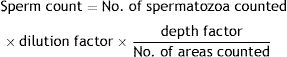Minocycline is a tetracycline with promising protective effects on different organs which are completely distinct from its antibacterial effects.
MethodsTo evaluate the effects of chronic administration of this agent on histological structure and sperm parameters of testes, forty adult male rats were randomly allocated into 2 equal groups I: control animals and II: treated animal that received 25mg/kg/day minocycline, orally. After 90 days of treatment, serum level of testosterone was assessed as well as sperm count, motility and morphology. Moreover, histological and histomorphometric evaluation of testes was performed including determination of height of the seminiferous germinal epithelium and perpendicular diameter of seminiferous tubules. Numbers of spermatogonia, primary spermatocytes, spermatids, Sertoli and Leydig cells were counted. Johnsen's scoring method was also performed.
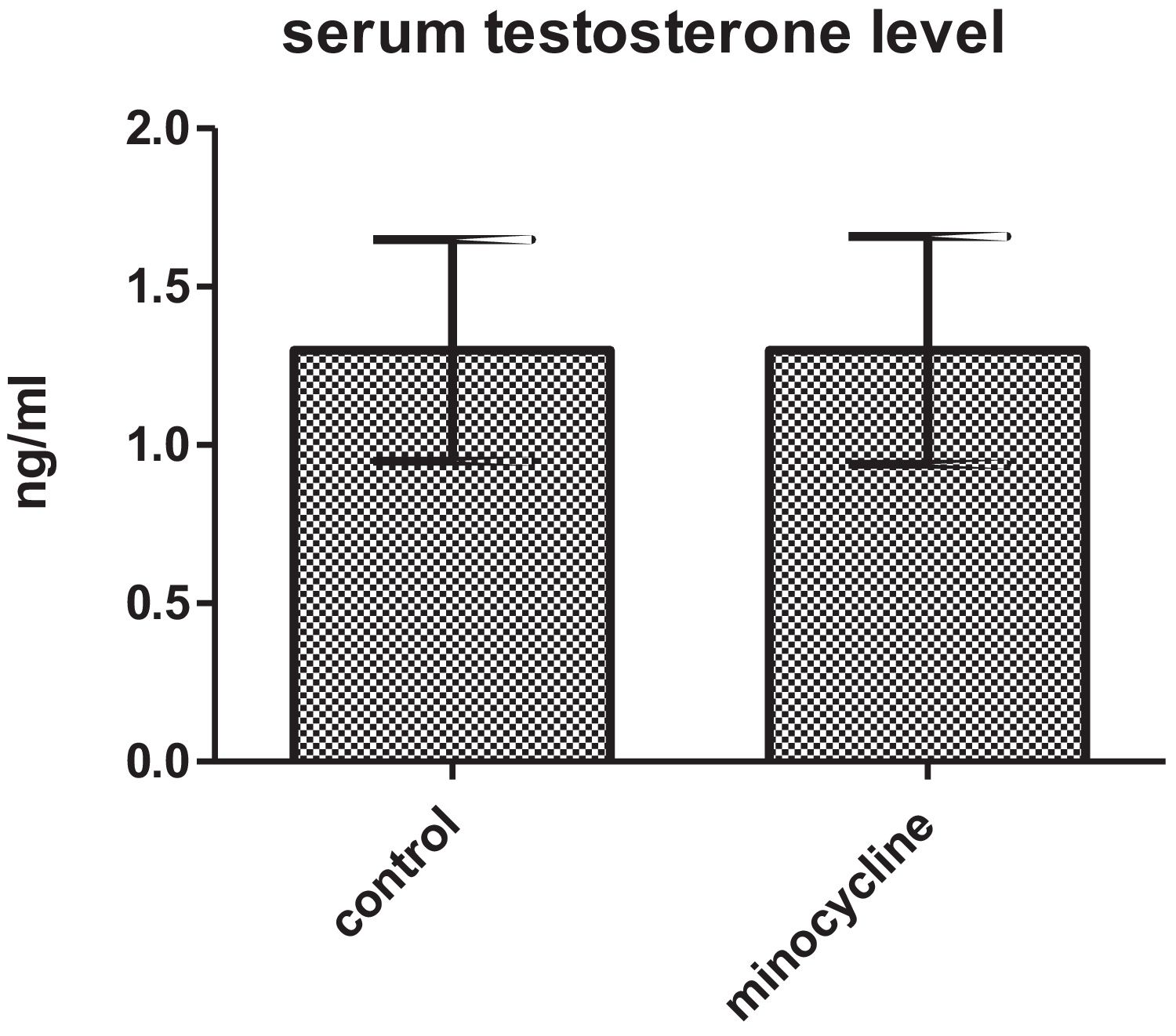
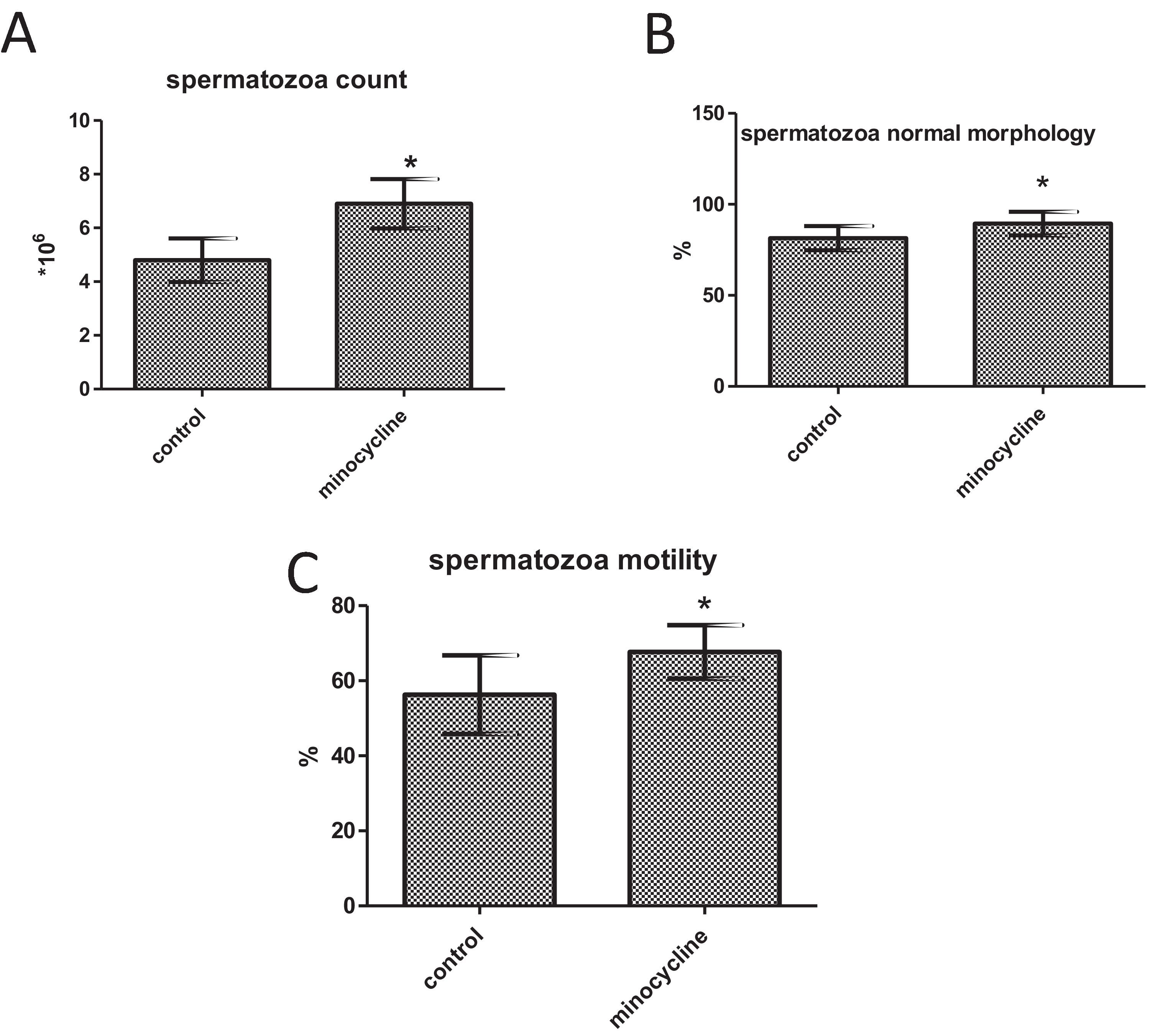
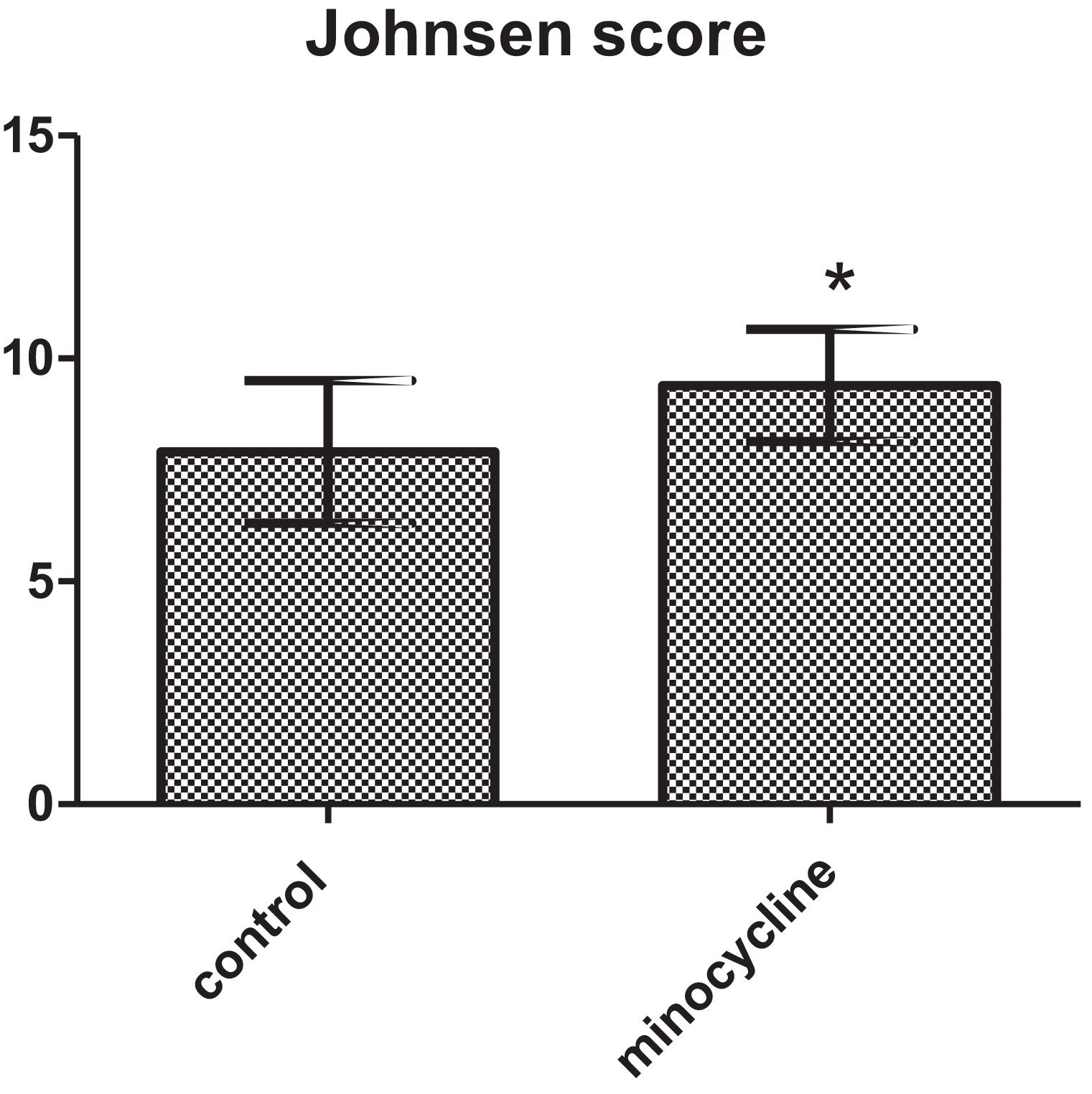
ResultsSperm parameters significantly improved in minocycline-treated animals. Moreover, number of germ cells in different stages of development significantly increased in treatment group as compared to control. This finding was associated with better Johnsen's score and thicker epithelium in seminiferous tubules. However, serum testosterone levels, Leydig and Sertoli cell count as well as tubular diameter did not show significant changes (p>0.05).
DiscussionChronic administration of minocycline is associated with improved spermatogenesis and sperm characteristics without affecting steroidogenesis in rats.
La minociclina es una tetraciclina con efectos protectores prometedores en diferentes órganos que son completamente distintos de sus efectos antibacterianos.
MétodosPara evaluar los efectos de la administración crónica de este agente sobre la estructura histológica y los parámetros espermáticos de los testículos, se asignaron al azar 40 ratas macho adultas en 2 grupos iguales: I, animales control; y II, animal tratado que recibió 25mg/kg/día de minociclina, por vía oral. Después de 90 días de tratamiento, se evaluó el nivel sérico de testosterona, así como el recuento, la motilidad y la morfología de los espermatozoides. Además, se realizó una evaluación histológica e histomorfométrica de los testículos, incluida la determinación de la altura del epitelio germinal seminífero y el diámetro perpendicular de los túbulos seminíferos. Se contó el número de espermatogonias, espermatocitos primarios, espermátidas, células de Sertoli y Leydig. También se realizó el método de puntuación de Johnsen.
ResultadosLos parámetros de los espermatozoides mejoraron significativamente en los animales tratados con minociclina. Además, el número de células germinales en diferentes etapas de desarrollo aumentó significativamente en el grupo de tratamiento en comparación con el control. Este hallazgo se asoció con una mejor puntuación de Johnsen y un epitelio más grueso en los túbulos seminíferos. Sin embargo, los niveles séricos de testosterona, el recuento de células de Leydig y Sertoli y el diámetro tubular no mostraron cambios significativos (p>0,05).
Discusión La administración crónica de minociclina se asocia con una mejor espermatogénesis y características de los espermatozoides sin afectar la esteroidogénesis en ratas.
Minocycline is a semisynthetic broad spectrum second-generation tetracycline with a history of use in sexually transmitted bacterial diseases and acne vulgaris (Nair1). Owing to its lipophilic nature, minocycline is readily absorbed, can cross biological barriers and is distributed throughout the organism.2
Minocycline shows distinct effects that are unrelated to its antibacterial properties. Promising effects of minocycline are reported in inflammatory and autoimmune diseases, neurodegenerative disorders and also malignancies among others.3 These new potential indications may necessitate the administration of minocycline in long-term regimens that may be associated with an exclusive profile of effects on different organs. For instance, Yau et al. showed that chronic minocycline treatment improves hippocampal neuronal structure in Fmr1 knockout mice.4
Testes, as the main organs of male reproductive system, have a high and constant proliferative function to produce spermatozoa. Numerous noxious agents such as toxic drugs, oxidative stress, etc. and even life style and daily encountered environmental factors may damage germ cells and disrupt spermatogenesis with resultant male infertility.5,6 Consequently, agents with an ability to protect testicular function and structure against noxious factors are demanded.
Unfortunately, our knowledge about the effects of minocycline on structure and function of male reproductive system are scarce, especially in long term regimens. In a pioneer study by Malallah and Zissis (1992), it was observed that administration of minocycline for one or two weeks to infertile men with high pus cell count in their seminal fluid is associated with increased sperm count and motility.7 Another related study was performed by Orazizadeh et al.8 These authors also evaluated short term effects of minocycline administration but in a mice model of testicular damage induced by dexamethasone.8
As far as we know, the effects of chronic administration of minocycline on testicular histology and function has not been fully addressed yet. Therefore, we evaluated the effects of chronic administration of this antibiotic on histological structure of testes and sperm parameters in rats.
Materials and methodsAnimalsAll procedures used in the study were in accordance with Animal Care and local Ethics Committee (No: IR.IAU.K.REC.1397.9). In this study, 40 male Sprague–Dawley rats with a body weight of 200–250g were purchased from laboratory animal house of the Faculty of Veterinary Medicine, Karaj, Iran. The rats were kept under standard conditions (12h dark/light with free access to food and tap water throughout the experiment).
Experimental designAfter a week of adaptation, animals were randomly divided into 2 groups (n=20):
Group I: control animals (only received distilled water by oral gavage)
Group II: treated animals that received 25mg/kg/day minocycline (Hexal AG, Germany) by oral gavage. To acquiring this dose, the highest recommended dosage for humans (4mg/kg/day) of minocycline was converted to animal equivalent dosage for rats based on the method described by Nair and Jacob (by multiplying human dosage by 6.2).9 Therefore, the dosage used in the study was animal equivalent dose for the recommended human dosage. The drug was administered as a suspension in distilled water.
After 90 days of treatment and overnight fasting, sampling was performed under deep anesthesia. For evaluations which are prone to subjective bias, the scores and qualitative assays were performed without having a knowledge on the grouping of the slides.
Blood sampling and hormone assayBlood sampling was performed from all animals by cardiac puncture under anesthesia. Sera were harvested by centrifugation (2500rpm for 30min). Samples were kept at −20°C until further analyses. To assay serum testosterone levels, a competitive ELISA method was used. The kit was prepared by Monobind Inc.® (California, USA), with the sensitivity of 0.038ng/ml.
Spermatozoa collection and assessmentSamples with equal length (roughly 1cm) were removed from the proximal part of the vas deferens and were cut into small pieces in PBS to let spermatozoa diffuse out of the tissue. To obtain an even distribution of cells, the suspension was moderately shaken at 37°C for 5min.
Spermatozoa countSpecimens were spread on a hemocytometer and the heads were counted manually under a light microscope. Data were expressed as total number of sperms/ml by using this formula.10
Spermatozoa motilityThe suspension of spermatozoa was prewarmed to 37°C on a microscopic placed on a slide warmer. Ten randomly-selected microscopic fields and 200–300 spermatozoa per animal were assessed. The motility of the spermatozoa was determined based on the following grading pattern: (1) rapid progression: quick movement in linear direction, (2) slow progression: slow movement, (3) no progression: only a circular motion, and (4) immotile: no movement was observed.11
Assessing of sperm morphologyStaining of sperm samples was performed by 1% Eosin Y for 5–10min. The percentage of spermatozoa with abnormalities in head or tail was calculated in each sample. In each sample the total number of spermatozoa that were counted was 200–300.11
Histological and histomorphometric evaluation of testesRight testes were immediately dissected and fixed in 10% buffered formalin. After fixation, 1-cm-thick samples were made from the testicular tissue in the transverse plane. Then 5-μm-thick cross sections were made serially from each specimen and stained with hematoxylin–eosin.
Height of the seminiferous germinal epithelium and perpendicular diameter of 10 seminiferous tubules were determined by a linear graticule under light microscope in each slide. The inclusion criteria were that only circular or relatively circular-shaped tubules with vertical and horizontal diameters length difference of less than 20% could be eligible for assessment.
Numbers of spermatogonia, primary spermatocytes, spermatids, Sertoli and Leydig cells in an area of 0.04mm2 were counted in two replicates per slide by100-latticed graticule. Johnsen's method with a score of 1–10 for each tubule cross section was used for evaluation.12
Statistical analysisStatistical difference between two groups was determined by using independent samples t-test. p<0.05 was considered as the level of significance.
ResultsSerum testosterone levelsWhen serum testosterone levels were compared between two groups, results were not statistically different for both control and minocycline-treated groups (p>0.05) (Fig. 1).
Spermatozoa count, morphology, and motilitySperm count (p<0.001), normal morphology (p=0.014) and motility (p=0.006) significantly increased in the minocycline administered group in comparison with the control group (Fig. 2A–C).
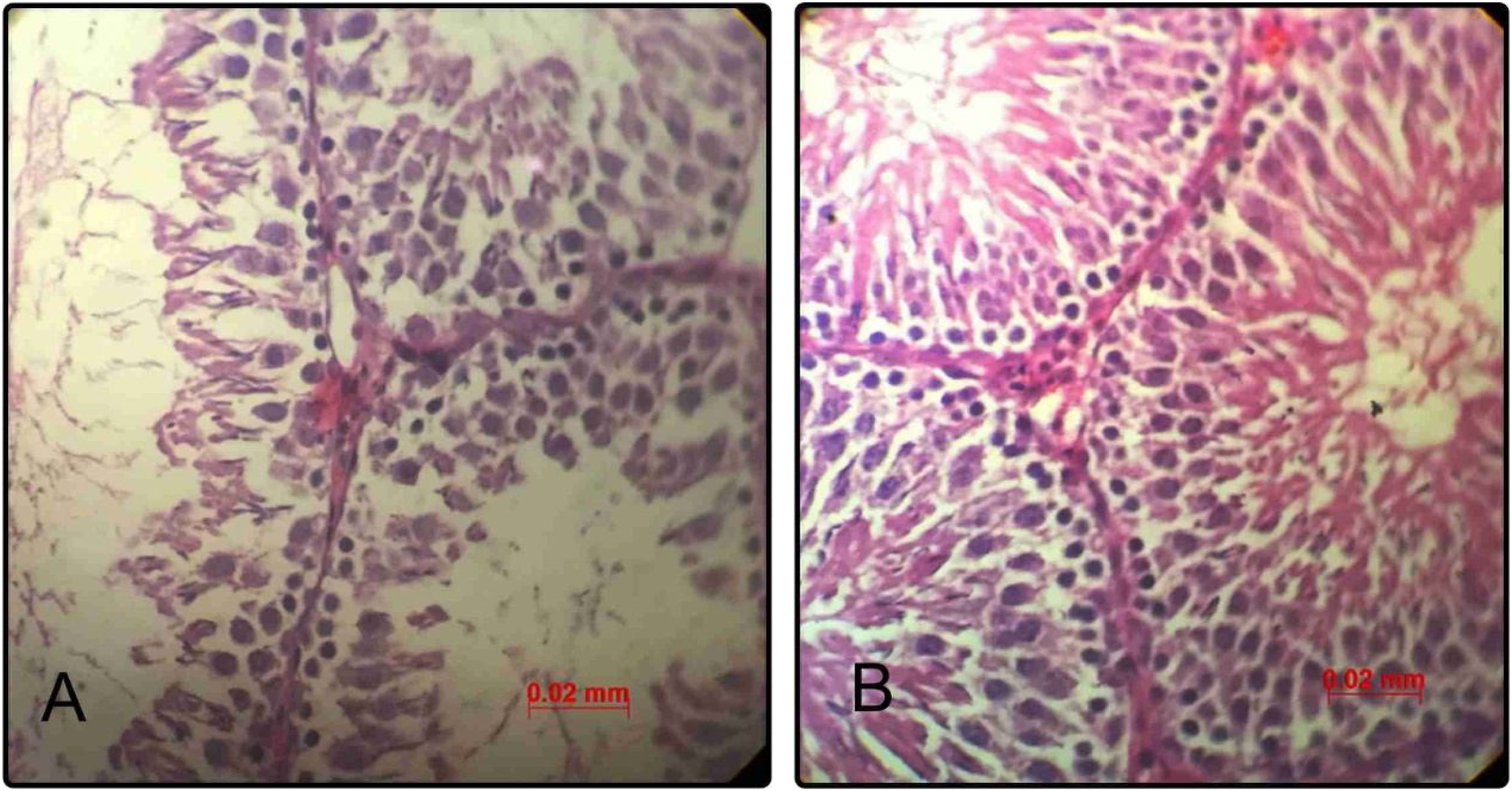
Histological and histomorphometric findingsNormal histology of seminiferous tubules was observed in testicular tissue of control group under light microscope. Epithelial cells and interstitial tissue showed completely normal appearance. Administration of minocycline was associated with considerable improvement in seminiferous and interstitial structures as compared to the control group in a way that in qualitative assessment, the epithelial layer of seminiferous tubules showed increased cellular intensity and order (Fig. 3).
Representative photomicrographs of seminiferous tubules in rats of control group (n=20) (A); minocycline group (n=20) (B) (hematoxylin–eosin, 400×). Scale bar=0.02. (A) The epithelial structure shows a normal cell density. (B) The epithelial structure shows some positive changes such as increased seminiferous cell count as compared to control group.
As shown in Table 1, in quantitative or histomorphometric evaluation, seminiferous tubules diameter in rats of control group were statistically the same with rats of the minocycline group (p≥0.05). Long-term administration of minocycline resulted in significantly thicker germinal epithelium of seminiferous tubules as compared to control (with p=0.035). Numbers of spermatogonia, primary spermatocytes, spermatid and spermatozoa appreciably increased in minocycline treated rats as compared to control rats (p=0.004, p=0.005, p=0.003 and p=0.003, respectively). Although Sertoli and Leydig cell numbers in rats of control group were statistically the same with rats of the minocycline group (p≥0.05) (Table 1).
Histomorphometric parameters (mean±SD) of testis in two groups.
| Tubular diameter (μm) | Germinal epithelium height | Number of spermatogonia | Number of spermatocyte | Number of spermatid | Number of spermatozoa | Number of Sertoli cells | Number of Leydig cells | |
|---|---|---|---|---|---|---|---|---|
| Control | 182.6±15.81 | 71.7±11.66 | 47.7±5.29 | 45.5±3.98 | 42.5±7.92 | 55±10.56 | 4.9±0.74 | 5.40±1.58 |
| Minocycline | 189±14.37 | 81.3±6.46* | 54.9±4.53* | 51.6±4.50* | 48.8±3.16* | 67.6±4.79* | 5.40±0.97 | 5.8±1.03 |
Values in a column with asterisk superscript had significant differences (p<0.05).
Number of spermatogonia, primary spermatocytes, spermatids and Sertoli cells were determined in an area of 0.04mm2.
The results of the Johnsen's scoring are reported in Fig. 4. The mean Johnsen's score was significantly increased in minocycline group in comparison with control (p=0.032) (Fig. 4).
DiscussionTestes, epididymis, and sex hormones collaborate in order to control the complicated process of reproduction in males. Constant spermatogenesis and steroidogenesis are the main functions of testes which comprise a major part of reproductive machinery.13 In the present study, we clearly demonstrated that chronic administration of minocycline can positively affect spermatogenesis as shown by increased number of germ cells in different stages of development without changing testosterone levels or Leydig cell number and morphology. Moreover, spermatozoa showed better characteristics including motility and normal morphology due to minocycline administration. As the most closely related study, Orazizadeh et al.8 evaluated the protective effects of short term (7 days) minocycline administration in mice with dexamethasone-induced testicular damage. In dexamethasone-treated mice, minocycline administration was associated with improved Johnsen score and testicular sperm count as well as reduced germ cell apoptosis. In contrast to our study, minocycline had no effect on these parameters in normal mice in comparison with those in control group that received saline, which may be related to short term vs. long term administration period of minocycline.
A strict balance among germ cell proliferation, differentiation, and apoptosis is mandatory for maintaining homeostasis in spermatogenesis.14 Reduced apoptosis can be associated with increased germ cell count. Anti-apoptotic effects of minocycline are established previously by different researchers and minocycline is shown to have cytoprotective effects. For instance, in a very recent study, Kaviani et al. showed that treating human pancreatic islet cells leads to a drastic increase in cell viability by inhibiting apoptosis.15 Same results are observed in germ cells, in a study by Castanares et al. it was observed that short term administration of minocycline to male rats prevents GnRH-A-induced germ cell apoptosis. Minocycline also prevented human male germ cell apoptosis induced by hormone free culture condition.16
Another related fact is that inducible nitric oxide synthase (iNOS) is important in the induction of male germ cell apoptosis and regulation of sperm number.17 Minocycline has an inhibitory effect on iNOS18 and this might have a role in prevention of apoptosis of germ cells. Jinzhao et al. reported that minocycline protects kidney epithelial cells against apoptosis induced by hypoxia, azide, cisplatin, and staurosporine. The protection occurred at mitochondria, involving the suppression of Bax (an important pro apoptotic protein) accumulation, outer membrane damage, and cytochrome-c release.19 Consistently, we observed that histological and histomorphometric parameters of testicular tissue in minocycline-treated rats show obvious improvements which comprise thicker tubular epithelium and increased germ cell count.
Therefore, it seems that the effect of minocycline on spermatogenesis as observed in the present study can be related to its anti-apoptotic effects by different mechanisms, although this needs to be established in future studies.
In conclusion, chronic administration of minocycline is associated with improved spermatogenesis and sperm characteristics without affecting steroidogenesis in rats.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Authors’ contributionsStudy design, statistical analysis, interpretation and preparation of the manuscript was performed by S. Hamedi. Both authors were involved in sample collection and data acquisition and have approved the manuscript.
FundingThis work was supported by Islamic Azad University, Karaj Branch, Karaj, Iran.
Conflict of interestThe authors declare that they have no conflict of interest.