Vascular access, arterial and venous, at peripheral and more central sites is a core skill, yet is not always well taught or in core training. Like many procedures, it can be simple to learn the basics, but hazards await inexperienced operators.
Many insertion steps are common to all procedures: time is needed to choose optimal devices and site dependent on clinical need, length of treatment, and patient wishes. Adequate explanation/consent is needed.
Asepsis is essential for all insertion and aftercare, because of direct access to the circulation. This is under increasing scrutiny 1. Prevention of needlestick injury is important as access needles carry a significant inoculum of blood. All but the smallest devices require Local Anesthesia (LA), topical or injected. For central access, wide infiltration is required (a minimum of 10-15mls for adults). Intravenous sedation is helpful for anxious patients. Some patients (e.g. children) will need general anaesthesia.
Cannulation is achieved by a number of techniques, including:
- •
Direct vision (e.g. superficial vessel or cut-down).
- •
Indirect vision (e.g. infrared devices).
- •
Palpation (arterial pulse, full vein).
- •
Landmark orientation (e.g. next to artery, clavicle).
- •
Ultrasound.
- •
X-ray (after contrast injection).
Needle entry is confirmed by backflow or aspiration of blood. A catheter or guidewire can then be passed. Catheter position is verified by aspiration/backflow of arterial or venous blood, flushing the catheter, measurements of pressures, ultrasound, X-ray and ECG guidance 2. Anchorage is needed to avoid catheter dislodgement, with adhesive dressings or sutures, or internal anchoring devices. Meticulous aftercare and observation is required to maintain safe effective catheter function. This includes regular flushing, removal before problems occur, and recognition and management of complications 3,4.
Most devices utilise percutaneous techniques, but surgical cut-down is still used in emergencies and small children. Usage has diminished due to operative time and skills needed, scars, potential greater damage to the cannulated vessel, and higher risk of local infection 5.
Peripheral vein cannulationThis is a core technique and skill is required in challenging cases, e.g. a small child, the very elderly with fragile veins, and when all obvious veins have already blocked. It is not risk free (Table 1).
COMMON COMPLICATIONS OF PERIPHERAL CANNULATION.
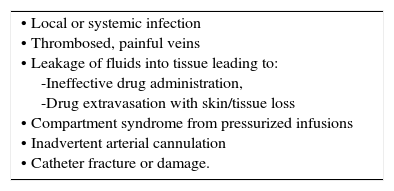
| • Local or systemic infection • Thrombosed, painful veins • Leakage of fluids into tissue leading to: -Ineffective drug administration, -Drug extravasation with skin/tissue loss • Compartment syndrome from pressurized infusions • Inadvertent arterial cannulation • Catheter fracture or damage. |
Discomfort is reduced by using the smallest feasible devices and effective LA. Avoid insertion over joint flexures. Aids to insertion are typically based on enhancing the size or visibility of vessels. Traditionally these included transillumination and local warming. High-resolution ultrasound aids procedures in all ages 6. Newer devices utilize the differentia absorption of infrared light, which penetrates deeper than visible light, by blood compared with tissues to generate an Image 7.
Intraosseous injectionThis route of access is widely used in adult and paediatric resuscitation. A needle with a trocar is inserted into the upper tibia to access venous sinuses. There are purpose-designed needles and powered drills available. Care must be taken to avoid extravasation, bony injury, and infection, and standard venous access should be sought soon after 8.
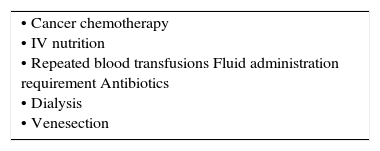
Central venous cathetersMany patients will require central venous catheterization in short or longer term (Table 2). More than an estimated 250000 are inserted annually in UK. Contraindications are relative including; limited sites for access, anatomical variants, venous stenosis, previous difficulties/complications, severe coagulopathy, and local sepsis at the insertion site.
INDICATIONS FOR CENTRAL VENOUS ACCESS.
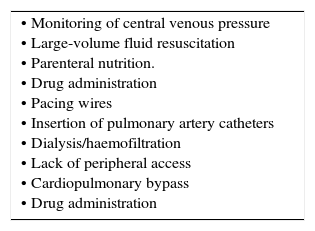
| • Monitoring of central venous pressure • Large-volume fluid resuscitation • Parenteral nutrition. • Drug administration • Pacing wires • Insertion of pulmonary artery catheters • Dialysis/haemofiltration • Lack of peripheral access • Cardiopulmonary bypass • Drug administration |
There is a wide choice of devices typically inserted via guidewire techniques. Commoner devices include; standard multilumen central venous catheters (CVC), a ‘long line’ or peripherally inserted central catheter (PICC), valved introducer sheaths, and dialysis-type catheters.
A range of fixed lengths is needed to suit each insertion site. For adults, use 15cm for the right internal jugular vein (IJV), 20cm for the left IJV and right axillary/subclavian vein, and 24cm for left axillary/subclavian and femoral veins. Use the narrowest gauge suitable to reduce insertion trauma. Large-bore catheters and dilators devices do not traverse corners easily, so use right IJV or femoral veins if possible. Compare the size of a vein on ultrasound with the catheter diameter. A catheter occupying more than 1/3rd of the diameter is associated with increased risk of thrombosis
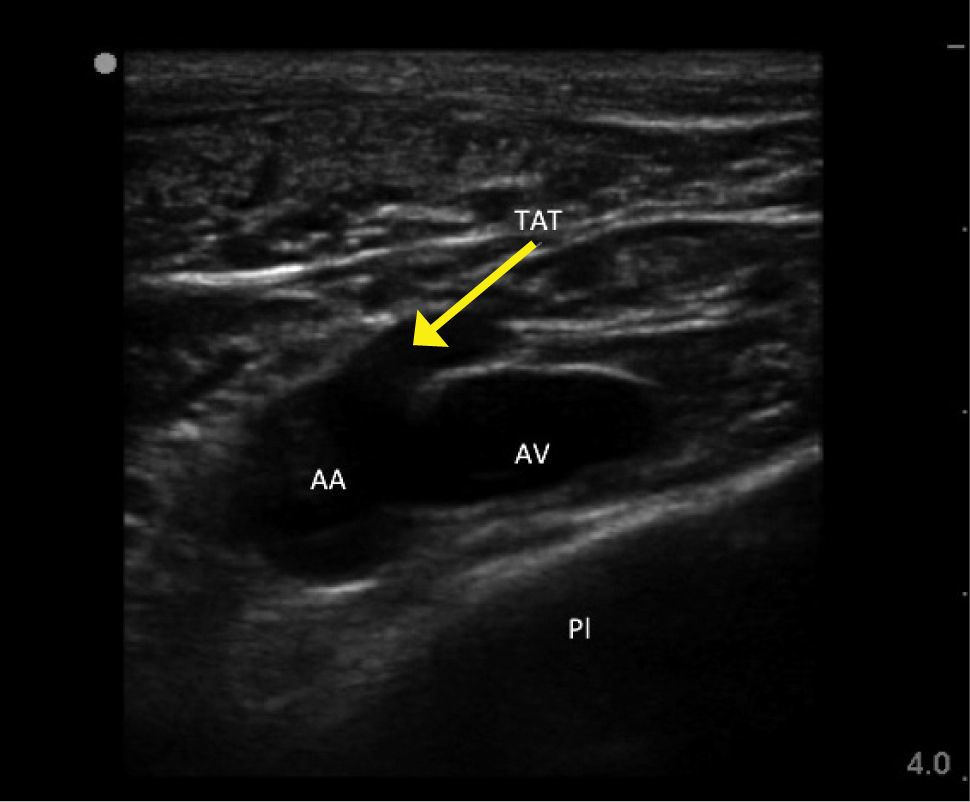
ROUTES OF ACCESSInternal jugular veinRight side access is linked to lower insertion complications and tip malposition. There is variation in carotid artery and IJV relation, and a dominant vein on one side. In sicker patients there is increased risk of infection due to proximity of oral secretions. Carotid puncture or catheterisation should be avoidable with ultrasound. Arteries like the thyrocervical trunk and branches, vertebral, and subclavian lie behind the vein (Figure 1) and can be hit on vein transfixion.
Landmark techniquesMany techniques of accessing the IJV are described 9. Typical approaches are from the apex of a triangle enclosed within the two heads of the sternomastoid. The neck is gently extended and turned a little to the opposite side. The carotid artery is felt at the level of the cricoid cartilage. The jugular venous pulse may be seen, and the vein if compressed will on release be seen to refill. The needle is inserted from the apex of the triangle at an angle of o and aimed towards the ipsilateral nipple. The vein often collapses under the needle, which then transfixes it, and puncture is not recognized. The vessel may then be located by aspirating as the needle is slowly withdrawn. The vein is usually less than 2cm from skin and can be located with a standard green ‘seeker’ needle. The vein can be cannulated in the semi-upright position in the case of heart failure or morbid obesity, if the venous pressure is high.
Ultrasound guidanceThere is strong evidence for use of ultrasound for IJV access to reduce complications and failures 10. Look for branches (e.g. facial vein), valves, and the carotid, subclavian, and branches thyrocervical trunk. The thyroid gland (cysts common) and large lymph nodes are visible. Choose a puncture site and needle direction to minimize overlap of vein and arteries.
EXTERNAL JUGULAR VEINThis site is used acutely when a standard peripheral cannula is placed under direct vision. CVCs passed via the external jugular vein traverse angles in fascial layers, which may give problems passing into the subclavian vein.
SUBCLAVIAN VEINLandmark approaches are linked with more risks compared with the IJV, e.g. pneumothorax and incorrect tip placement. It is more comfortable for the patient and a potentially cleaner site.
Avoid access on the side of arteriovenous fistulae as there is high pressure in vein and potential risk to fistula from thrombosis.
Landmark techniquesThe needle is passed under the clavicle at the junction of its medial third and lateral two-thirds, and then redirected towards the suprasternal notch, with continuous aspiration until blood is seen. The vein may be transfixed, so aspiration is important on needle withdrawal 11.
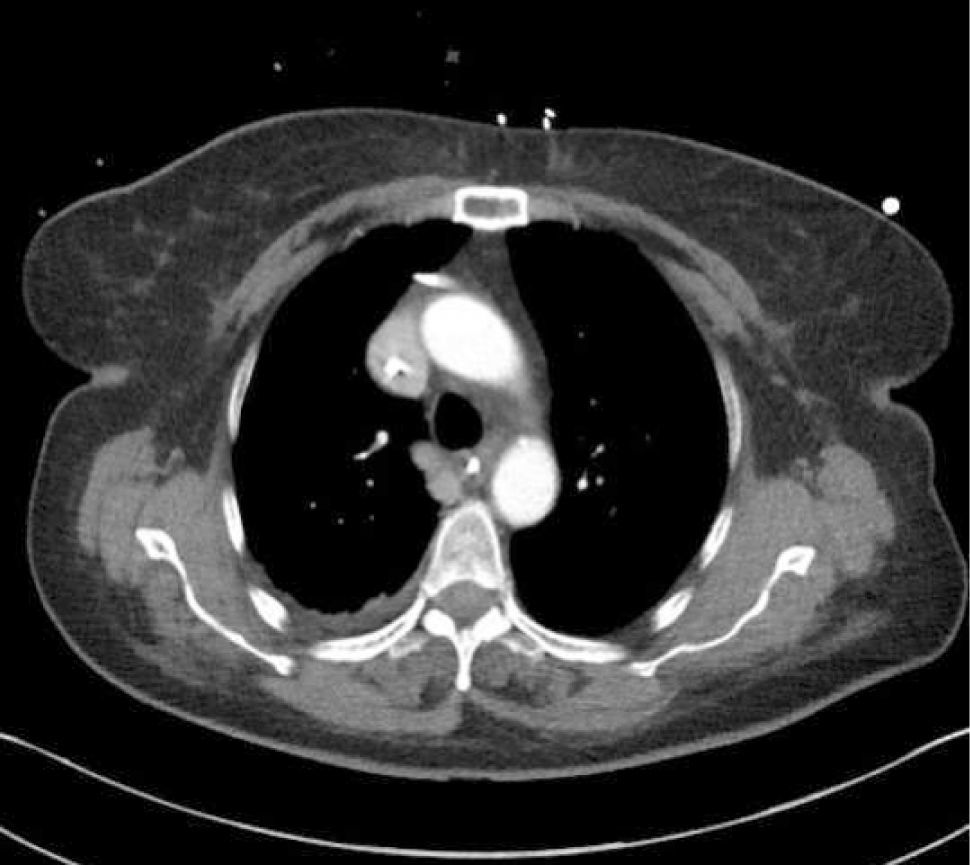
Ultrasound guidanceThe clavicle impedes ultrasound, requiring a more lateral infraclavicular axillary or supraclavicular approach 12. Recent studies have shown benefits of ultrasound 13,14. Ultrasound allows avoidance of the nearby pleura, axillary artery (and the thoracoacromial trunk branches passing anterior to vein), cephalic vein and brachial plexus (Figure 2). It may be difficult to visualize and access a deep vein in obese or muscular patients.
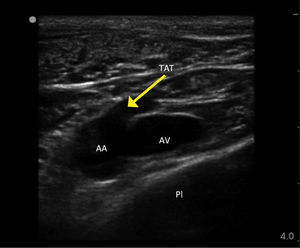
FEMORAL VEINThe anatomy is more complex than the side-to-side orientation of vein and artery in textbooks, which is only relatively true at the level of the inguinal ligament. Femoral vein access is useful in patients unable to tolerate head-down position, in children and emergency situations.
Landmark techniquePalpate the femoral artery and introduce the needle just medial to the femoral artery close to the inguinal ligament (not palpable, but represented by a line from iliac crest to pubis tubercle). It is a common mistake to go too low where the superficial femoral artery partially overlies the vein.
Ultrasound guidanceIdentify the femoral vein and long saphenous vein, and the common femoral artery dividing into deep and superficial branches. Higher punctures risk incompressible damage to vessels, to cause occult bleeding into the peritoneal or retroperitoneal space.
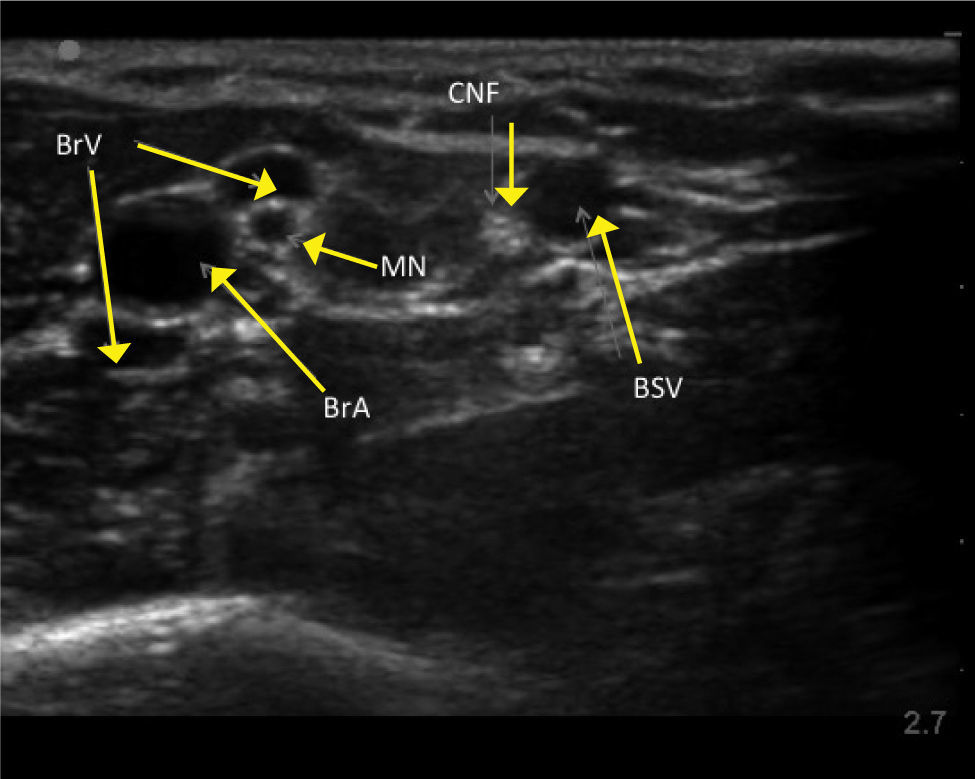
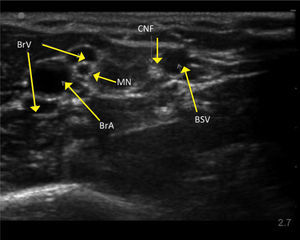
UPPER ARM VEINSHigh resolution ultrasound easily identifies deeper veins in the proximal upper arm to aid access in difficult case, and PICC insertion in the upper arm to avoid elbow flexure PICCs can be inserted from antecubital fossa with direct vision. The basilic, brachial, and cephalic veins are seen with arteries and nerves (Figure 3). Note close relation of median nerve and brachial veins, and cutaneous nerve of forearm and basilic vein. The cephalic vein runs a tortuous course to enter the axillary vein leading to difficulties in passing catheters. There is wide anatomical variation.
Access is easiest with a sharp small-bore needle (20g), fine guidewire, dilator, and sheath (micropuncture set). The catheter is measured for length externally, from X-ray screening or ECG guidance 15.
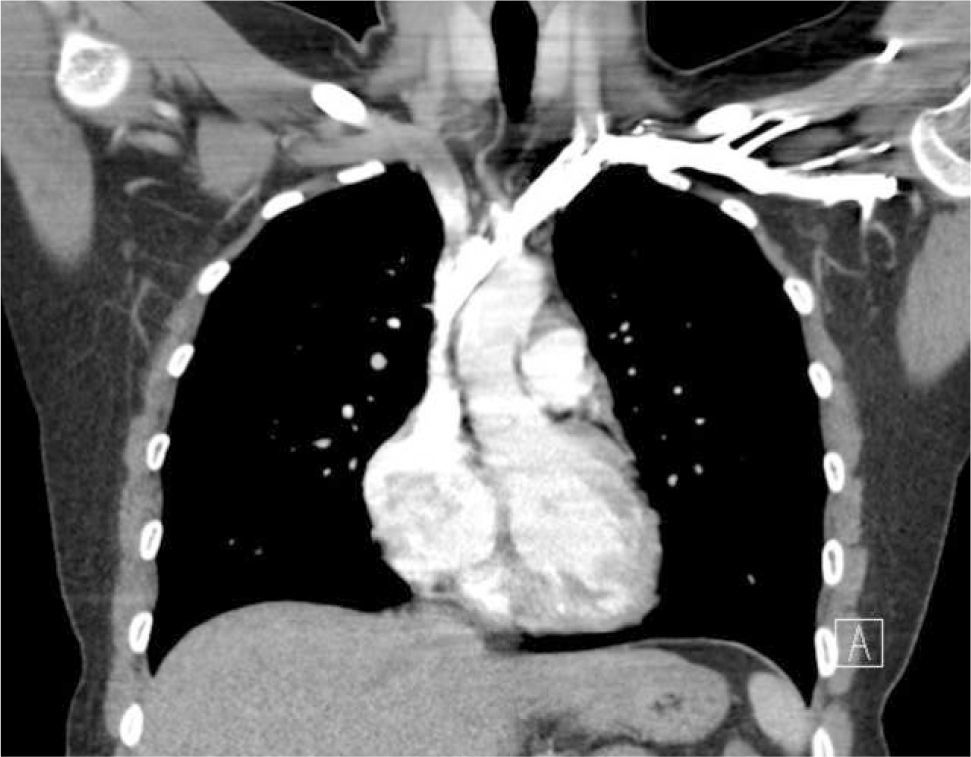
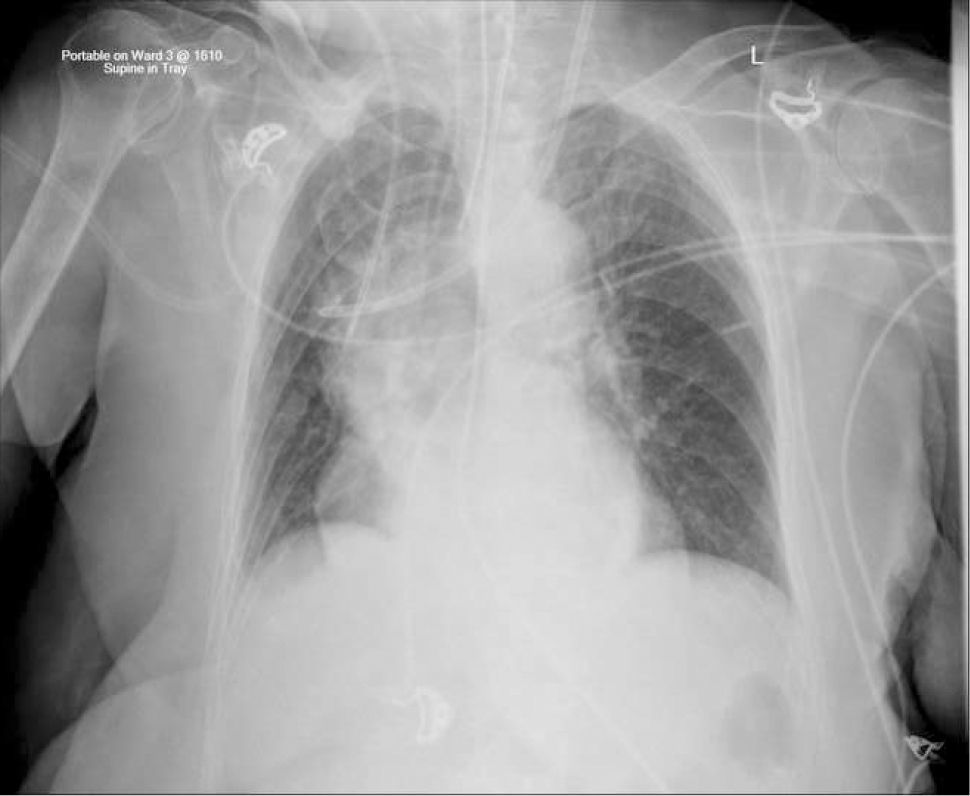
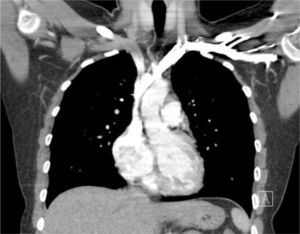
Applied anatomy of the superior vena cava (SVC)The lower SVC is the target for catheter tips from the upper-body and applied anatomy is important 16. It is formed by the two brachiocephalic veins behind the first right costal cartilage. It is approximately 2cm in diameter and 7cm long with no valves and descends to the right atrium (Figure 4). Its right border is partially visible on chest X-ray but it is difficult to visualize the junction with right atrium.
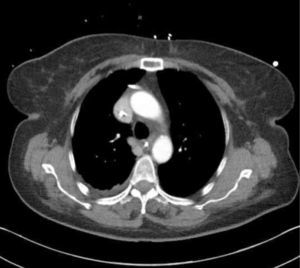
The upper right border of the SVC bulges into the low pressure pleural space so a tear can cause major bleeding. Abutting catheter tips (particularly from the left) can erode the vein wall and cause a hydrothorax (Figure 5). The lower section of the SVC is within the pericardium so a perforation risks cardiac tamponade.
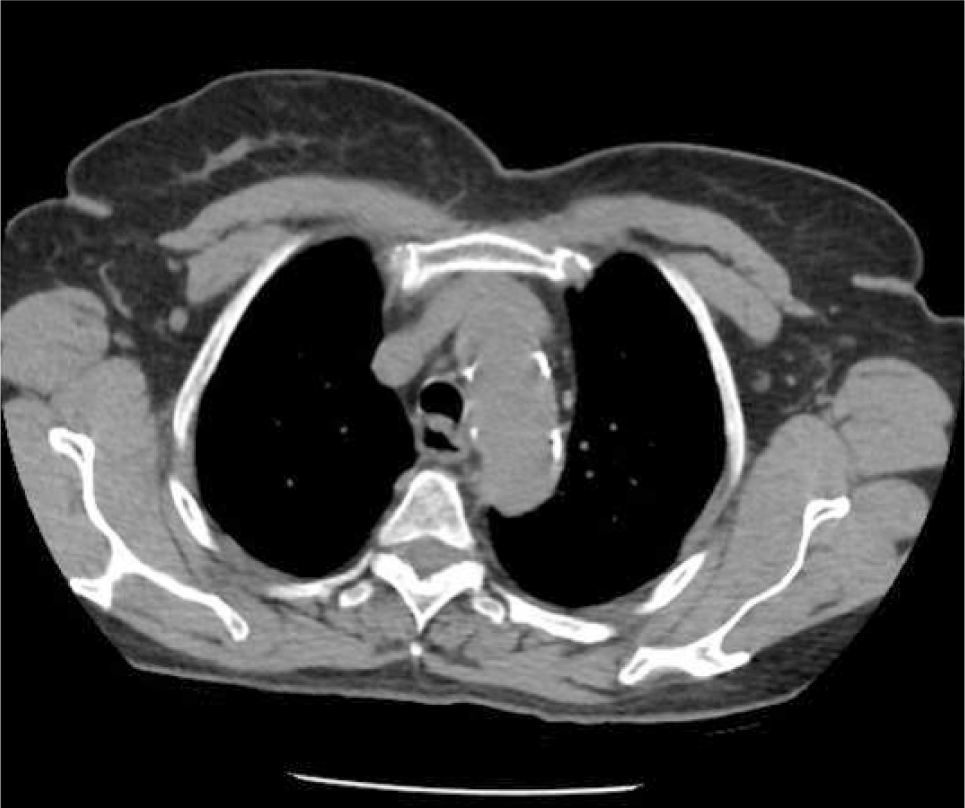
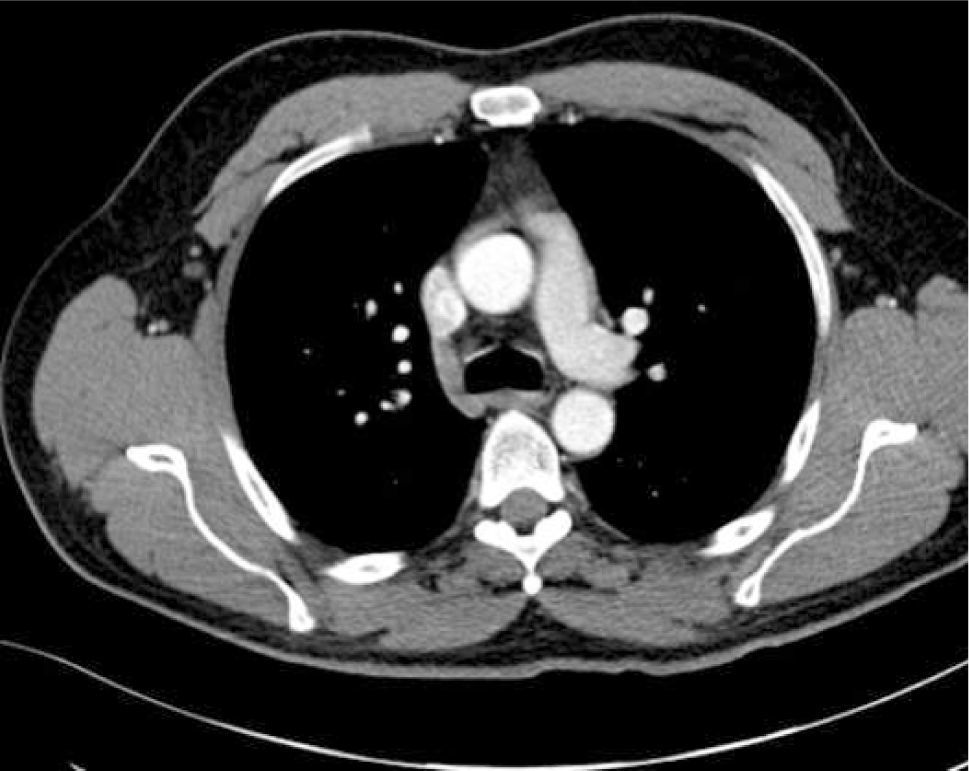
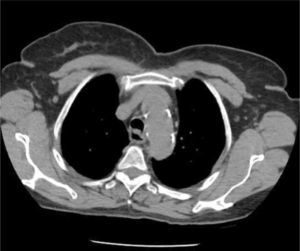
The azygous vein ascends on the right side in the posterior mediastinum, passes anteriorly to join the mid-section of SVC above the hilum and is a site for tip malposition. Left sided catheters traverse one or more corners to pass to the SVC, making tip placement more difficult, particularly if the left innominate vein curves anteriorly (Figure 6).
AXIAL CT OF CHEST SHOWING THE LEFT INNOMINATE VEIN CURVING ANTERIORLY TO ADD FURTHER CORNERS FOR LEFT SIDED CATHETER TO PASS THROUGH TO ACCESS THE SVC
This patient had distorted aortic arch from aneurysm but this curvature is seen without obvious disease. It is a common reason for difficulty in positioning left sided catheters.
The commonest SVC variant is a left sided vein, which occurs with or without a normal right SVC (0.5% population, and higher with cardiac defects). A left SVC crosses the arch of the aorta and left pulmonary hilum, and enters the right atrium via an enlarged coronary sinus. It can be used for access if entering right atrium, but may open into the left atrium with risks of systemic embolism 17. The IVC can show similar double variation.
Patients with dextrocardia have the heart orientated in reverse so that it lies over to the right. It can be associated with the reversal of abdominal/chest organs and blood vessels, so-called situs inversus, in which case the SVC and IVC also lie to the left.
AcquiredAcute SVC compression from tumour can cause oedema and venous engorgement in the upper body (SVC syndrome). Stenosis or thrombosis is common with long-term access, and is often asymptomatic due to collateral vein formation. This can present as a failure to pass a guidewire or catheter.
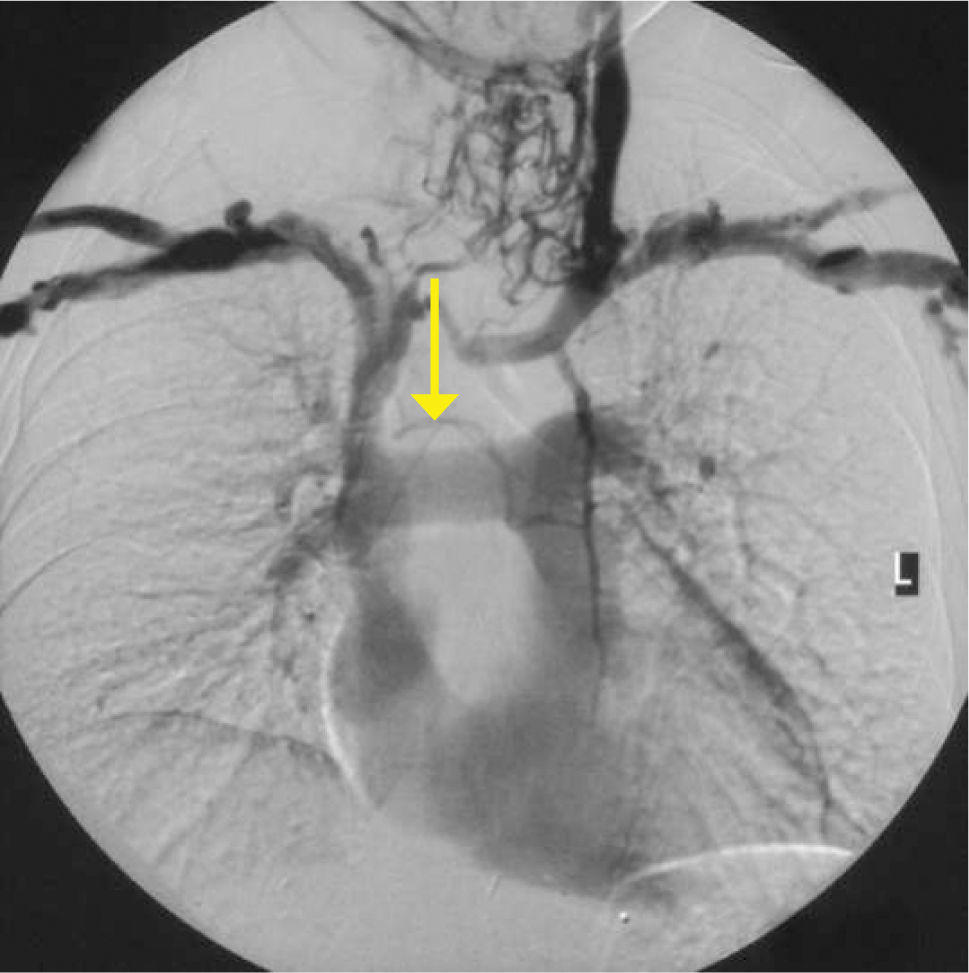
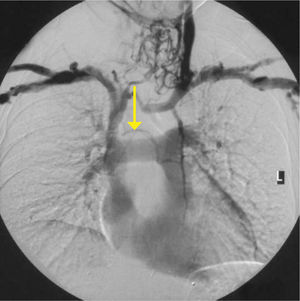
Obvious venous collaterals on the chest wall, difficult to compress veins on ultrasound, or higth venous pressure on cannulation suggest this problem. Confirmation is by venography, CT, or Doppler ultrasound studies (Figure 7). Mediastinal shift from effusions, lung collapse, or pneumonectomy will shift mediastinal structures including the SVC. In the event of IV blockage, the azygous system enlarges to provide drainage.
Catheter tip positionPoor tip position increases risks of: thrombosis, arrhythmias, perforation of vein wall (causing hydrothorax, cardiac tamponade, extravasation), catheter failure, pain on injections, and stenosis. A adequate length of catheter, in long axis of the SVC, with its tip above the pericardial reflection is traditionally considered ideal, and this approximates to the level of the carina. This is often not possible, particularly with left-sided catheters 18. A frequent problem is a short catheter with its tip abutting vein wall with an acute angle (Figure 5).
Most practitioners now aim to have catheter tips at the cavo-atrial junction (two vertebral body units below carina) 19.
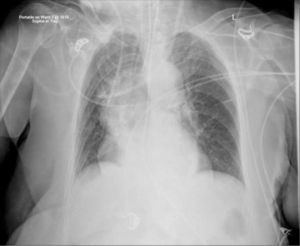
ImagingShort-term CVCs are usually inserted without real-time imaging, with a CXR to confirm positioning. Catheter tips move with changes from lying to sitting/standing, or on deep breathing 20. ECG guidance or electromagnetic sensors are increasingly used, but do not confirm either arterial, venous, and mediastinal placement, or a coiled tip.
Misplaced cathetersCatheters may be misplaced within the venous system following an abnormal path to the neck, the arm or across the midline and need repositioning unless for very short term use. Alternatively patients may have a normal variant of anatomy or acquired stenosis of the great veins leading to misplaced catheters. Catheters are typically easily recognized in such positions and specialist advice is not generally required before revision, use or removal.
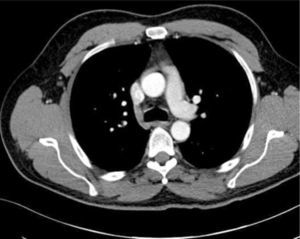
Catheters may be in an obviously incorrect position outside the vein or appear to follow an approximate normal path on CXR, but are not correctly sited in the SVC. Axial CT images show how catheters in the SVC, right pleural space, right internal mammary vessels, azygous system, ascending aorta, or mediastinum cannot be distinguished on one plane imaging (Figure 8). CXR can only confirm central catheter passage, kinking or procedural complications.
Catheters in an unusual position or malfunctioning need further investigation before use or removal due to the risks of thrombosis (CVA) if intra-arterial, pneumothorax, haemothorax or cardiac tamponade. Bedside tests include; pressures in all lumens (fluid column or transducer), and aspiration of blood from the lumens for estimation of haemoglobin (e.g. systemic blood vs pleural collection), and oxygen partial pressure. None of these bedside tests are entirely reliable. If in doubt, definitive localisation requires contrast studies (linogram) or cross-sectional CT imaging 21. “If in doubt, don’t pull it out! seek specialist advice.
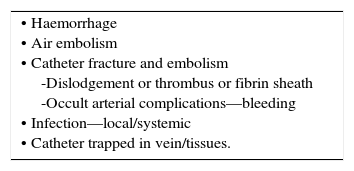
ComplicationsAny anatomical structure adjacent or connected to vessels may be damaged during insertion procedures, or later from thrombosis, perforation, or infection (Table 3) 22.
COMPLICATIONS OF CVC INSERTION.
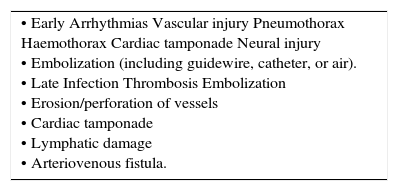
| • Early Arrhythmias Vascular injury Pneumothorax Haemothorax Cardiac tamponade Neural injury • Embolization (including guidewire, catheter, or air). • Late Infection Thrombosis Embolization • Erosion/perforation of vessels • Cardiac tamponade • Lymphatic damage • Arteriovenous fistula. |
Some procedural complications are particularly life-threatening and a frequent cause for high- value legal claims 23,24. These typically relate to local pressure effects from an arterial haematoma, massive bleeding into the chest/abdomen, and strokes from carotid cannulation.
COLLATERAL DAMAGE FROM NEEDLE PLACEMENTTypical examples are damage to arteries, lung, pleura, and nerves. Even with correct needle direction, veins often are transfixed leaving structures behind vulnerable, e.g. neck arteries.
Arteries also overlie veins e.g. superficial femoral artery and thoracoacromial trunk branches anterior to the axillary vein 25. This is generally avoidable by routine use of ultrasound.
Central insertion of devicesGuidewires may go astray from all access sites, including the IJV. Without imaging, there is often no certainty that they have not passed across the midline, into branches, down an arm, or out of the vein. Excessive force can easily push wires through the vein wall into the pleura, mediastinum, or other structures. Dilators and catheters passed over a guidewire will then enlarge a tract to their diameter or larger if the vein tears. If a guidewire is kinked or acutely angulated, and further force is applied to the dilator/catheter, it may tear the vein. The guidewire should be repeatedly checked to ensure it moves freely through the dilator/catheter, to ensure no distortion or false passage. If resistance is felt, the procedure must be stopped, or further imaging obtained to guide alternative approaches. Image intensifiers provide the optimal tool, but are rarely used during short term CVC insertions.
Arterial damageInsertion of needles, guidewires, dilators, and catheters may damage arteries at the puncture site or more centrally. A haematoma or false aneurysm may cause skin/tissue loss, nerve damage and airway compression. Arterial dissection, thrombosis, embolus, and unintentional cannulation may cause ischaemia, with particular relevance to the carotid artery. If there is needle puncture only, then removal and pressure for 5–10min will usually suffice.
It is recommended not to remove catheters larger than 9 Fr without percutaneous or surgical closure. In the short term, it is generally safe to leave dilators, catheters, and guidewires in situ, particularly in a heparinized patient, while the situation is evaluated with radiologists and surgeons. If in doubt don’t take it out!
Accidental arterial catheter placement may be missed clinically, as malfunction, back-bleeding, infusion pump alarms, and signs of thrombosis (e.g. stroke) are attributed elsewhere. Bleeding is not necessarily seen until catheter removal.
Removal of catheters from the carotid risks of thrombus and emboli to the brain, and bleeding. Systemic heparinization (if not bleeding) and removal of the device, either surgically or radiological stenting/closure, are preferred options 26. Removing devices and pressing for 20 mins to prevent bleeding, risks brain ischaemia from haematoma, emboli, and a lack of blood flow.
Pleural collectionsPneumothorax usually follows needle damage during subclavian puncture, but can occur from guidewire or catheter damage. It should be avoidable with ultrasound. Pleural catheter placement allows infusions to cause a pleural effusion, and if the catheter traverses the vein a haemothorax may develop on catheter withdrawal.
Haemothorax/peritoneumMinor tears of central veins are probably more frequent than realized as low venous pressure allows connective tissue, muscle, or other structures to halt bleeding. Massive haemorrhage develops when a tear connects to low-pressure pleural space 27. Veins adjacent to pleura include the SVC (right border), azygous system, hemi-azygous system (on left), and internal mammaries (Figura 4).
Arterial damage causes similar issues, and a needle hole can cause major haemorrhage. Subclavian arteries protrude into the pleural space. A more distant enlarging arterial bleed may extend into the pleural space. Similar mechanisms apply in the peritoneal space. Management relies on drainage, leaving dilators/catheters in place to reduce bleeding, and urgent repair by surgery or radiology.
Lymphatic leaks are rarely seen with current techniques. There may be an external leak of lymph, a lymphocoele (localized collection), or a chylothorax as a result from direct damage to major thoracic lymph vessels where they join the IJV or SCV, or vein thrombosis causing back pressure. Problems are more frequent with the larger left thoracic duct 28.
Nerve damageNerve trunks are present at all central access sites, which are at risk during insertion procedures or stretching from a haematoma. Reported sites include: phrenic nerve, sympathetic chain, femoral nerve, brachial plexus, and median nerve. At some sites, nerves (e.g. Median nerve and PICCs) can be visualized and avoided with ultrasound.
Cardiac tamponadeTwo mechanisms are reported:
- 1.
Needle puncture to proximal branches of aortic arch during subclavian access cause bleeding into the pericardial (extends up to aortic arch) 29.
- 2.
Perforation by catheters via the lower SVC or RA, allowing pressurized infusion into pericardium 30. Series suggest this is more common than bleeding.
Tamponade is often a post-mortem finding. Suspicion is necessary to prompt, and confirm diagnosis by echocardiogram. Aspiration of fluid through the catheter may be successful. Urgent pericardiocentesis, stenting, or surgery repair are needed.
Removal of cathetersMost catheters can be pulled out easily. The negative pressure in central veins can entrain air via an open/damaged catheter or insertion tract. This is a higher risk with large-bore devices, and established short tracts (e.g. jugular dialysis catheter), and is a rare cause of collapse and death.
Position the patient head-down, with applied pressure, and an occlusive dressing to entry site.
There are other potential complications (Table 4.).
Occasional long-term devices cannot be removed due to tight constriction of a fibrin sleeve, or organized clot anchoring catheter centrally. Do not persist to catheter breakage or vessel damage, seek specialist advice. Some are cut off and left in situ.
REMOVING A CUFFED TUNNELED CATHETERS AND IMPLANTED PORTSCuffed catheters can be pulled out if less than 3-4 weeks old, before adhesions form, or if infection has broken down adhesions. Anchored catheters need a cut-down to release the cuff 31.
The cuff can be found with palpation or ultrasound. Perform a cut down (1cm length) on venous side of cuff under LA, and with blunt dissection free and remove the venous section first to avoid catheter embolism. A thin fibrin sheath will need to be incised. Then sharp dissect around the cuff to free adhesions and remove it. Similar principles apply to ports encased in a fibrous capsule with sutures.
Short versus long-term venous accessPeripheral vein cannulae last only a few days. Long term devices last months/years, and are increasingly used in and out of hospital for predicted use longer than 6 weeks. Basic knowledge of function (Table 5) is valuable as devices are used for routine and emergency use. Some devices (e.g. Groshong) have a valve in tip or proximal hub.
Ports are accessed with non-coring Huber tip needles, although acutely any small bore needle can be used. Needles need a firm push through the thick rubber membrane to then hit the back wall.
Many long term devices are inserted by anaesthetists and other non-surgical specialists as standalone procedures. Points to consider re devices include: Indications and duration of treatment, location for therapy (hospital/community), clinical status (e.g. coagulation, sepsis), self- administration of infusions. Early use of devices is used to save peripheral veins, prevent pain and discomfort, and complications from repeated attempts at peripheral cannulation 32.
Site of accessSome studies suggest right-sided catheters have lower risks of thrombosis, because of straighter route to SVC, with easier tip positioning. Site choice takes in patient factors, previous vein access, and clinician experience, evidence of vein thrombosis, previous scars, or venous collaterals. Central vein imaging (venogram, CT, MRI) is helpful in difficult cases.
Tips for insertion of long-term devicesCentral passage of guidewire/catheter is aided by requesting awake patients to inspire during insertion. Measurements catheter length can be made from a guidewire or uncut catheter (with fluoroscopy), or measure predicted length externally on chest wall.
Stiff sheaths/dilators risk vein damage, and are typically longer than required and don’t need to be inserted fully. Open sheaths will bleed back and risk air embolism so need obstructing by pinching, use of a valve and rapid insertion of the catheter tip. Sheaths easily kink and may need to be pulled back to allow catheter passage.
Long, thin, coated guidewires (70+cm) can be passed via a sheath or catheter to aid central placement. Venography can be used via needles, sheaths, or catheters if difficulties ensue. For fixed-length catheters (e.g. dialysis type) plan the length of tunnel tract and exit site to provide right length to insert into vein.
Ports can be inserted under LA +/- sedation, or GA. The incision and pocket size can be reduced by placing anchor stiches in the pocket first, then sliding the port in. Subcuticular sutures provide a good scar.
AftercareExternal anchoring devices should retained for 3-4 weeks to allow tissue ingrowth into cuff (slowed with chemotherapy or general debility). PICCs have no internal anchor and need an external suture, adhesive device, or hooked anchor device.
If the patient is considered at high risk of thromboembolism, therapeutic dose anticoagulation may be indicated. Some units lock dialysis type cannullae with heparin 1000 units/ml, this needs aspirating before use. Thrombosed/blocked catheters or fibrin sleeves may be unblocked, or prevented with low doses of thrombolytic agents 33.
DEVICESMedium termMid-lines (10–20cm) are inserted in the upper arm, with the tip in the upper third of the basilic/cephalic vein or axillary vein, short of the central great veins, and are suitable for up to 3 weeks of non-irritant solutions.
Long termPICCs are advanced centrally from the antecubital fossa/upper arm vein. Cuffed catheters are tunnelled from the insertion site to chest/abdominal wall. A woven cuff allows tissue ingrowth for anchorage. These may be soft narrow bore or larger dialysis type devices. Subcutaneous totally implanted ports are inserted surgically on the chest, abdomen, or upper arm. Devices are made with single and multiple lumens, some are CT compatible, rated as suitable for high pressure (325 psi) injection of X-ray contrast.
Arterial accessRelevant indications include the following:
- •
Cardiovascular monitoring
- •
Repeated arterial sampling
- •
Pulse contour analysis
- •
Aortic balloon pumps
- •
Extracorporeal circuits
Common access sites include the radial, ulnar, brachial, dorsalis pedis, and femoral arteries.
The presence of an arteriovenous fistulae requires consideration.
Applied anatomy Peripheral arterial access is usually performed via the radial artery in the non-dominant forearm. A patent ulnar artery provides alternative flow to the forearm and hand so that if the artery is thrombosed, tissue loss does not usually occur 34. The brachial artery can be used, but as it is an end artery, distal ischaemia is a risk with occlusion.
Detailed anatomy and variants may be under-recognized 35.
Superficial radial and ulnar arteries may be cannullated during attempted venous access 36. Variation in the upper arm and forearm may not be obvious on palpation at the elbow (e.g. high bifurcation of the brachial artery)).
Patients with blocked brachial, radial, or ulnar arteries rely on collateral supply. This should beidentifiable clinically, and with ultrasound. Careful assessment of perfusion is needed. Allen's test; compression of the radial/ulnar artery and assessing hand blood flow is useful conceptually, but is not proven clinically 37.
The femoral artery is widely used for diagnostic and interventional procedures. In the context of more prolonged catheterization, it carries increased risks of infection and thrombosis. Higher damage can lead to hidden bleeding into the abdomen. There is increasing evidence for use of ultrasound to cannulate the common femoral artery 38.
Practical insertion tipsMultiple needle passes at poor distal vessels may represent a higher risk than cannulating a larger end artery more proximally. The femoral and brachial arteries are useful in shocked patients. In deep arteries (femoral and brachial) a short catheters can be dislodged with movement. Seldinger techniques have a higher success than catheter over needle devices in routine and challenging cases.
Vessels may be calcified, making cannulation difficult and it may be impossible to close the vessels with pressure after removal. Other vessels may have aneurysmal changes or dissection. If difficulties ensue, consider a surgical cut down to lessen the risk of vessel injury. Large sheaths in situ need systemic heparinization to avoid clot formation.
RemovalAfter catheter removal, press firmly on the site for at least 5min. Persistent bleeding may require a fine suture (e.g. 5/0 nylon), to close the skin wound and stabilize clot. Radiological occlusion devices are greatly improved and required for removal of devices larger than 9Fr, in the presence of severe coagulopathy, or in areas where pressure cannot be applied.
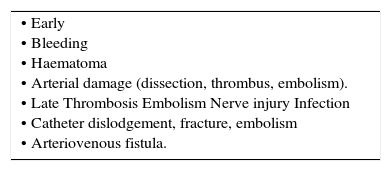
ComplicationsComplications can be divided into early and late, but some will be delayed in presentation (Table 6). Vascular compromise may occur at any stage. Accidental arterial injection of drugs is an important avoidable complication. Risks of infection increase with time and arterial catheters can cause catheter related blood stream infections. If concerns arise as to arterial patency and distal circulation, then urgent referral to vascular surgery is indicated.
ULTRASOUND GUIDANCEUltrasound imaging is not yet used routinely to cannulate arteries, although studies are increasingly suggesting benefits, not just in difficult cases 39. Such guidance is useful with low blood pressure, atheromatous vessels, stenosis, dissections, thrombosis, oedema, obesity and variations in anatomy. It is likely that the frequency of procedural and infectious complications is related to the number of needle passes. Other deeper sites, for example, the radial and ulnar arteries in the mid forearm can also be used.
General Principles of ultrasound guidanceThere is a strong evidence for use of ultrasound in all ages and most sites of access 40, particularly for the IJV in terms of first pass success and complications 10, with the following advantages
- •
Direct imaging of vessels and adjacent structures
- •
Imaging of thrombosis, valves, dissection, atheroma, or anatomical variants
- •
Identification of optimal target vessel
- •
First-pass access avoiding adjacent structures
- •
Confirmation of guidewire and catheter in vessel
- •
Reduces procedural complications
Veins show respiratory variation (with a free connection to right atrium), and are easily compressible. Arteries are round and non-compressible, and become clearer to image with pressure. Peripheral arteries have characteristic double vena comitantes. If in doubt, use colour Doppler to differentiate pulsatile from a more continuous venous signal. Limb veins will showenhanced signal if the distal limb is squeezed or the patient contracts muscles.
The display should anatomically be in the orientation as seen from the position of the operator.
Correct orientation ensures that the image moves in a logical direction when the probe is moved and that the needle moves in the same direction in the patient as on the display.
Precise needle tip imaging is vital. The needle and ultrasound probe may be arranged in the ‘short (out-of-plane)’ or ‘long (in-plane)’ axis and the image axial or longitudinal. An axial vessel view and short-axis needle insertion gives good visualization of surrounding structures but it takes experience to achieve good needle tip imaging as the shaft can be mistaken for the tip.
Better needle images are seen if the needle inserted in the long axis but if the vein is imaged longitudinally concurrent images of surrounding structures are not seen. Some needles have their distal section machined to increase echogenicity 41. Training and accreditation issues related to ultrasound are important 42.
CONCLUSIONVascular access is a core skill needing anatomical knowledge and practical skills. Recognition and management of complications is essential. The increasing use of ultrasound, ECG guidance, and X-ray screening, and improved design of devices allows safe and successful procedures. Many patients now benefit from early use of long-term access devices.
The author declares no conflicts of interest, in relation to this article.