Intrathecal chemoprophylaxis prevents central nervous system relapses in patients with acute lymphoblastic leukaemia (ALL) but few studies have addressed the predictive markers of relapse. Among these, traumatic lumbar puncture (TLP) has been associated with lower disease-free survival (DFS).
To investigate the risk posed by TLP on relapse and DFS, we assembled a retrospective cohort including 79 patients with ALL who received intrathecal chemoprophylaxis during 2009 to 2013. One TLP per patient was recorded in 49 cases, and more than one TLP in 3. Mean follow-up was 283 (22–1118) days with an overall DFS of 68%. DFS was significantly lower in the group that had experienced TLP (58% vs 100% [P=.070]). Multiple TLP posed a greater risk of relapse than single TLP (P=.001). In conclusion, TLP in adults constitutes a major risk factor, greater than that reported in the large paediatric series.
La quimioprofilaxis intratecal evita la recaída a sistema nervioso central en pacientes con leucemia linfoblástica aguda (LAL), aunque poco se ha estudiado sobre marcadores predictivos de recaída. Entre estos, la punción lumbar traumática (TLP) se ha asociado con una menor supervivencia libre de la enfermedad (EFS).
Para investigar el riesgo que infiere la TLP sobre la recaída y la EFS, se formó una cohorte retrospectiva compuesta por 79 pacientes con LAL que recibieron quimioprofilaxis intratecal durante 2009 a 2013. Se registró una TLP por paciente en 49 casos y más de una en 3. La media de seguimiento fueron 283 (22–1118) días, con EFS global del 68%, significativamente menor en quienes recibieron alguna TLP (58% vs 100% [p=0.070]). Múltiples TLP aportaron mayor riesgo que TLP única (p=0.001). En conclusión, las TLP en adultos son un factor de riesgo preponderante, con mayor relevancia de lo reportado en las grandes series pediátricas.
At present acute lymphoblastic leukaemia with B or T precursors is treated using block sequential chemotherapy regimens in conjunction with immunotherapy and tyrosine kinase inhibitors, and unlike other leukaemias, it requires prophylaxis therapy due to the high risk of central nervous system relapse.1–4 In its infancy, prophylaxis consisted of radiation therapy to the head followed by high doses of methotrexate plus intrathecal chemotherapy. Most of our knowledge on prophylaxis and relapse has been gained from paediatric protocols. Conter and collaborators in the AIEOP group substituted chemotherapy for a regime of high doses of intrathecal methotrexate (5g/m2 for four courses) and achieved a relapse-free survival of above 90%, recording a recurrence rate of only 5%.5 This strategy has been validated by other authors around the world with similar relapse rates,6,7 and therefore intrathecal chemotherapy became the standard prophylaxis therapy for the central nervous system (CNS), the triple combination strategy being the most popular.8 There are few studies in adults in this regard, and most of them agree that adequate prophylaxis prevents CNS relapse.9 There are also few predictive markers of relapse. Sancho and collaborators only managed to identify lactate-dehydrogenase as a predictive marker of relapse.10 Traumatic lumbar puncture as a CNS relapse risk is another factor of interest. In general, most series agree that the presence of traumatic cerebrospinal fluid with blasts shortens relapse-free survival and that CNS1 and CNS2 liquids behave similarly.11,12 In our hospital, triple-drug therapy (cytarabine, methotrexate and hydrocortisone) has been effective in controlling isolated CNS relapses.13 This is because our institutional protocol uses intensive doses of intrathecal chemotherapy, and the objective proposed was to establish whether there is an association between traumatic lumbar puncture and the risk of CNS relapse.
Patients and methodsProtocol designAn observational study based on a retrospective cohort of patients with de novo acute lymphoblastic leukaemia treated in the haematology department of the General Hospital of Mexico between December 2009 and July 2013. These patients had received first-line treatment and prophylaxis directed at the central nervous system using intrathecal chemotherapy. Patients who were to receive radiotherapy or who did not have basal cerebrospinal fluid for analysis were excluded from the study.
TreatmentInstitutional protocol HGMLAL07 has been available in our hospital from 2007 to the present date as first-line treatment for patients with adult acute lymphoblastic leukaemia. This protocol includes a pre-induction phase with steroids similar to protocol GIMEMA ALL0087 and intensification phases during the maintenance phase. Central nervous system-directed prophylaxis was given in four doses of intrathecal chemotherapy during the induction of remission phase, followed by monthly doses during the six consolidation blocks and then bimonthly doses over the 2 years of maintenance. The chemotherapy regimen included 15mg methotrexate, 40mg cytarabine and 8mg dexamethasone. The patients who were treated with the Hyper-CVAD regimen received chemotherapy on days 2 and 8 of each course with 15mg methotrexate and 100mg cytarabine respectively.
The cerebrospinal fluid was examined by cytospin (ROTOFIX 32 A, ZENTRIFUGEN brand at 6000rpm), it was then stained with Wright–Giemsa and examined at increases of 10× and 100×.
The status for considering central nervous system infiltration was based on the following criteria: CNS1, non-traumatic lumbar puncture (<10 erythrocytes/μL); CNS2, non-traumatic lumbar puncture with a white blood cell count of less than 5μL and blasts in the cerebrospinal fluid; CNS3, non-traumatic lumbar puncture with >5 white blood cells and blasts in the CSF. For the statistical analysis, it was considered traumatic fluid (TLP) if it had >10 erythrocytes/μL and lumbar puncture with infiltration if blasts were present in the cerebrospinal fluid.
Statistical analysisCentral nervous system relapse-free survival was considered from the date of diagnosis until the date of relapse, death or the last date recorded on the medical record. The Kaplan–Meier method was used for distribution and the two groups were subsequently compared using the Log-Rank test. The factors that might influence central nervous system relapse were analysed using Cox regression analysis. The hypothesis contrast test was performed using the chi square test, a value of ≤0.05 being considered significant, analysed at a 95% confidence interval. The entire analysis was performed using IBM SPSS Statistics for Windows version 20.0 (Armonk, New York).
Ethical considerationsThis study has been approved by the ethics and research committees of the General Hospital of Mexico.
ResultsPatients included in both the institutional protocol HGMLAL07 and the Hyper-CVAD regimen were studied from December 2009 to July 2013. Twelve patients were excluded from the study because they had a sample from follow-up but no sample of cerebrospinal fluid from diagnosis. 79 patients were studied for the final analysis. The mean follow-up of the patients was 315 days (range of 22–1118 days). After analysis of cerebrospinal fluid at the time of diagnosis, central nervous system infiltration was recorded in only one case (1.3%). On diagnosis, the mean white blood cell count was 40.86×103/mcl, when the cases were divided only 15.2% (n=12) of the patients had a white blood cell count of more than 100×103/mcl at the time of diagnosis, and in general the majority of the patients had counts of less than 30×103/mcl (64.6%). The mean age was 31 (range from 16 to 65) the majority being patients under 35 (67.1%). All the patients were classified according to the type of risk of early relapse (age <35, white blood count >30×103/mcl, central nervous system infiltration on diagnosis, expression of Philadelphia chromosome, resistance after 4 weeks of treatment). Of the 79 patients, 46.8% (n=37) were considered usual risk and 53.2% (n=42) high risk. Immunophenotyping on diagnosis showed that 89.9% (n=71) of cases corresponded to a precursor B and 10.1% (n=8) to a precursor T lymphoblastic leukaemia. Contrary to expectations, the mean white blood cell count in T leukaemias was lower compared to the B leukaemias (14.11 vs 43.85×103/mcl) but this difference is not significant (P=.235, 95% Confidence Interval).
Association between traumatic lumbar puncture and central nervous system relapse. The mean of the fluids collected as part of the central nervous system prophylaxis protocol was 7 (range 1–32 punctures), and at least one traumatic puncture was recorded in 64.6% of cases (n=51). When the cases were divided, 62% (n=49) had experienced one single traumatic lumbar puncture and 3.8% (n=3) more than one traumatic lumbar puncture during the follow-up protocol. A total of 8 cases of central nervous system infiltration were recorded during follow-up (10.1%). The χ2 test showed a statistically significant result between the presence of a traumatic puncture and central nervous system relapse (P=.024, 95% CI).
Relapse-free survival (RFS) between the groups. The mean relapse-free survival (RFS) for this cohort was 68% (mean follow-up 283 days, range 22–1118 days). When the central nervous system relapse-free survival rates between the two groups (traumatic and non-traumatic) were established, those who had experienced a traumatic puncture had lower RFS compared to those who had experienced a non-traumatic lumbar puncture (100% vs 58%). The difference was established using the Log-Rank test, the P value being P=.070 (Fig. 1).
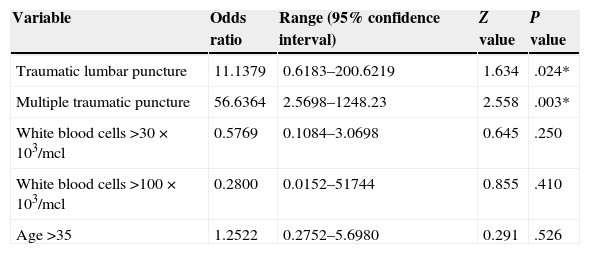
Association between different factors and central nervous system relapse. A model was created to determine whether traumatic lumbar punctures represented a risk of central nervous system relapse, considering the risk of the presence of a single traumatic lumbar puncture or multiple traumatic lumbar punctures (two or more punctures) throughout the entire follow-up. Other variables were also considered such as the white blood cell count on diagnosis (30 vs 100×103/mcl), age (limit of 35) along with the risk of bone marrow relapse after a traumatic lumbar puncture. The main risk was established in patients with traumatic lumbar punctures but it was considerably greater in patients who had experienced multiple traumatic punctures. The odds ratio results are shown in Table 1. The odds ratio value of traumatic lumbar puncture on bone marrow relapse is 2.2963; this is not statistically significant (P=035, 95% CI [range 0.40–13.16]).
Multivariable analysis of the risk factors for central nervous system relapse.
| Variable | Odds ratio | Range (95% confidence interval) | Z value | P value |
|---|---|---|---|---|
| Traumatic lumbar puncture | 11.1379 | 0.6183–200.6219 | 1.634 | .024* |
| Multiple traumatic puncture | 56.6364 | 2.5698–1248.23 | 2.558 | .003* |
| White blood cells >30×103/mcl | 0.5769 | 0.1084–3.0698 | 0.645 | .250 |
| White blood cells >100×103/mcl | 0.2800 | 0.0152–51744 | 0.855 | .410 |
| Age >35 | 1.2522 | 0.2752–5.6980 | 0.291 | .526 |
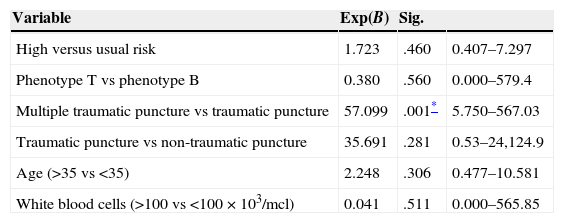
A Cox regression analysis was used to assess the influence of the different variables on relapse-free survival considering all the risk variables and the two types of traumatic CSF (single or multiple traumatic CSF). Both traumatic lumbar puncture and multiple traumatic punctures were the two variables that presented a greater risk of central nervous system relapse. Table 2 shows the summary of the model.
Cox regression analysis of the risk factors, taking CNS relapse as the final event.
| Variable | Exp(B) | Sig. | |
|---|---|---|---|
| High versus usual risk | 1.723 | .460 | 0.407–7.297 |
| Phenotype T vs phenotype B | 0.380 | .560 | 0.000–579.4 |
| Multiple traumatic puncture vs traumatic puncture | 57.099 | .001* | 5.750–567.03 |
| Traumatic puncture vs non-traumatic puncture | 35.691 | .281 | 0.53–24,124.9 |
| Age (>35 vs <35) | 2.248 | .306 | 0.477–10.581 |
| White blood cells (>100 vs <100×103/mcl) | 0.041 | .511 | 0.000–565.85 |
Leukaemic infiltration of the CNS is one of the main complications associated with the treatment of acute lymphoblastic leukaemia. Although flow cytometry can identify monoclonal B-cells in up to 0.01% of the entire population, it is not yet a validated test, and morphological analysis of cerebrospinal fluid is used instead.14,15 It has been difficult to identify markers to predict central nervous system relapse, in general only DHL >1000U/L or a high tumour load on diagnosis have been identified as predictive markers.10,5 Some authors suggest that markers that express normally in CSF may also be useful. One of these is osteoprotegerin, a cytosine receptor that is a member of the superfamily of tumour necrosis factor receptors,16 and it has also been isolated in leukaemia and myeloma cell lines.17 Other cell markers are surface markers. Cannizzo and collaborators identified the expression of CD56+ in a case of isolated CNS relapse,18 like Matsubara and collaborators who also found the expression of this marker, but in bone marrow blasts at the time of diagnosis.19,20 Because it is difficult to identify any useful marker, there is major interest now in the risk associated with traumatic lumbar puncture and central nervous system relapse. Howard and collaborators analysed around 5609 lumbar puncture procedures in St Jude Children's Research Hospital, and identified both modifiable and non-modifiable risk factors for the presence of a traumatic lumbar puncture, the latter included being of black ethnicity and aged under 1 year, and the main modifiable factors are a history of lumbar puncture 2 weeks earlier performed by a trained doctor and with adequate transfusion support, primarily with a count lower than 50×103/mcl.21 The hypothesis that leukaemic cells might implant in the meninges has already been corroborated in in vivo models. Barone and collaborators injected leukaemic T cells intrathecally in their experimental model in rats. The histopathological study of the rats, sacrificed between days 1 and 11 after injection of the leukaemic cells, showed a highly dispersed pattern of leukaemic cells throughout the entire subarachnoid space, brain and cerebellum. This was greater in the rats sacrificed on day 11 post-injection. Between 28% and 100% of the animals also showed molecular expression of the leukaemic cells.22 Perhaps the most significant finding was the capacity of the blasts to invade the subarachnoid space as well as the Virchow–Robin space. This is of major significance since blasts that are introduced and scattered via lumbar puncture have a capacity to proliferate and invade which is very similar to bone marrow. Therefore various series establish that traumatic lumbar punctures with blasts are associated with lower relapse-free survival. Similarly to our study, Shaikh and collaborators analysed cases with traumatic lumbar punctures with blasts, and identified a decreased relapse-free survival compared to those with sterile liquids (77% vs 93%), but unlike our study, a history of a traumatic puncture was found not to pose as much risk (1.43 vs 56.6).23 This is why it is increasingly being recommended that lumbar puncture should not be take place at the time of diagnosis but should be performed when circulating blasts are no longer found (e.g. day 8 or day 9).24–26
In conclusion, knowledge on acute lymphoblastic leukaemia has been gained from paediatric protocols, division of risk, early intensification,27 and now traumatic lumbar punctures constitute strategies to enable improved adult protocols.
FundingNone.
Conflict of interestThe authors have no conflict of interest to declare.