Chronic pain and especially neuropathic pain are a major challenge to clinical practice and basic science. Neuropathic pain syndromes are characterised by the occurrence of spontaneous ongoing and stimulus-induced pain. Stimulus-induced pain (hyperalgesia and allodynia) may result from sensitisation processes in the peripheral (primary hyperalgesia) or central (secondary hyperalgesia) nervous system. The traditional underlying pathophysiological mechanisms of pain perception says thatpain involves a direct transmission system from somatic receptors to the brain. The amount of pain perceived, moreover, is assumed to be directly proportional to the extent of injury. The peripheral and central neural networks that mediate nociception show extensive plasticity in pathological disease states. Disease-induced plasticity can occur at both structural and functional levels and is manifest as changes in individual molecules, synapses, cellular function and network activity. Recent research has indicate a better understanding of communication within the neural matrix of physiological pain and has also brought important advances in concepts of injury-induced hyperalgesia and tactile allodynia and how these might contribute to the complex, multidimensional state of chronic pain. Clinical and experimental evidence shows that noxious stimuli may sensitize central neural structures involved in pain perception. Salient clinical examples of these effects include amputees with pains in a phantom limb that are similar or identical to those felt in the limb before it was amputated, and patients after surgery who have benefited from preemptive analgesia which blocks the surgery-induced afferent barrage and/or its central consequences.
Sensory stimuli act on neural systems that have been modified by past inputs, and the behavioral output is significantly influenced by the ”memory” of these prior events. An increased understanding of the central changes induced by peripheral injury or noxious stimulation should lead to new and improved clinical treatment for the relief and prevention of pathological pain.
However, the cerebral processing of hyperalgesia and allodynia is still controversially discussed. In recent years, neuroimaging methods (functional magnetic resonance imaging, fMRI; magnetoencephalography, MEG; positron emission tomography, PET) have provided new insightsinto the aberrant cerebral processing of neuropathic pain. Thepresent paper reviews different cerebral mechanisms contributing to chronicity processes in neuropathic pain syndromes. These mechanisms include reorganisation of cortical somatotopic maps in sensory or motor areas (highly relevant for phantom limb pain and CRPS), increased activity in primary nociceptive areas, recruitment of new cortical areas usually not activated by nociceptive stimuli and aberrant activity in brain areas normally involved in descending inhibitory pain networks. Moreover, there is evidence from PET studies for changes of excitatory and inhibitory transmitter systems. Finally, advanced methods of structural brain imaging (voxel-based morphometry, VBM) show significant structural changes suggesting that chronic pain syndromes may be associated with neurodegeneration.
Pain is a complex awareness state. The sensation of pain starts in the brain, and als the cronification of pain. Actually we understand the mechanism of the chronic pain pathways. A part of the mechanism of the chronic pain takes place in the brain According to conservative estimates, it is assumed that approximately 2–4% of the total population in Western countries sufferfrom neuropathic pain (1).
The prevalence increases with age. Neuropathic pain leads to a significant restriction of the quality of life and functioning in everyday life (2). The neuropathic pain is defined as ”Pain disorder or disease with affection of the somatosensory system” (3). In recentyears, several working groups have tried to investigate cerebral activation patterns in neuropathic pain through the use of imaging techniques. Where the magnetencephalography (MEG), fMRI and the Positron Emission Tomography (PET) are leading methods. Essentially six main mechanisms have emerged, which are involved in the chronicity of neuropathic pain. This should be considered:
- 1.
Cortical reorganization and maladeptive neuro-plasticity.
- 2.
Activity increases in primary nociceptive areas.
- 3.
Recruitment of new cortical areas.
- 4.
Modified endogenous pain modulation.
- 5.
The neurochemistry change.
- 6.
Structural changes of the cortex.
Phantom pain arise after amputation of extremities. They are associated with different phantom phenomena, which occur for equently often after amputation. For example, the sensation of the presence of the amputated limb and discomfort in the amputated limb include the phantom phenomena. Pain in a part where no longer existi extremity occurat 50–80% of patients (4, 5). The cause is particular cerebral reorganization phenomena, in addition different peripheral mechanism (such as ectopic discharges of the stump Neuroma, or the Perikarien of the spinal ganglion, a pathological sympathico afferent coupling) (4, 6). The observation of transferred sensations, like sensations in the phantom limb during tactile stimulation in the face (7) gave occasion to investigate the somatotope organization of the primary somatosensory cortex (S1) in amputees. Thereby a shift of the mouth area could agree in several MEG-Studies the hand area of S1 will be shown (8–10). The extent of shift of the mouth area is closely related to the intensity of the phantom pain (8,9), but not with the presence of figurative sensations (9). Establishing a ”pain memory” is discussed here by permanent nociceptive inlet before the amputation, with consecutive neuro plastic changes, and after the amputation nociceptive attracts influx from neighbouring regionsof neurons in the deafference area (11). This leads to the perception of the phantom pain.
This thesis is supported by studies in other painful conditions, such as chronic back pain. In a MEG study in patients with this disease, was an increased cortical activation in S1 with tactile irritation in the painful area measured and observed an enlarged representation of this region in S1 (11,12). Further demonstrated by Nikolajsen et al. (13), that the presence of preprocessor amputation pain positive 3 months after amputation correlated with the presence of phantom limb pain.
Therapeutic interventions can partly modify this neuroplastic changes. So could the cortical reorganization by a behavior-related sensory discrimination training on the butt area be reduced. The regression of the cortical time adaptation process was accompanied by a reduction of phantom pain and improving the sensory discrimination ability in the butt area (9). Reorganization phenomena could be displayed using fMRI for the primary motor cortex in phantom limb pain. While the location of the motor mouth area in the direction of the former hand area (9) moves comparable with the S1 changes. This work also shows that the use of a myo-electric prosthesis goes with a reduction by phantom pain and cortical reorganisation.
Complex regional pain syndromeComplex regional pain syndrome (CRPS) often occur after trauma and are characterized by the onset of pain, which go well beyond the coverage area of a single peripheral nerve or Dermatoms. The clinical presentation consists of sensitive, motor and autonomic disorders (14). In addition to a neurogenic facilitation and pathological sympathico-afferent coupling there is now convincing evidence that changes in the central nervous system in the pathogenesis of CRPS are involved, within the somatosensory system. With MEG and fMRI a reduction of cortical representation of the hand was demonstrated consistently counter lateral to the CRPS affected arm (15–17). Predictors were the perceived pain intensity and the degree of mechanical hyperalgesia for the cortical reorganization. Interestingly the cortical reorganization can be undone by S1 by a sufficient pain management (15,17). A reduction in pain is associated with the recovery of a normal somatotopie which (8, 18) represents the modified somatotopie in acute CRPS and the normalization of representations as an example S1 after successful therapy.
Cortical reorganization mechanisms can explain some of the clinical signs of CRPS, such as the distribution of sensitive errors in a glove - or sock-shaped pattern, the occurrence of projected sensations and hemisensible déficits (19). In addition to these sensitive changes, there is increasingly evidence on changes of central motor system in CRPS. Over 70% of patients with CRPS have paresis muscle of the affected region, fine motor skills errors and reduction of active range of motion (20). About half of the patients has a holding or action tremor. Other symptoms include movement disorders such as dystonia, and myoclonus. In addition, a neglect-like syndrome can lead to a reduce use of the limb (10). It is unlikely that these motor changes appear only by a peripheral mechanism (E.g. influence of sympathetic nervous system on the neuromusculartransmission or the contractility of skeletal muscles)(20). In recentyears, it was shown that more changes at the cerebral level represent the cause of motor disturbances in CRPS. Electrophysiological studies with MEG and Transcranial Magnetic Stimulation (TMS) (21) could indicate a deficient inhibition and an increased excitability in the motor cortex counter lateral to the affected limb. Interestingly abnormalities of inhibitory mechanisms were also observed in the ipsilateral motor cortex (21), which may be accompanied by a minor motor impairment in the non-affected half of the body (21). In a recent fMRI work of our working group (20) succeeded in identifying a cortical network, which correlates with the individual degreesof motor dysfunction in CRPS. The analysis of purposeful movements in CRPS patients already suggested a disturbed sensorimotor integration in the posterior parietal cortex in this study. This cortex is essential for spatial orientation.
Interference, forexamplebya stroke, often leadstoa neglect. The extent of the motor throttling correlated with CRPS with false activations in motor and parietal brain areas (20). Therefore new approaches to therapy for the neuro-rehabilitation of CRPS patients could result.
Carpal tunnel syndromeCarpal tunnel syndrome (CTS) is a common bottleneck syndrome of Nervus medianus with discomfort and pain in the first 3 fingers. Two imaging studies investigated the cortical representation of the affected and non-affected fingen One study with MEG reveals significant correlation between the clinical severity of carpal tunnel syndrome and the latency of the early S1M20. In addition, it was shown as a treatment with acupuncture to a correlated with the degree of clinical improvement regression of cortical reorganization phenomena (24).
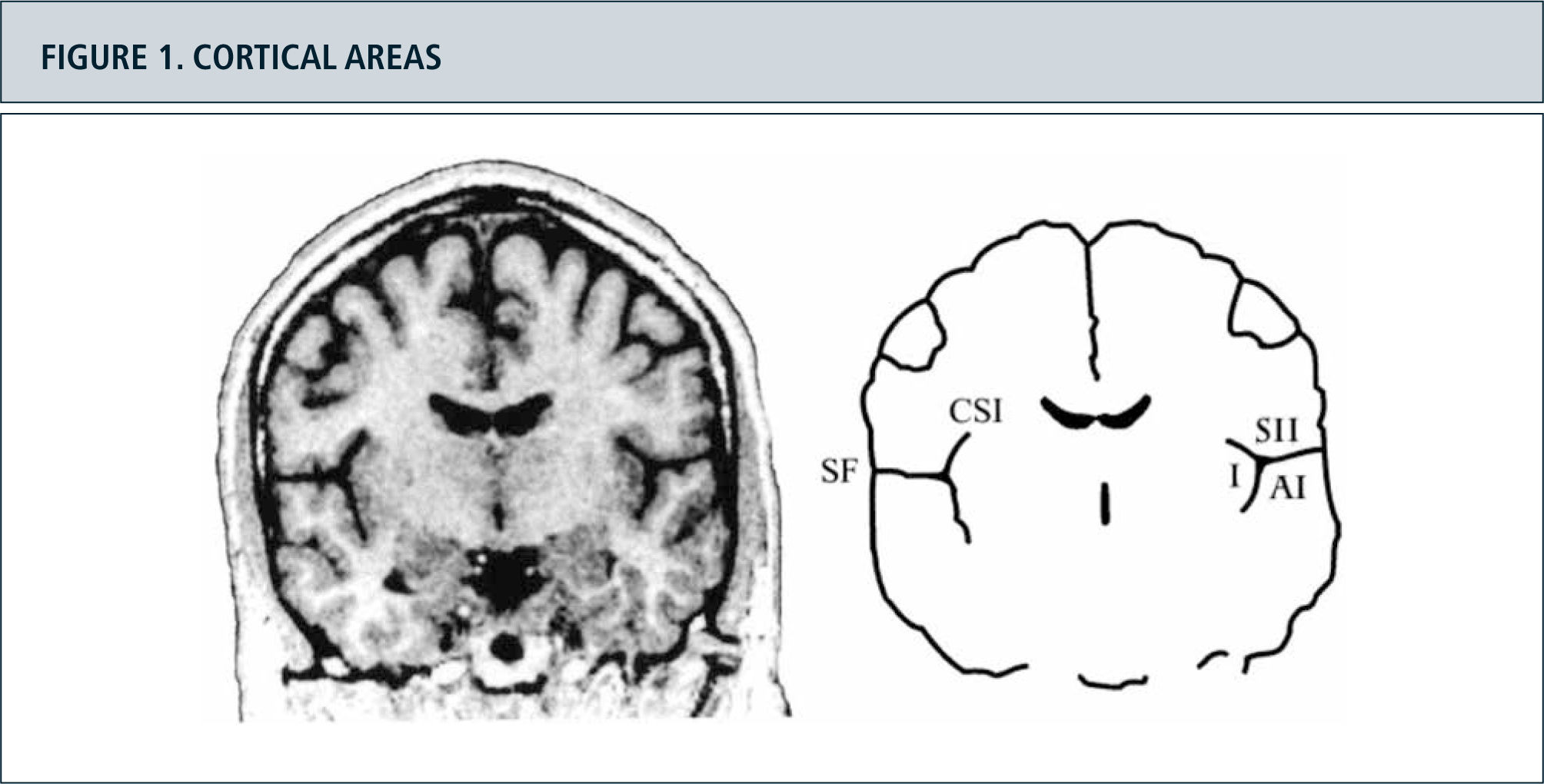
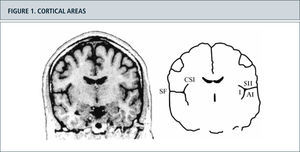
Activity increase in primary nociceptive areasMany articles have examined the cerebral processing of acute pain stimuli and hyperalgesia and allodynia in surrogate models in healthy volunteers as well as neuropathic pain in patients with modern imaging techniques. A major result of these studies was that there is not ”a pain Center”, but that nociceptive input activates a complex network of brain areas. Primary nociceptive areas are often know as the ”pain-neuro matrix” (11,25). This pain matrix (Fig. 2) (26) consists of the primary and secondary somatosensory Cortices (S1 and S2), the insula the anterior Cingulum (ACC), the prefrontal cortex (PFC) and the thalamus (25,26). The corresponding areas have different tasks and process various sub components of the sensation of pain. In S1 and S2, the sensory discriminative sub component is processed mainly in ACC and PFC the affective motivational dimension. So, you can very simply distinguish between a lateral (S1 and S2) and a medial (ACC and PFC) pain system (26). Insula occupies an intermediate position here not only anatomically and functionally. An anatomical representation of the involved cortical areas shows in Figure 1.
Figura 2. Cortical areas around the Sylvian fissure in the human brain. (Left) Magnetic resonance image of a coronal section through the brain of a healthy human subject. (Right) Schematic outlines of this section. The secondary somatosensory cortex (SII) is situated in the upper bank of the Sylvian fissure (SF). The primary auditory cortex (AI) is situated in the opposite, lower bank of the Sylvian fissure, in Heschl's transverse gyrus. On the medial side, SII is situated close to the insular cortex (I), which lies on the opposite bank of the circular sulcus of the insula (CSI). The cortex above the Sylvian fissure contains multiple somatosensory areas, the functions of which are largely unknown. Nociceptive areas in this region overlap only partly with tactile areas and the classical SII region. Nociceptive areas are also found in parts of the insular cortex. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus.
The altered cerebral activation patterns observed in neuropathic pain compared to physiological Nociceptive pain can be divided into 2 basic phenomena:
- 1.
increase in activity primary nociceptive areas (areas of the so called ”pain matrix”) and
- 2.
the recruitment of additional cortex areas (i.e., regions of the brain, that are not members of the ”pain matrix” primary).
In the analysis of the processing of neuropathicpain in the central nervous system must take account also of variety of symptoms, because diverse pathophysiological mechanisms underlying the individual symptoms of the overall phenomenon ”neuropathicpain”. Forthe classification of the symptoms and imaging studies we can distinguish between:
- 1.
Spontaneous pain, which may be continuous or paroxysmal, and
- 2.
Pain such as evoked pain, dynamic mechanical allodynia, mechanical hyperalgesia, thermal allodynia and thermal hyperalgesia.
A spontaneous pain during neuropathic pain frequently arises in the peripheral nervous system through ectopic discharges of degraded primary afferents (27,28) with consecutive awareness of rear horn neurons, or in the central nervous system by DIS inhibition phenomena (6,12).
Cerebral activity of spontaneous pain were examined in particular in PET studies. PET enables the measurement of basal brain activity in patients compared to healthy control subjects and comparing the healthy while v. a. with the sick side. The application of fMRI is fraught in this context with methodological problems. The PET, however allows the continuous registration of regional cerebral blood flow (rCBF) and above comparison can reveal brain areas activated or disabled. By PET, a reduced rCBF in the contralateral thalamus was consistent with spontaneous pain of patients with Mononeuropathien found (29). However, an increased rCBF in the insula ACC, posterior visceral cortex and PFC, but not in S1 and S2 was measured (29). As an explanation for the talassemic reduction of rCBF one discussed an inhibition of excessive nociceptive input or a decoupling of the rCBF of the neuronal activity (18,29). A recently published study with fMRI investigated spontaneous pain in patients with chronic low back pain (6). Chronic back pain can often be associated with a neuropathic component (12). The patients had in the MRI scanner phases with high and low spontaneous pain, what advantage was made for the analysis of fMRI data. The authors found increased activity in the PFC and the rostral ACC during high periods of spontaneous pain. In phases of pain building however, i.e. in increasing pain intensity, was activated classic ”Pain neuromatrix” measure (30). The evoked pain is for many patients particulary distressing and often regarded as the leading sympton of nerophatic pain. For the physician, the understanding of the underlying cerebral processing of this phenomenon is of great scientific interest. The hyperalgesia is divided in primary (in the injured tissue area) and secondary hyperalgesia (into the surrounding tissue). An awareness level of Nociceptors is the primary hyperalgesia (existent for various Submodalities, such as heat, cold, mechanical stimuli) v. a. underlying. Secondary hyperalgesia, however is due to an awareness of nociceptor input at the spinal level. This awareness of the dorsal horn neurons is mainly inducedby C-fiber input, especially from the so-called ”silent nociceptors”. Nociceptor input on the sensitized dorsal Horn neuron results in increased activity in this tate. Alternatively, a hyperalgesia (especially a cold hyperalgesia) generated by lesion-induced desinhibitions and desintagration phenomena at different levels of the neuro axis (18). To differentiate from the hyperalgesia is the dynamic mechanical allodynia, where normally the tactile system-related A-β-fiber input obtained pathological connection to the nociceptive system.
A summary of the results of presented in the following studies on stimulus-induced pain can be found in (31).
The incidence of reported activation of particular brain areas from the studies served as base to weigh the size representation of Cortex areas. This figure is therefore a first impression what Cortex areas are frequently activated allodynia and hyperalgesia (31).
The cerebral processing of allodynia and hyperalgesia was investigated in ten patients with neuropathic pain due to peripheral or central nerve lesion, and in patients with complex regional pain syndrome (CRPS) (1,3, 11,18). When dynamically-mechanical allodynia are notably areas in the lateral pain system activated. Activations in the ACC were not, however, observed in all studies. The dynamic mechanical allodynia was examined in the following studies:(-peripheral neuropathic pain (9,18, 32), - central neuropathic pain (33) - CRPS (119,20,34) -heterogeneous patient population with peripheral and central neuropathic pain syndromes (35). 6 patients with Syringomyelia have been included in the study of Ducreux (33) and activations in S1 and S2, found in the Dorsolateral PFC, parietal Assosiationscortex, thalamus and the basal ganglia. Activations in S2, anterior Insula and the orbitofrontal cortex were measured in the study of Witting (35) in 9 patients with peripheral nerve lesion. These studies are common, they found no activation of the ACC and predominantly activations in the lateral pain system were observed. This pattern is thus in contrast to the activations observed in nociceptive pain and ”Pin-prick hyperalgesia ”. The cause forthe missing ACC activation in these studies is unclear, be discussed a differential cerebral processing of dynamic mechanical allodynia as a result of the pathologically related A-β fiber input. Five other studies found cingula activations with tactile allodynia. One of them is the work of Schweinhardt et al. (37), in 8 patients, an activation of the ACC was measured with peripheral nerve lesion, in addition activations in S1, S2, Insula, PFC and the posterior parietal cortex were found. Another study of ourworking group in 12 patients with CRPS found also activations in the ACC and all areas of the pain matrix (19). Also activation of the ACC, as well as all other areas of the pain matrix was measured in a study by Becerra (32) in 6 patients with trigeminal neuropathy. In a further work by Peyron (35) in a very heterogeneous patient population with peripheral and Central pain syndromes also a recruitment were observed in tactile allodynia in addition to the activations in contralateral S1, S2, and Insula of ipsilateral Cortex areas in S1, S2, and insula.
A symptom of another, less often examined in patients is ”Pin-prick hyperalgesia”. A study investigated 12 patients with complex regional pain syndrome (13,14). Multiple activations were found in all areas of the pain matrix and activations in addition recruited areas outside of the pain matrix there. By ”pin-prick hyperalgesia” enabled areas seem thus to distinguish itself from the activation patterns with regard to the increased activation in the medial pain system observed in dynamic mechanical allodynia, a direct comparison is however pending.
Thermal hyperalgesia has been tested in 2 trials in patients with neuropathic pain: cold hyperalgesia has been tested with fMRI in 6 patients with Syringomyelia and observed activations in the middle and posterior Insula, the ACC and the PFC and PA and SMA (33). In the already mentioned study by Becerra (32), the brain activations in 6 patients with trigeminal neuropathy were measured with fMRI in heat and cold hyperalgesia. This multiple activations in prefrontal Cortex areas and in the basal ganglia by stimulation on the affected side compared to the unaffected side and more activations when heat and mechanical stimulation in the Insula found in refrigeration and mechanical stimulation.
Additional information about the cerebral cortical pain processing arise from works with surrogate models of neuropathic pain. Because clinical neuropathic pain syndromes with certain heterogeneity of the symptoms are surrogate models offer the advantage of isolated and under controlled conditions in healthy subjects with imaging techniques to examine individual symptoms. It must be remembered however that the models offer no complete substitute for a clinical neuropathic pain syndrome and usually they don’t meet their complexity. Surrogate models are the experimental generation of evoked pain which have found application in studies on the functional imaging methods, the capsaicin hyperalgesia model (1,14, 38), the menthol hyperalgesia model (39) and the UV-B (39) hyperalgesia model. One of the works conclude that different types of hyperalgesia in a human surrogate model of ¡nflammatory pain produce different brain activation patterns (39). This ”network of hyperalgesia”, consists of ACC, bilateral anterior Insula and bilateral inferio-rem frontal cortex (IFC). It seems to play a key role in awareness-raising processes.
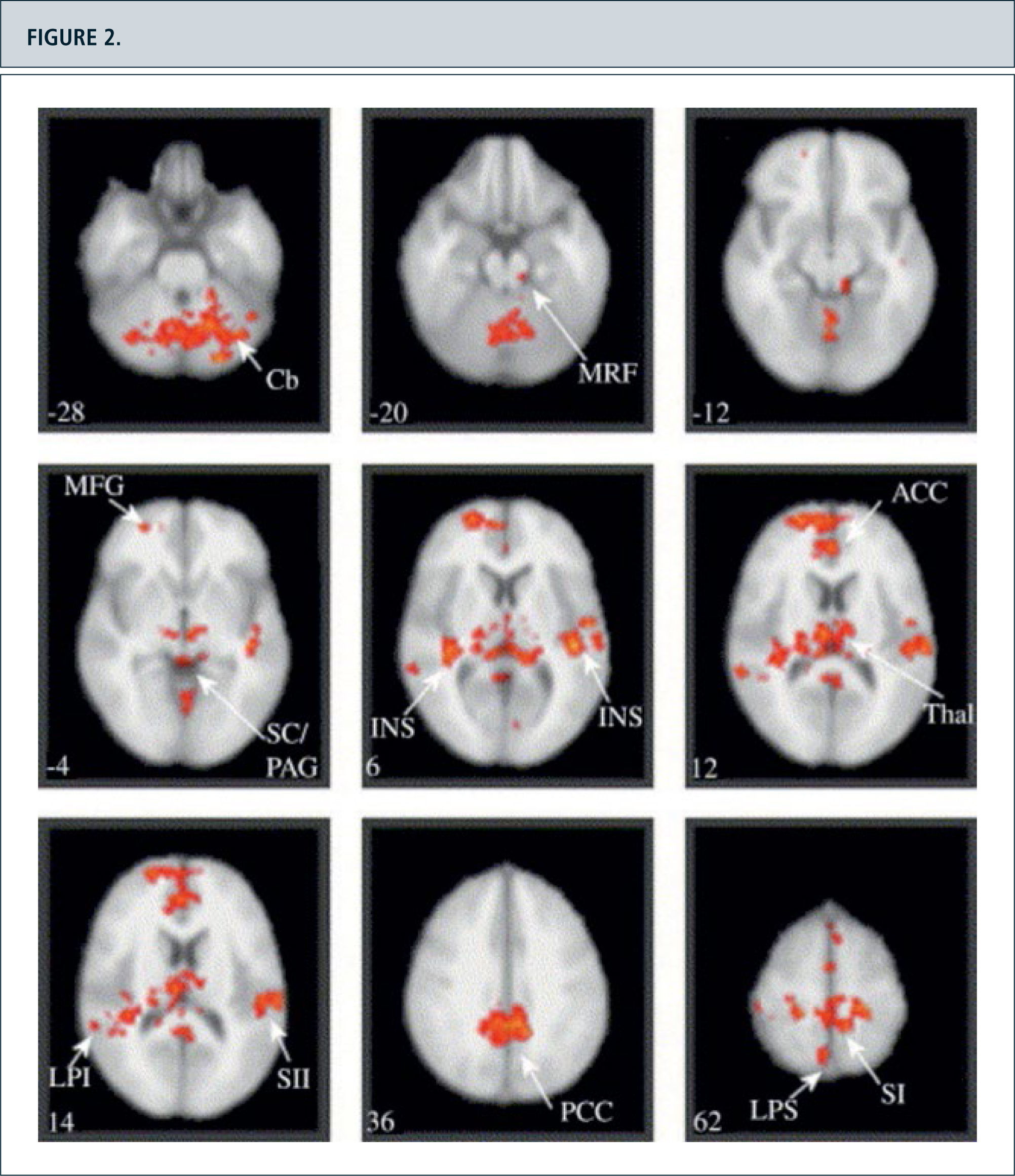
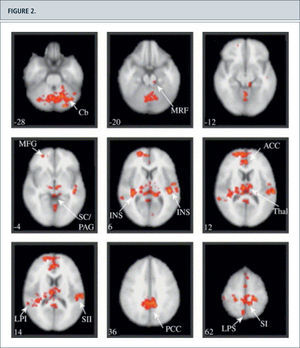
The observable in neuropathic painBeyond the Pain -neuromatrix ”individual pain signature” (40) is influenced by multiple factors such as underlying pathological pain condition, expectations, attention, affect and mood. These areas include frontal Cortex areas such as the dorsolateral prefrontal cortex (16,17), as well as a slew of brainstem nuclei, which are involved in modulating pain networks. So were Seifert and Maihofner (15,16,19,20,39) in the model of the cold hyperalgesia induced by menthol parabrachialis activations of the nucleus of the brainstem (Fig. 2).
Figura 4. Paired test between the brain activation maps in response to punctate stimulation of the secondary hyperalgesic area and control site stimulation. Group activations registered onto the MNI standard brain in axial view, Z score >2.3, cluster corrected P <0.01. Specific coordinates and Z scores for each cluster are listed in Table 1. Activation was detected in: the cerebellum (Cb), the midbrain reticular formation (MRF) with a lateral cluster corresponding to the location of nucleus cuneiformis (NCF) and a rostral midline cluster consistent with the periaqueductal gray/superior colliculi (SC/PAG), bilateral insula (INS) and thalamus (Thal), anterior and posterior cingulate cortex (ACC and PCC), primary (SI) and secondary (SII) somatosensory cortex, superior (LPS) and inferior (LPI) parietal cortex. The number on the bottom left corner of each panel represents the Z coordinate in standard space, i.e. the superior-inferior location of the slice. Images are displayed in radiological convention.
These findings support the assumption that brain stem structures can effectively modulate the nociceptive transmission.
Changes of endogenous pain modulationIn patients with neuropathic pain syndromes research imaging to the endogenous pain modulation by attentional, cognitive, or emotional processes are still outstanding, however, is to assume that basic mechanisms of pain modulation by healthy volunteers on patients can be transferred.
Changing the neurochemistryTwo methods of functional imaging allows the non-invasiveinvestigation of regional neurochemistry in the human brain. These are the PET and the magnetic resonance spectroscopy (MRS). The PET can measure regional cerebral blood flow (rCBF) and regional glucose metabolism. In addition, the regional distribution of certain receptors can be detected with the ligand PET. For ligand-PET examinations in the context of pain studies ligands for the opioid system are used. The methodologyenables also the study of dynamic changes of the receptor inset through the natural ligands (38) the local receptor distribution. A significant opioid -receptor binding potential is found in all areas of the neuromatrix-pain (33). In the context of neuropathic pain, this binding profiles can be significantly changed. So a decreased ligand binding in the thalamus, PFC, ACC and Insula, visceral Assoziations-cortex could be detected in patients with trigeminal neuralgia and patients with central neuropathic pain (”post stroke pain”) (38–40). Also in fibromyalgia patients, there is evidence of a reduced number of free opioid receptors in the brain (41). These SE findings can explain partly why opioids don’t always work for neuropathic pain. As underlying mechanisms is the down-regulation of opioid-receptors, but also a change in the binding capacity by endogenous opiates discussed (42).
Structural changes of the brainStructural change of cerebrums in vivo can be measured with voxel-based morphometry (VBM). In a study by Draganski, a decrease in the contralateral thalamic gray matter could be measured in 28 patients after limb amputation. However, these thalamic changes are not correlated with the presence or intensity of phantom pain. However, a decrease of the gray matter in the PFC, Cingulum, SMA and dorsal midbrain (43) were positively correlated with the intensity of the pain. In another study in patients with chronic back pain, a loss of gray matter on one globally on the whole brain level and on the other hand regional bilateral PFC and right thalamus was measured. The decrease of the gray matter at the brain level correlated positively with the duration of the disease, thereby has been pronounced in a sub group of patients with neuropathic pain component (12,30) in the dorsolateral prefrontal cortex region. Another work for chronic back pain revealed localised changes with increased grey matter in the basal ganglia and the thalamus and reduced grey matter in the brain stem and somatosensory cortex (40). A reduction is found in patients with fibromyalgia of the total volume of gray matter and a lower density of gray matter in pain-related brain regions such as PFC, Cingulum and anterior Insula (37). A second work in fibromyalgia patients showed an increase in the gray matter, however, in striatum and OFC and a decrease in the thalamus and superior of temporal gyrus (44).
Thus the results of VBM studies in chronic pain are still heterogeneous and sometimes difficult to interpret, co-morbidity justified among other things also in the frequently small case numbers, differences in drug treatment, and of the functional status and affective comorbidity. Nevertheless chronic pain can lead to significant structural changes in the gray matter in the brain. Future studies with larger numbers of cases and advanced methodology will show whether there are characteristic patterns of changes of the cortex in individual diseases.
ConclusionNeuromagnetic recordings have a relevant value in providing information on the excitability, extension, localisation and functional hierarchy of sensorimotor brain areas during motor learning, as well as sensorimotor integration in both the healthy and in pain patients.
La autora declara no tener conflictos de interés, en relación a este artículo.