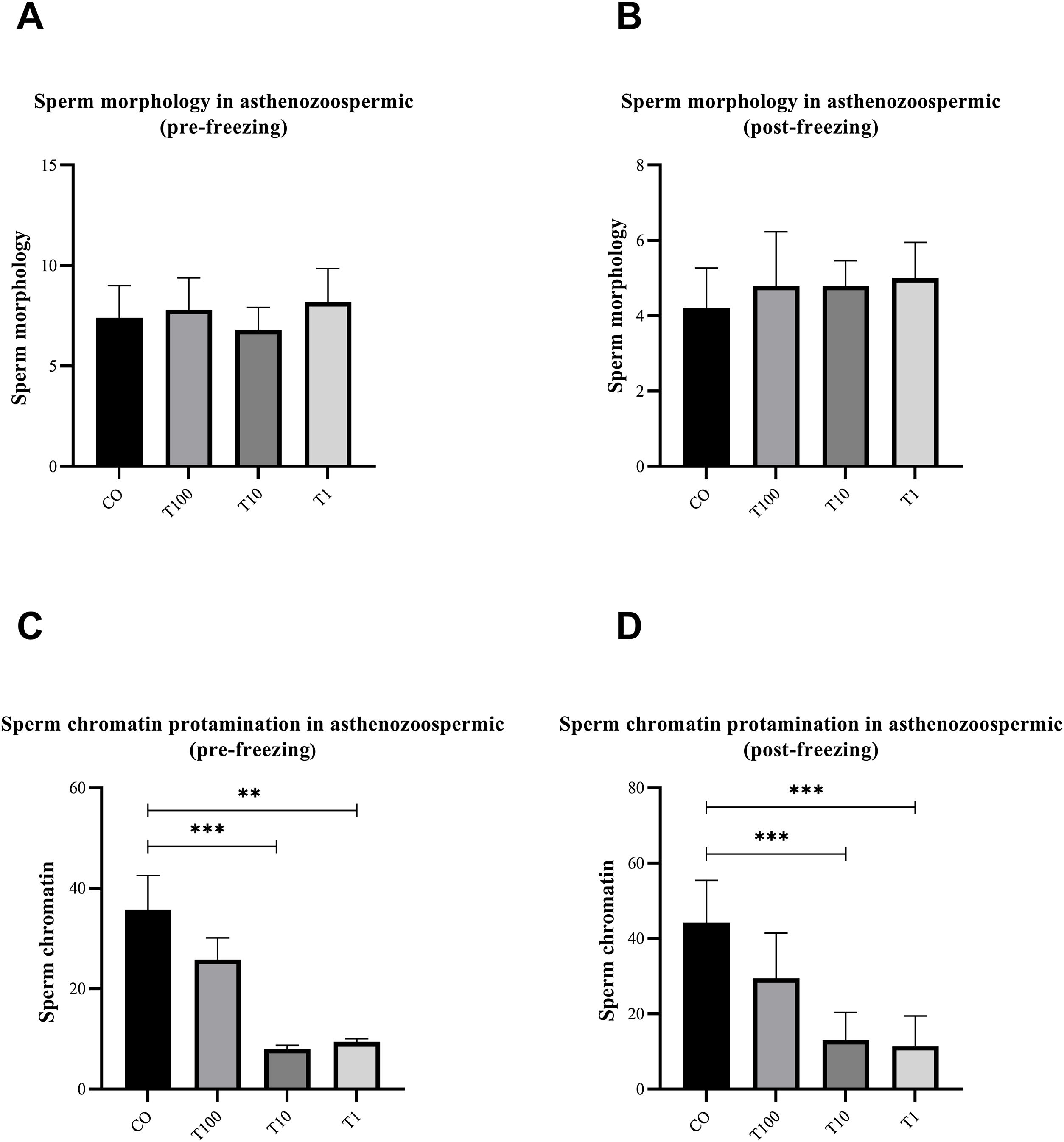
ResultsThe normospermic samples showed no significant differences in the motility, viability and morphology of sperm before and after freezing, (p˃0.05) (Fig. 1). But, regarding chromatin protamination of sperm, normospermic sample showed the only significant difference at concentrations of 10nM of testosterone compared with the control group before freezing (p=0.006) (Fig. 2). This result also was significant after freezing in two concentrations of 1 and 10nM (p=0.001 and p=0.0009, respectively) (Fig. 2). In addition, the asthenospermic samples with different concentrations of testosterone, showed no significant difference on sperm parameters, before and after freezing, in comparison to control group (p˃0.05) (Fig. 3B, Fig. 4, and Fig. 5A-B), but this group showed lower amount of protamine deficiency, in both concentration of 1 and 10nM, before (p=0.0014 and p=0.0009, respectively) and after freezing (p=0.0002 and p=0.0003, respectively) (Fig. 5C-D).
The effect of different concentration of free testosterone on sperm motility (A and B) and sperm viability (C and D) before and after freezing in normospermic individuals (there was no significant differences between groups). Data are presented as mean±SD for three independent experiments; one-way ANOVA followed by Tukey's post-hoc test was used for comparison between groups (T: testosterone).
The effect of different concentration of free testosterone on sperm morphology (A and B) and sperm chromatin protamination (C and D) before and after freezing in normospermic individuals (the sperm morphology between groups was not significant before and after freezing). Data are presented as mean±SD for three independent experiments; one-way ANOVA followed by Tukey's post-hoc test was used for comparison between groups (**p≤0.01, ***p≤0.001; T: testosterone).
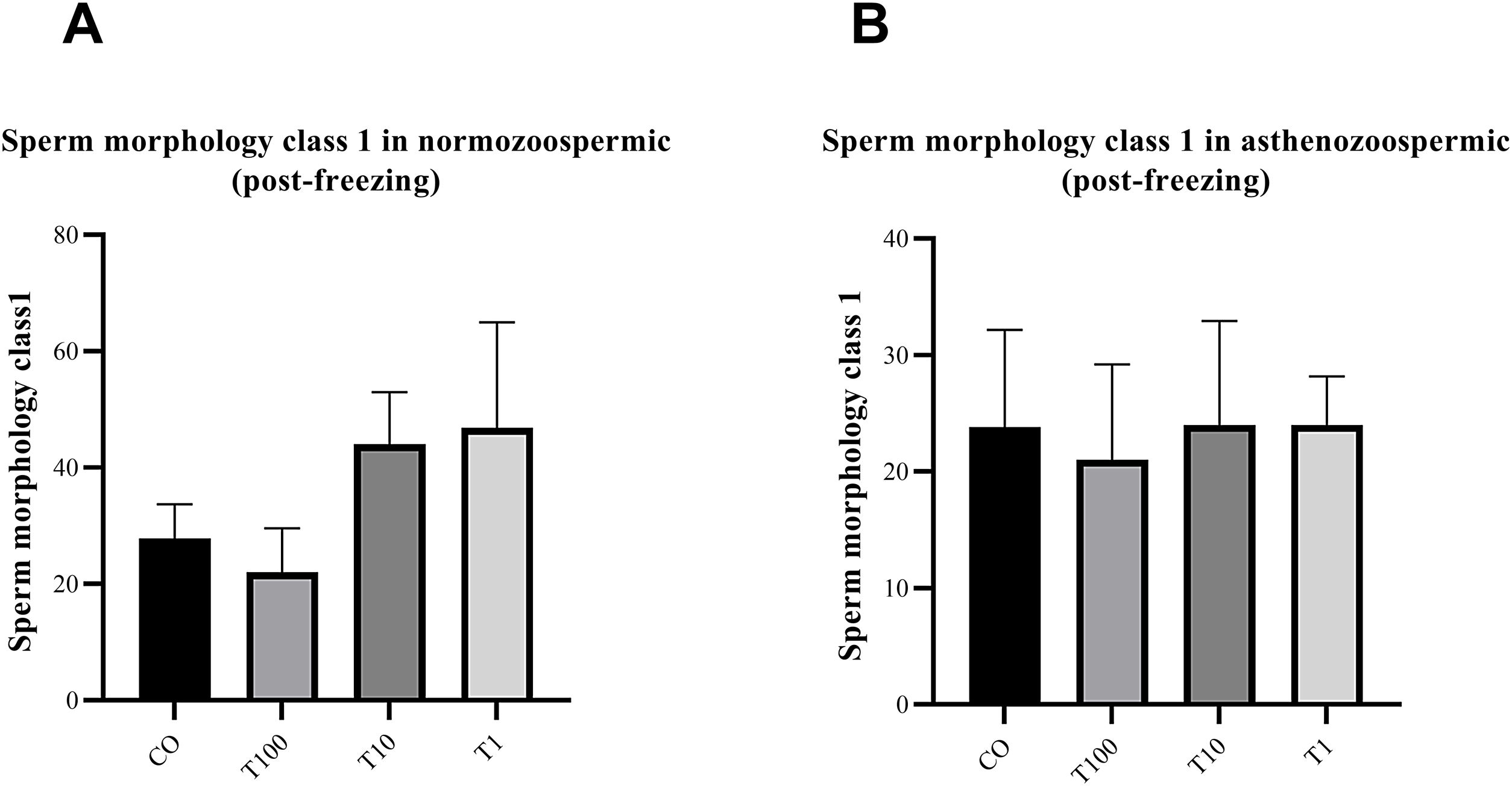
The effect of different concentration of free testosterone on sperm morphology class 1 of MSOM after freezing in normospermic individuals (A) and asthenospermic individuals (B) (there was no significant differences between groups). Data are presented as mean±SD for three independent experiments; one-way ANOVA followed by Tukey's post-hoc test was used for comparison between groups (T: testosterone).
The effect of different concentration of free testosterone on sperm motility (A and B) and sperm viability (C and D) before and after freezing in asthenospermic individuals (there was no significant differences between groups). Data are presented as mean±SD for three independent experiments; one-way ANOVA followed by Tukey's post-hoc test was used for comparison between groups (T: testosterone).
In this study, the effect of testosterone, an important steroid hormone in the male reproductive system, on sperm parameters, and sperm chromatin quality were investigated in two groups of normospermic and asthenospermic individuals before and after sperm freezing. Based on our literature review, studies similar exist that investigated the effect of free testosterone on the sperm culture medium, but these studies are in the field of animal sperm. Hence, in this study, the effect of different concentrations of free testosterone on human sperm parameters and chromatin quality was investigated. Due to the limited studies in connection with testosterone in sperm culture medium, the interpretation of the results was based on the characteristics of this hormone. In this study, low-doses of testosterone had fewer protamination defects than the control group. Since, one of the important factors in improving fertilization process and the development of the embryo is dependent on chromatin quality.27,28 Using low doses of testosterone (1 and 10nm) may have a positive effect on sperm parameters than high concentration of testosterone androgen receptors are located in the middle of the sperm in the mitochondrial region.29–31 Testosterone has a strong tendency to bind to its receptor.32 The presence of a low hydroxyl group in the structure of testosterone causes less binding of this hormone to the plasma membrane and facilitates its entry into the cell.33–35 By binding to its receptor, this hormone not only exerts its anti-apoptosis properties but also strengthens some important signals in sperm metabolism.36–38 But in a large concentration of testosterone, due to the abundant and uncontrolled entry of hormones to the cell, it may reduce or alter the targeted base structure or signaling pathways. As a result, the anti-apoptotic properties of this hormone are inhibited. So, the activation of caspases and the release of apoptotic agents, severe damage is caused to sperm chromatin.39,40 Therefore, the lower the dose of this hormone helps to preserve energy (required for sperm motility), and its anti-apoptotic properties by binding to its receptor. It also protects the membrane of the treated sperm against free radicals before and after the freezing process. Studies show that testosterone strengthens the genes of intracellular enzymes and counteracts the increase of free radicals in cells.9,10 Also, estrogen and testosterone affect the mitochondrial electron transport chain, cell morphology, and gene expression, and cause the strengthening or maintaining mitochondrial function under pathological/stress conditions by activating signaling pathways such as PI3K/Akt, MAPKs.11,41 Therefore, using low doses of testosterone in culture media may activate these activities in the cell and improve sperm function. Twenty-five percent of urologists in the United States still prescribe androgens to treat male infertility, an imbalance of the hormone in men that causes infertility.3,42 Testosterone may increase the viability and motility of sperm by suppressing or inducing the expression of certain genes.43 We have not found a study that mentions a specific dose of testosterone in the human sperm culture medium. The only animal and human studies that we found were those performed by intravenous injection or oral administration. The effect of the dose results obtained in vivo is very different from the hormone dose used in vitro conditions (Fig. 5).
The effect of different concentration of free testosterone sperm morphology (A and B) and sperm chromatin protamination (C and D) before and after freezing in asthenospermic individuals (the sperm morphology between groups was not significant before and after freezing). Data are presented as mean±SD for three independent experiments; one-way ANOVA followed by Tukey's post-hoc test was used for comparison between groups (**p≤0.01, ***p≤0.001; T: testosterone).
Another study also found that using testosterone as an exogenous substance in humans’ samples causes azoospermia or oligospermia.42,44 While other articles showed that low serum testosterone levels were significantly associated with asthenospermia, oligospermia, and azoospermia in infertile males.45 However, according to the results of the present study, the significant effects of this hormone when compared to the control group, a positive result for chromatin quality in a lower dosage. In a study with similar results, it was found that varicocele mice that received testosterone exogenously had a lower level of ROS, as well as a higher expression of the Hsp70 gene than those who did not receive this treatment. Because varicocele is an important factor that leads to sperm DNA damage, prescription of exogenous testosterone can increase HSP70 gene expression and subsequently decrease sperm DNA damage, in the treated group.46
ConclusionAlthough we had some limitation such as low number of the patients, data from the present study showed that using a low dose of testosterone (1 and 10nm) in the sperm culture medium, has positive effects on chromatin quality.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestThere is no any conflict of interest to be declared.
The authors thank the staff from the Research and Clinical Center for Infertility, Shahid Sadoughi University of Medical Sciences, Yazd, Iran, for their skillful technical assistance during the course of this study.