The minor BCR-ABL (e1;a2) transcript oncogene is the most common genetic alteration in adults with acute lymphoblastic leukaemia (ALL). It is associated with a poor prognosis.
AimTo determine the frequency of minor BCR-ABL (e1;a2) transcript oncogene expression in ALL patients in Mexico.
Material and methodsA cohort of 411 patients with de novo ALL were tested for the oncogene using reverse transcription polymerase chain reaction (RT-PCR).
ResultsThe oncogene was found in 14% (n=57) of the study population. Mean age was 29 years, and 53% were male. Median leucocyte count was 53×103μl.
ConclusionPrevalence of BCR-ABL expression by RT-PCR has not previously been reported in Mexico. Our laboratory found a higher prevalence than that reported in Latin-American series, but lower than that reported for the European population.
El oncogén BCR-ABL (e1;a2) rompimiento menor constituye la alteración de mayor frecuencia en la leucemia aguda linfóblastica (LAL) del adulto. Su presencia se asocia con pronóstico adverso.
ObjetivoDeterminar la frecuencia de la expresión del oncogén BCR-ABL (e1;a2) en portadores de LAL en México.
Material y métodosSe estudiaron 411 pacientes con diagnóstico de LAL de novo para la búsqueda del oncogén mediante Reacción de cadena de polimerasa por Punto final (RT-PCR).
ResultadosEl 14% (n=57) de la población estudiada presentó expresión positiva. La edad promedio fue 29 años, el 53% correspondió al sexo masculino, la mediana de leucocitos fue 53×103μl.
ConclusiónEn México no hay reportes de la frecuencia de expresión de BCR-ABL por RT-PCR, nuestro laboratorio encontró una frecuencia mayor que lo reportado en las series Latino-Americanas y menor a lo reportado para población europea.
Acute lymphoblastic leukaemia (ALL) is one of the most common types of cancer found in Mexico, with an average incidence of 5 cases per 100,000 inhabitants.1 On average, 70 new cases of ALL are admitted to the Haematology Department of the General Hospital of Mexico each year. Several cytogenetic abnormalities are involved in the development of this type of cancer. The t(9;22) (q34;q11) translocation, known as the Philadelphia chromosome or Ph gives rise to the BCR-ABL fusion transcript. This transcript, together with abnormalities such as t(4:11), is associated with an adverse prognosis.2 Incidence of this gene varies; reports suggest it to be 5% in the paediatric population,3–5 and 25–50% in adults.6–9 The minor BCR-ABL transcript codes for a chimeric protein (190kDa) with tyrosine kinase activity, which is implicated in both the activation of various cell signalling pathways (RAS-GTP) and cell apoptosis (PI3K).10–13 The BCR-ABL transcript has been associated with an adverse prognosis in most international studies.14 The introduction of therapies that act on specific molecular targets, such as BCR-ABL tyrosine kinase (TK) inhibitors (Glivec©, Novartis) has improved overall survival rates when compared to traditional chemotherapy. There are various methods for isolating the BCR-ABL transcript, the most common being conventional karyotyping, fluorescent in situ hybridization (FISH), and polymerase chain reaction.15–17 In Mexico, the Philadelphia chromosome is found in around 3.8% of the paediatric population18 and 16.7% of adults,19 isolated by reverse transcription polymerase chain reaction (RT-PCR) and conventional cytogenetics, respectively. In our laboratory, we amplify the BCR-ABL fusion transcript by means of RT-PCR, and perform around 60 tests on ALL patients each year. In this study, we describe the frequency of minor BCR-ABL expression in ALL patients compared with the international literature.
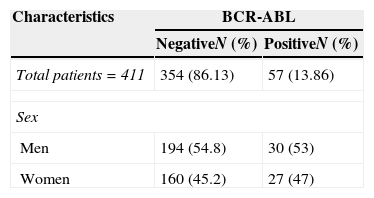
Materials and methodsAn experimental, prospective, longitudinal study conducted from February 2000 to January 2010 in the molecular biology laboratory of the Haematology Department. The study was approved by the institution's independent ethics committees. Male and female patients with de novo diagnosis of ALL that agreed to give peripheral blood samples after having signed the informed consent form were included in the study. ALL was diagnosed in accordance with the French–American–British (FAB) classification systems, with the help of immunophenotyping and cytochemistry assays. Clinical data were sourced from the patient's medical records (Table 1).
General clinical characteristics of patients with acute lymphoblastic leukaemia.
| Characteristics | BCR-ABL | |
|---|---|---|
| NegativeN (%) | PositiveN (%) | |
| Total patients=411 | 354 (86.13) | 57 (13.86) |
| Sex | ||
| Men | 194 (54.8) | 30 (53) |
| Women | 160 (45.2) | 27 (47) |
| Median (range) | Median (range) | |
|---|---|---|
| Median age (years) | 29 (16–62) | 25 (18–56) |
| Laboratory tests | ||
| Baseline leucocyte count (×103/μl) | 55.9 (0.7–789) | 54 (1.2–207) |
| Haemoglobin (g/dl) | 7.18 (4–10.9) | 7.05 (5.4–11.5) |
| Platelets (×103/μl) | 53 (0.88–388) | 45 (2–432) |
| N (%) | N (%) | |
|---|---|---|
| FAB classification | ||
| L1 | 7 (2) | 0 (0) |
| L2 | 347 (98) | 57 (100) |
| Immunophenotype | ||
| B-cell | 111 (81.6) | 12 (100) |
| T-cell | 25 (18.3) | 0 (0) |
| Nervous system infiltration | 7 (2) | 0 (0) |
Bone marrow samples were collected from ALL patients that had signed the informed consent form. Samples were collected in heparinized tubes containing Lymphoprep (Nycomed Pharma AS, Oslo, Norway) and centrifuged to obtain mononuclear cells.
Reverse transcription polymerase chain reaction (RT-PCR)Total-cell RNA was isolated with Trizol (Life Technologies, Paisley, UK), and 1μg of RNA was used for cDNA synthesis by means of MMLV (Life Technologies, Paisley, UK). The CMLB primers 5′ATCTCCACTGGCCACAAAATCATACA3′.
ALLA 5 AGATCTGGCCCAACGATGGCGAGGGC3 were used for PCR amplification. Results were validated by sequencing two positive samples (ABI PRISM 3100, Applied Biosystem, San Francisco, USA). Each cDNA was tested by PCR using primers specific for the constituent β2microglobulin gene. PCR cycles of 1min 94°C, 1min 55°C, 1min 72°C were repeated 35 times. The PCR products were stained with ethidium bromide and visualized in a 1.5% agarose gel.
ResultsPatient characteristicsA total of 411 patients with a mean age of 29 years (range 16–62) were studied. By morphology, most (n=98%) presented acute lymphoblastic leukaemia (ALL-L2), with 81.6% corresponding to the B-cell immunophenotype. Only 2% showed central nervous system infiltration at diagnosis.
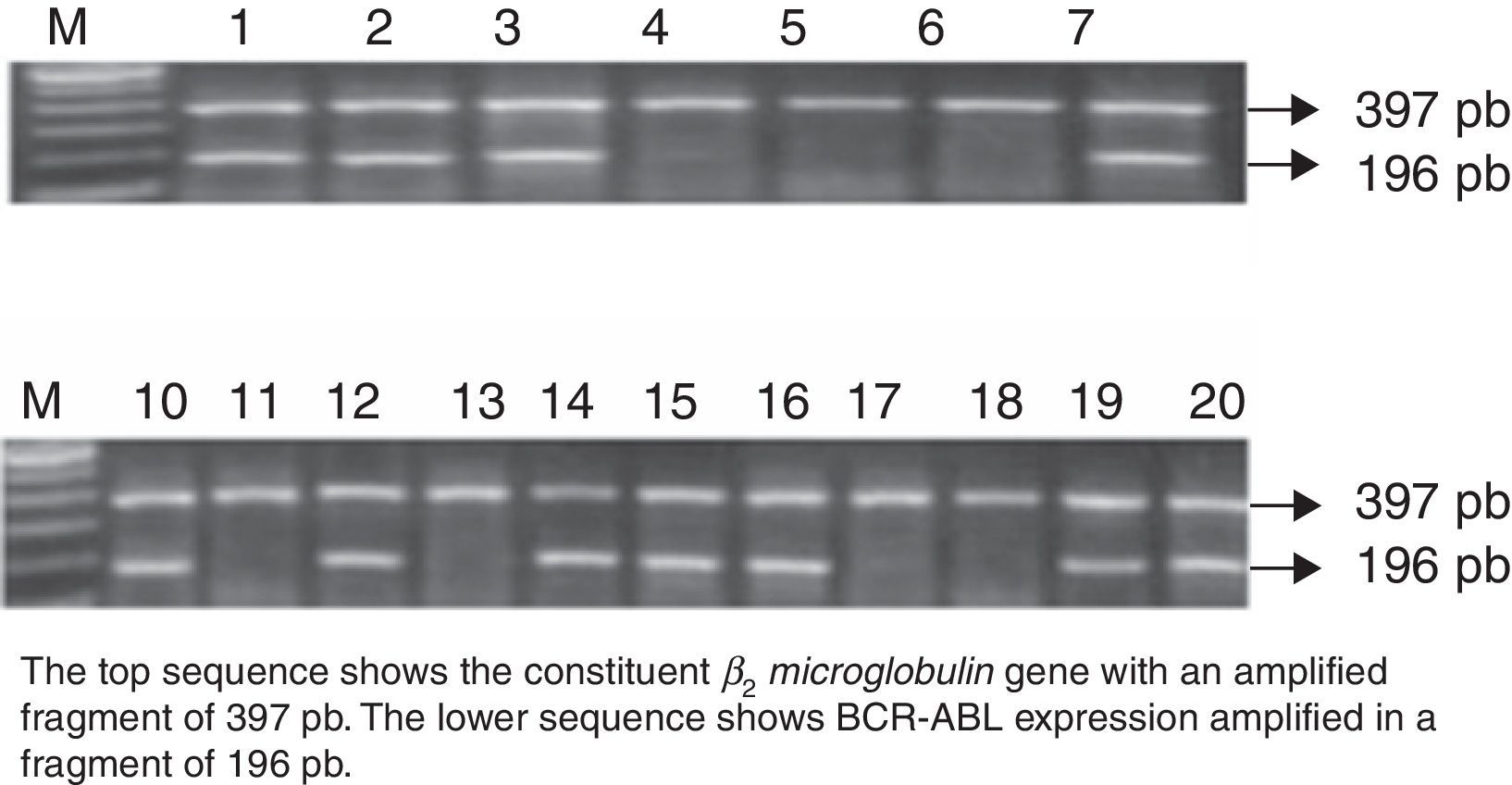
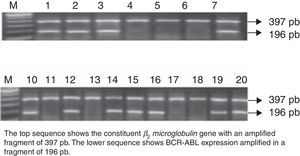
Expression of the minor BCR-ABL oncogeneAll 411 de novo ALL cases were studied for BCR-ABL oncogene expression. RNA quality was evaluated by amplification of the constituent β2microglobulin gene, which amplifies a fragment of 397bp by RT-PCR. BCR-ABL was isolated in 57 patients, amplifying a fragment of 196bp. This represents 13.8% of the study population.
Mean age of the 57 BCR-ABL-positive patients was 25 years (range 18–56); 53% (n=30) were men, and 47% (n=27) were women. Mean leucocyte count at diagnosis was 54×103/μl (range 1.2–207×103/μl). All (100%) patients were of the B-cell immunophenotype, and none showed central nervous system involvement (Fig. 1).
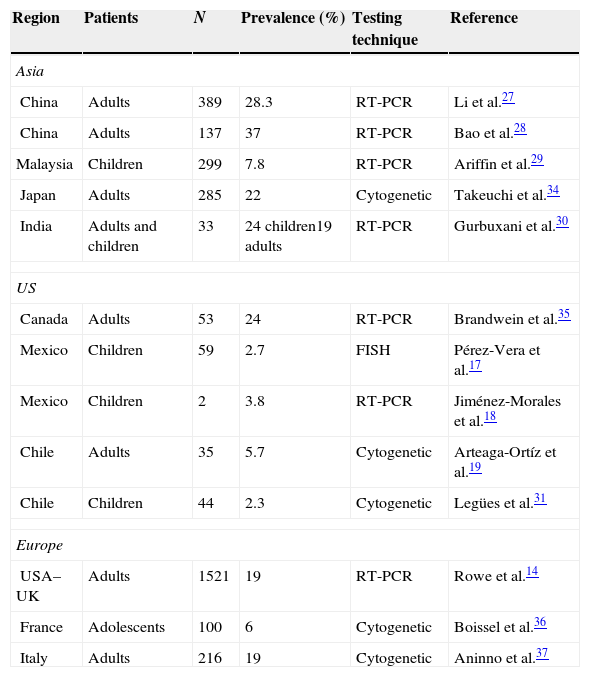
DiscussionIn this study, we evaluated the prevalence of the BCR-ABL transcript fusion (e1;a2) in a population of ALL patients in Mexico using reverse transcription polymerase chain reaction (RT-PCR). This technique has been used since the 1990s by various international groups in both the diagnosis and follow-up or ALL Ph+. The first studies in RT-PCR reported a prevalence of the minor BCR-ABL transcript of 50%, with no difference in either prognosis or clinical presentation.20,21 Researchers in the GIMEMA 0496 trial reported a prevalence of minor vs. major breakpoint of 58.5% and 41.5%, respectively.22 Prevalence continues to vary across Latin America, ranging from 5.7% in adults and between 2.3% and 2.7% in the paediatric population.22–24 Prevalence in ALL patients in the US is estimated at 19%,25 and from 25% to 39% in Asia (Table 2).26–29 Very few studies in BCR-ABL prevalence in children have been conducted in Mexico, and none in adults. In our laboratory, we found prevalence to be greater than that reported for Latin America, and lower than that reported for the American and European population. These discrepancies could be due to the genetic diversity of the Latin American population.30 Nowadays, it is particularly important to isolate the BCR-ABL transcript in ALL patients due to the potential benefits of tyrosine kinase (TK) inhibitors, such as Imatinib, nolotinib, or Dasatinib.31–34
Prevalence of the BCR-ABL Ph+ oncogene worldwide, by cytogenetic and RT-PCR testing.
| Region | Patients | N | Prevalence (%) | Testing technique | Reference |
|---|---|---|---|---|---|
| Asia | |||||
| China | Adults | 389 | 28.3 | RT-PCR | Li et al.27 |
| China | Adults | 137 | 37 | RT-PCR | Bao et al.28 |
| Malaysia | Children | 299 | 7.8 | RT-PCR | Ariffin et al.29 |
| Japan | Adults | 285 | 22 | Cytogenetic | Takeuchi et al.34 |
| India | Adults and children | 33 | 24 children19 adults | RT-PCR | Gurbuxani et al.30 |
| US | |||||
| Canada | Adults | 53 | 24 | RT-PCR | Brandwein et al.35 |
| Mexico | Children | 59 | 2.7 | FISH | Pérez-Vera et al.17 |
| Mexico | Children | 2 | 3.8 | RT-PCR | Jiménez-Morales et al.18 |
| Chile | Adults | 35 | 5.7 | Cytogenetic | Arteaga-Ortíz et al.19 |
| Chile | Children | 44 | 2.3 | Cytogenetic | Legües et al.31 |
| Europe | |||||
| USA–UK | Adults | 1521 | 19 | RT-PCR | Rowe et al.14 |
| France | Adolescents | 100 | 6 | Cytogenetic | Boissel et al.36 |
| Italy | Adults | 216 | 19 | Cytogenetic | Aninno et al.37 |
Research suggests that the combination of BCR-ABL and TK inhibitor therapy reverses the disease by providing a specific molecular target. In contrast to previous interpretations, this marker is now thought to indicate a good prognosis. In conclusion, ALL is one of the most common malignancies seen in the Haematology Department. Isolation of BCR-ABL in ALL patients is of primordial importance, particularly in view of the potential action of tyrosine kinase inhibitors.35–37 Advances in molecular biology, such as real time PCR, will allow clinicians to monitor BCR-ABL transcript levels more closely. An understanding of the prevalence of this fusion gene in the Mexican population will give greater insight into ALL, improve management and monitoring of the disease, and introduce more specific TK-based therapy.
FundingThis research was partially supported by the Institute of Science and Technology Mexico 162269; Novatis Oncology Mexico CST1571AMX10T; Research Office of the General Hospital of Mexico DIC/09/204/03/131, DIC 08/204/04/017.