Few studies compare the therapeutic efficacy of different iron deficiency anaemia treatments.
AimEvaluate the therapeutic response of the most common iron preparations.
Material and methodsRetrospective, observational-analytical study based on medical records from the Haematology Department, conducted from March to October 2014, including 121 adults with ferropenic anaemia and 3-month follow-up. Patients with comorbidities or pregnancy were excluded.
Results85.8% were women (n=103) and 14% men (n=17), with a mean age of 42 (16–83) years. Seventy patients (58.3%) started with oral administration; the rest received intravenous iron. Efficacy was similar among all the iron preparations, with no significant differences (p>0.05). Iron sucrose was most effective in rapidly replenishing body iron stores.
ConclusionsDespite comparable efficacy among treatments, ferrous fumarate had the lowest treatment failure and was the therapy of choice.
Escasos estudios comparan la eficacia terapéutica entre tratamientos para anemia ferropénica.
ObjetivoEvaluar el efecto terapéutico de las formulaciones de hierro más empleadas.
Materiales y métodoEstudio retrospectivo, observacional-analítico basado en los expedientes del Servicio de Hematología desde marzo a octubre de 2014, incluyendo 121 adultos con anemia ferropénica con seguimiento de 3 meses, excluyendo casos con comorbilidades y embarazadas.
ResultadosEl 85.8% fueron mujeres (n=103) y 14.2% hombres (n=17), con edad media de 42 (16-83) años. Setenta pacientes (58.3%) iniciaron tratamiento vía oral, el resto vía endovenoso. La eficacia fue mejor para fumarato ferroso (p=0.001) y hierro sacarosa (p=0.000). Hierro sacarosa restituye rápida y óptimamente las reservas corporales de hierro.
ConclusionesA pesar de la efectividad equiparable entre tratamientos, fumarato ferroso fue el predilecto con menor falla terapéutica.
Nearly half of all reported cases of anaemia worldwide are secondary to iron deficiency. Despite the existence of guidelines for the management of anaemia, controversy still surrounds the diagnosis and treatment of this disease, and this uncertainty contributes to an average of 841,000 deaths each year, and 35,057,000 disability-adjusted life years.1 In contrast to other pathologies, iron deficiency anaemia can present at all stages of life, either as a primary or comorbid disease (for example, in chronic kidney failure or restless legs syndrome).2 Situations such as pregnancy, menstruation, or body growth are most commonly associated with the development of iron deficiency anaemia. Recently, however, other pathologies such as heart failure or inflammatory bowel syndrome have also been linked to a high prevalence of iron deficiency. Standard treatment is oral administration of iron, usually in the form of ferrous sulphate (325mg [65mg of elemental iron] 3 times daily).3 Oral iron can cause side effects, such as reflux, constipation, diarrhoea, nausea or flatulence, and is not effective in some patients. In these cases, parenteral administration of iron should be considered.4 The benefits of intravenous iron supplementation have mainly been evaluated in patients with chronic kidney failure. The most commonly used formulations are iron dextran, ferric gluconate, iron sucrose, ferumoxytol, iron isomaltose, or carboxymaltose.5 In Mexico, iron dextran is the intravenous therapy of choice, although dextran-derived ligands or compounds can cause anaphylactic shock.6 In a recent study, Alvarado-Ibarra et al.7 reported a 0.26% prevalence of adverse events associated with iron dextran infusion. Mexico has a high prevalence of iron deficiency, particularly among the more underprivileged population groups (20% vs.14%).8 Nevertheless, few studies have evaluated the therapeutic efficacy of the different formulations available or the management of patients with iron deficiency anaemia not secondary to pregnancy or chronic kidney failure. Diagnosis and follow-up should not be limited exclusively to normalizing haemoglobin levels,9,10 as these alone have low sensitivity and specificity for diagnosing and monitoring iron deficiency anaemia. Instead, other haematological parameters such as mean corpuscular volume (MCV) and Wintrobe indices, together with other recently discovered biomarkers such as zinc protoporphyrin and reticulocyte haemoglobin should be used. All these are highly effective screening tools, and help quantify anaemia severity and guide the initial management strategy.11–13 Greater insight into iron metabolism has shown that haemoglobin and mean corpuscular volume are the last parameters to be affected in iron deficiency anaemia; serum ferritin and iron levels are altered first, and are therefore invaluable markers in the diagnosis of iron deficiency anaemia and the estimation of body iron reserves.14–16
The primary aims of this study were to evaluate the efficacy of different iron therapy regimens in increasing both haemoglobin levels and red blood cell count, and their relationship with iron kinetics.
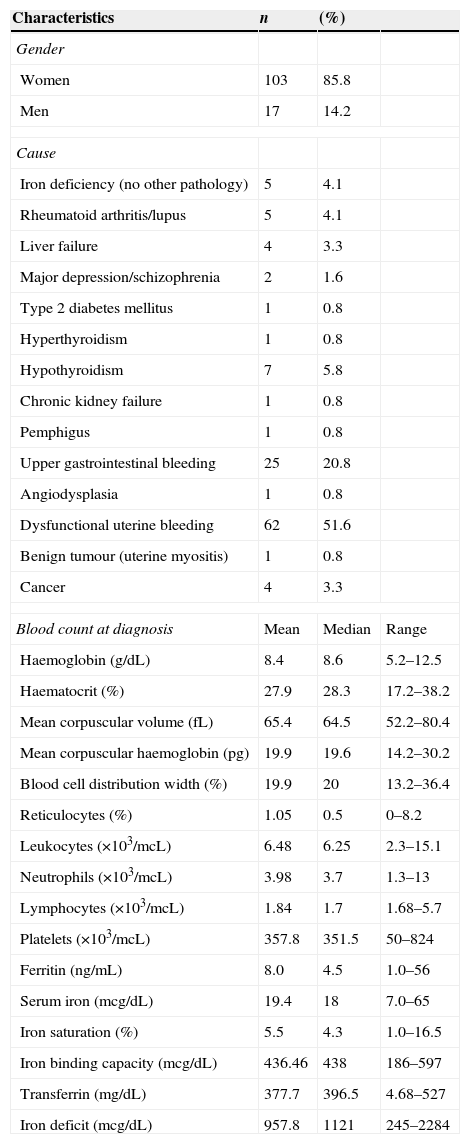
MethodsStudy designA retrospective, analytical observational study based on the clinical records of patients treated in the Haematology Department between March and October 2014. Patients that met diagnostic criteria for iron deficiency anaemia (Table 1) were included. Only patients with previous diagnosis of chronic kidney failure, cancer, or pregnancy were excluded. Convenience sampling methods were used, so all cases that met the study criteria were included. Cases with incomplete follow-up, defined as loss to follow-up up to 3 months after start of treatment, were excluded. Treatment response was defined as a 2g/dL increase in haemoglobin levels over a period of 3–4 weeks; treatment failure was defined as an increase of less than 1g/dL over a period of 4 weeks. The formulation used and the route of administration were chosen at the discretion of the attending physician.
Diagnostic criteria for iron deficiency anaemia.
| Criterion | Men | Women |
|---|---|---|
| Haemoglobin | <14g/dLa | <13g/dLa |
| Reference range: | 16.1±0.8 (+1)g/dL | 14.3±0.68 (+1)g/dL |
| Hematocrit | <39%a | <36%a |
| Reference range: | 47.6±2.5 (+3)% | 42.4±2.13 (+3)% |
| Serum ferritin | <20ng/mL | <20ng/mL |
| Reference range: | 40–300ng/mL | 20–200ng/mL |
| Serum iron | <60ng/mL | <60ng/mL |
| Reference range: | 60–170ng/mL | 60–170ng/mL |
a Values adjusted for the altitude of Mexico City (2250m above sea level. Factor used shown in brackets).
Study data were analysed using IBM SPSS version 20.0 (Armonk, New York) for Windows. The mean and mode of the haemoglobin levels and red blood cell count resulting from different treatment regimens were analysed descriptively. The mean difference in haemoglobin levels among intravenous and oral therapies was calculated using the Student's t test. The mean difference between different therapies used was calculated using the single factor ANOVA test. The therapeutic failure hypothesis was tested using the chi-square test, with a significance of p≤0.05 and a 95% confidence interval.
Ethical considerationsThe trial was submitted to and approved by the Independent Ethics Committee of the General Hospital of Mexico. Due to its retrospective nature, no signed informed consent forms were required, and patient privacy and data anonymity were preserved at all times, in accordance with prevailing laws.
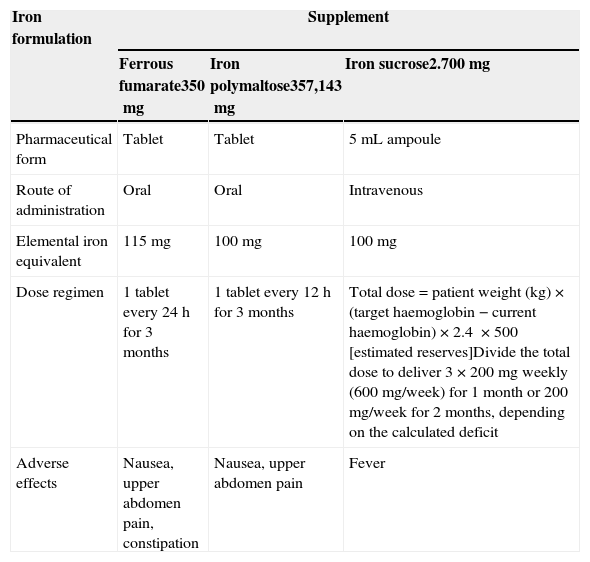
ResultsIn total, 120 patients met inclusion criteria and their records were analysed for the purpose of this study; 85.8% were women (n=103) and 14.2% were men (n=17). The overall mean age was 42 years (range 16–83 years); the mean age of men was slightly higher than that of women (52 vs. 41 years). Over 50% of patients presented symptoms associated with anaemia, although less specific symptoms, such as pica, were found in 44.2% of cases (n=53). The most common findings on physical examination were pallor and hair loss; koilonychia was found in 45.5% of cases (n=55). The aetiology, complete blood count, and iron kinetics at the time of diagnosis are shown in Table 2. The most common aetiology was abnormal uterine bleeding (51.2%), in most cases associated with uterine myomatosis. This was followed by upper gastrointestinal bleeding (20.6%), in most cases due to gastric ulcers or erosive gastritis. The least common causes included psychiatric disorders, in 1.6%, and hypothyroidism in around 5.8% of patients. Iron deficiency preceded diagnosis in 0.08% of cases of diabetes mellitus, chronic kidney failure, and even pregnancy. Iron deficiency was significantly associated with diagnosis of digestive cancer (colon cancer) in 2.5% of cases. Most of these patients were men (n=3). Oral and intravenous therapy dose regimens are shown in Table 3. The main adverse effects associated with oral administration were: nausea, upper abdomen pain and constipation; intravenous iron administration caused fever in 4 patients.
Main patient characteristics and results of whole blood count at diagnosis.
| Characteristics | n | (%) | |
|---|---|---|---|
| Gender | |||
| Women | 103 | 85.8 | |
| Men | 17 | 14.2 | |
| Cause | |||
| Iron deficiency (no other pathology) | 5 | 4.1 | |
| Rheumatoid arthritis/lupus | 5 | 4.1 | |
| Liver failure | 4 | 3.3 | |
| Major depression/schizophrenia | 2 | 1.6 | |
| Type 2 diabetes mellitus | 1 | 0.8 | |
| Hyperthyroidism | 1 | 0.8 | |
| Hypothyroidism | 7 | 5.8 | |
| Chronic kidney failure | 1 | 0.8 | |
| Pemphigus | 1 | 0.8 | |
| Upper gastrointestinal bleeding | 25 | 20.8 | |
| Angiodysplasia | 1 | 0.8 | |
| Dysfunctional uterine bleeding | 62 | 51.6 | |
| Benign tumour (uterine myositis) | 1 | 0.8 | |
| Cancer | 4 | 3.3 | |
| Blood count at diagnosis | Mean | Median | Range |
| Haemoglobin (g/dL) | 8.4 | 8.6 | 5.2–12.5 |
| Haematocrit (%) | 27.9 | 28.3 | 17.2–38.2 |
| Mean corpuscular volume (fL) | 65.4 | 64.5 | 52.2–80.4 |
| Mean corpuscular haemoglobin (pg) | 19.9 | 19.6 | 14.2–30.2 |
| Blood cell distribution width (%) | 19.9 | 20 | 13.2–36.4 |
| Reticulocytes (%) | 1.05 | 0.5 | 0–8.2 |
| Leukocytes (×103/mcL) | 6.48 | 6.25 | 2.3–15.1 |
| Neutrophils (×103/mcL) | 3.98 | 3.7 | 1.3–13 |
| Lymphocytes (×103/mcL) | 1.84 | 1.7 | 1.68–5.7 |
| Platelets (×103/mcL) | 357.8 | 351.5 | 50–824 |
| Ferritin (ng/mL) | 8.0 | 4.5 | 1.0–56 |
| Serum iron (mcg/dL) | 19.4 | 18 | 7.0–65 |
| Iron saturation (%) | 5.5 | 4.3 | 1.0–16.5 |
| Iron binding capacity (mcg/dL) | 436.46 | 438 | 186–597 |
| Transferrin (mg/dL) | 377.7 | 396.5 | 4.68–527 |
| Iron deficit (mcg/dL) | 957.8 | 1121 | 245–2284 |
Exogenous iron supplements administered.
| Iron formulation | Supplement | ||
|---|---|---|---|
| Ferrous fumarate350mg | Iron polymaltose357,143mg | Iron sucrose2.700mg | |
| Pharmaceutical form | Tablet | Tablet | 5mL ampoule |
| Route of administration | Oral | Oral | Intravenous |
| Elemental iron equivalent | 115mg | 100mg | 100mg |
| Dose regimen | 1 tablet every 24h for 3 months | 1 tablet every 12h for 3 months | Total dose=patient weight (kg)×(target haemoglobin−current haemoglobin)×2.4 ×500 [estimated reserves]Divide the total dose to deliver 3×200mg weekly (600mg/week) for 1 month or 200mg/week for 2 months, depending on the calculated deficit |
| Adverse effects | Nausea, upper abdomen pain, constipation | Nausea, upper abdomen pain | Fever |
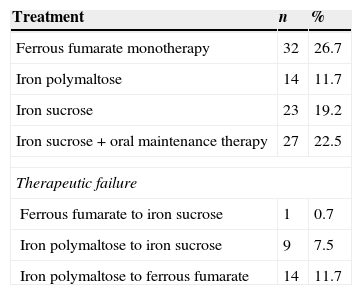
Approximately 58.3% of patients (n=70) were started on oral therapy, and 41.6% (n=50) on intravenous administration. The preparations most commonly used were ferrous fumarate and iron polymaltose. The only intravenous formulation used was iron sucrose (iron deficit was calculated on the basis of the following formula: total dose=patient weight (kg)×(target haemoglobin−current haemoglobin)×2.4×500 [estimated reserves]). In 19.9% of cases therapy was changed (n=24) due to treatment failure; iron polymaltose therapy failed in 19.2%, and therapy was either changed to intravenous iron sucrose or to oral ferrous fumarate, the latter with a failure rate of 0.7% (Table 4).
Percentage use of different therapies.
| Treatment | n | % |
|---|---|---|
| Ferrous fumarate monotherapy | 32 | 26.7 |
| Iron polymaltose | 14 | 11.7 |
| Iron sucrose | 23 | 19.2 |
| Iron sucrose+oral maintenance therapy | 27 | 22.5 |
| Therapeutic failure | ||
| Ferrous fumarate to iron sucrose | 1 | 0.7 |
| Iron polymaltose to iron sucrose | 9 | 7.5 |
| Iron polymaltose to ferrous fumarate | 14 | 11.7 |
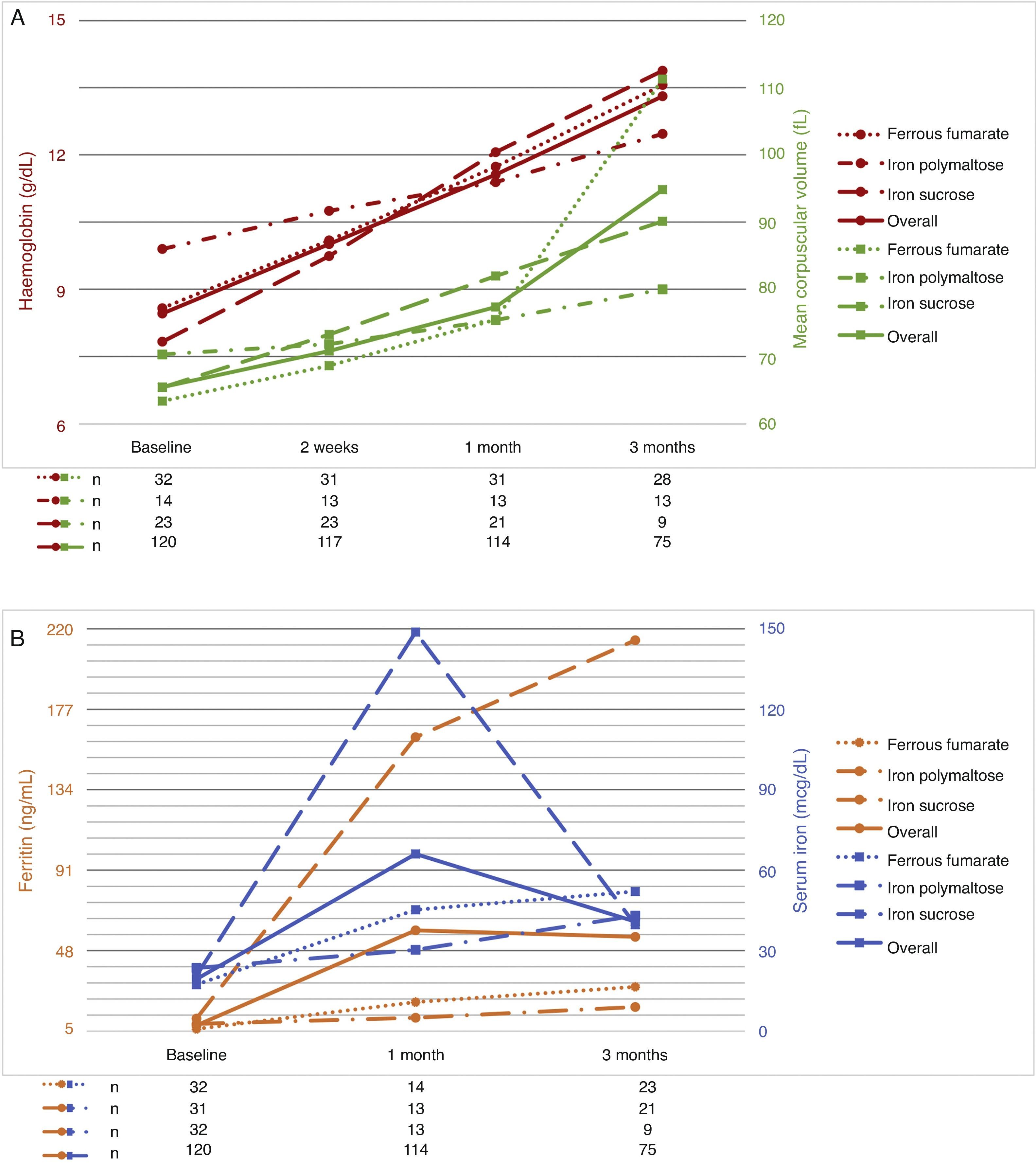
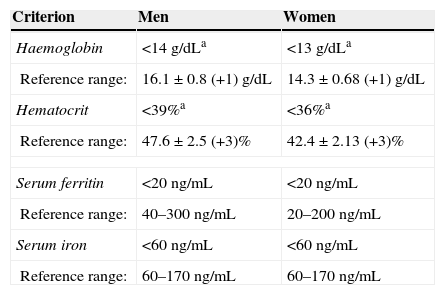
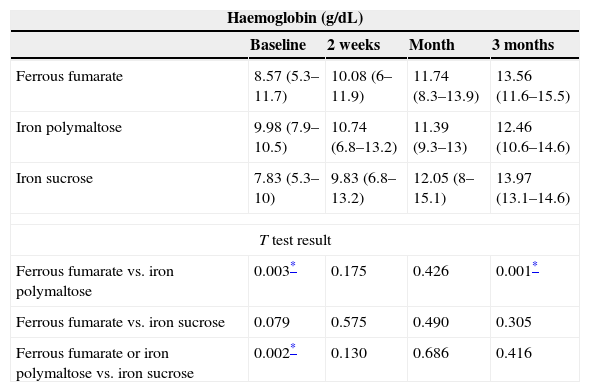
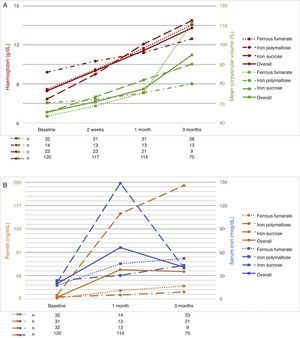
The follow-up period was 3 months. Patients were evaluated at 2 weeks after start of treatment, then at 1 month, and finally at 3 months. As a result of the therapies used, haemoglobin levels increased by 1–2g/dL at 2 weeks after start of treatment; the best responses were achieved with ferrous fumarate and iron sucrose. No differences in haemoglobin levels were found between ferrous fumarate and iron sucrose (p=0.575 at 2 weeks, p=0.490 at 1 month, p=0.490 at 3 months). Ferrous fumarate showed better response compared with iron polymaltose at 3 months (p=0.175 at 2 weeks, p=0.426 at 1 month and p=0.001 at 3 months). Increase in ferritin levels was greater with ferrous fumarate and iron sucrose overall; in comparison, ferrous fumarate performed better than iron polymaltose (p=0.004 and 1 month and p=0.001 at 3 months). Response with intravenous iron (iron sucrose) was superior to oral iron (p=0.000 at both 1 and 3 months) (Table 5). Fig. 1A shows haemoglobin levels and mean corpuscular volume. Fig. 1B shows serum ferritin and iron levels.
Comparison of haemoglobin and ferritin levels with different therapies over 3-month follow-up.
| Haemoglobin (g/dL) | ||||
|---|---|---|---|---|
| Baseline | 2 weeks | Month | 3 months | |
| Ferrous fumarate | 8.57 (5.3–11.7) | 10.08 (6–11.9) | 11.74 (8.3–13.9) | 13.56 (11.6–15.5) |
| Iron polymaltose | 9.98 (7.9–10.5) | 10.74 (6.8–13.2) | 11.39 (9.3–13) | 12.46 (10.6–14.6) |
| Iron sucrose | 7.83 (5.3–10) | 9.83 (6.8–13.2) | 12.05 (8–15.1) | 13.97 (13.1–14.6) |
| T test result | ||||
| Ferrous fumarate vs. iron polymaltose | 0.003* | 0.175 | 0.426 | 0.001* |
| Ferrous fumarate vs. iron sucrose | 0.079 | 0.575 | 0.490 | 0.305 |
| Ferrous fumarate or iron polymaltose vs. iron sucrose | 0.002* | 0.130 | 0.686 | 0.416 |
| Ferritin (ng/mL) | ||||
|---|---|---|---|---|
| Baseline | 2 weeks | Month | 3 months | |
| Ferrous fumarate | 6.20 (1.2–26.9) | NA | 20.51 (7.8–44.2) | 28.68 (10.5–56) |
| Iron polymaltose | 8.89 (2.4–26) | NA | 12.20 (4–28.2) | 17.90 (9.3–36) |
| Iron sucrose | 11.71 (1.2–56) | NA | 162.08 (34.6–512) | 213.86 (63–724.8) |
| T test result | ||||
| Ferrous fumarate vs. iron polymaltose | 0.161 | NA | 0.004* | 0.001* |
| Ferrous fumarate vs. iron sucrose | 0.031 | NA | 0.000* | 0.000* |
| Ferrous fumarate or iron polymaltose vs. iron sucrose | 0.367 | NA | 0.000* | 0.000* |
Iron deficiency anaemia is a public health problem in both developed and developing countries. It affects people at all stages of life, but is most prevalent in women of childbearing age, pregnant women, and children under 2 years of age. The most common cause of iron deficiency anaemia in adolescent and adult women is vaginal haemorrhage due to dysfunctional uterine bleeding or secondary to uterine myomatosis. In adults of both sexes, iron deficiency anaemia is caused by chronic pathologies: gastrointestinal haemorrhage (erosive gastritis, gastric ulcers, polyps, intestinal diverticula or colon cancer).17,18 In our study, we found a predominance of female patients. The most common cause of anaemia was dysfunctional uterine bleeding secondary to uterine myomatosis. This was followed by upper gastrointestinal bleeding (Helicobacter pylori gastritis), autoimmune diseases such as hypothyroidism, and colon cancer, predominantly in men, consistent with literature.19,20 Patients present the typical symptoms of anaemia, such as weakness, headaches, irritability, tinnitus, phosphenes and koilonychia, although many patients were asymptomatic. The clinical manifestations were largely similar in all study patients, the only notable finding being koilonychia in 45.5% (n=55). Diagnosis of iron deficiency anaemia was made on the basis of haemoglobin levels, Wintrobe indexes, and more importantly, mean corpuscular volume, the latter being the most important marker in red blood cell indices. Other findings used to confirm iron deficiency anaemia are higher red blood cell distribution width and low ferritin levels, all of which were present in study patients. Some patients presented platelet counts of up to 800,000, an unusual finding in patients with iron deficiency anaemia.
The aim of therapy is to normalize haemoglobin levels and Wintrobe indices, and replenish iron reserves. Iron deficiency therapy should start with dietary strategies.21,22 When anaemia is severe, exogenous oral or intravenous iron supplements should be started. Several different formulations based on different iron salts are available (ferrous sulphate, ferrous fumarate, iron polymaltose, iron dextran, iron sucrose, and ferric carboxymaltose).14,23,24 The recommended dose for oral therapy in adults is 100 to 200mg of elemental iron daily24,27. In the Haematology Department of the Hospital General of Mexico, we give our patients ferrous fumarate, iron polymaltose and iron sucrose. Iron dextran has not been used for 6 years, due to a higher risk of anaphylactic shock. In this study, we compared treatment response over 3 months in order to evaluate the efficacy of each of these 3 preparations. Ferrous fumarate and iron sucrose were equally effective and better than iron polymaltose in normalizing haemoglobin and serum iron levels and mean corpuscular volumes, while ferritin level increase was greater with iron sucrose vs. all oral formulations. Comparing ferritin increase in oral formulations (ferrous fumarate and iron polymaltose), better results were obtained with ferrous fumarate.
In conclusion, oral and intravenous administration of iron salts are similarly effective in anaemia therapy (normalization of haemoglobin levels and MCV); iron sucrose is more effective in restoring iron reserves. However, we believe that oral iron supplements should be continued for 3 months in order to restore total iron reserves, and the best oral supplement is ferrous fumarate. Controlled clinical trials are needed to confirm this observation.
Conflict of interestThe authors declare that they have no conflict of interests.