The depression is the most prevalent state throughout the life of the bipolar patient. Ketamine has been shown to be an effective and rapid treatment for depression. The objective of the present work is to perform a systematic review on the efficacy and safety of ketamine as treatment of bipolar depression, as well as its different patterns of administration. The search found 10 relevant manuscripts that met the inclusion criteria: one clinical trial, 5 cohort studies, and 4 case reports. Intravenous infusion was used in 60% of the studies. According to data, ketamine seems to be an effective and safe treatment for bipolar depression, although the length of its effect is short. Adverse effects observed generally occurred at the time of infusion, and tended to completely disappear within 1–2h. Therefore, more studies are necessary to explore new patterns of administration, as well as on its safety and adverse effects.
El componente depresivo es el más prevalente del trastorno bipolar. La ketamina ha mostrado eficacia y rapidez como tratamiento de episodios depresivos. El objetivo del presente trabajo es realizar una revisión sistemática sobre la eficacia y seguridad de la ketamina como tratamiento de la depresión bipolar, y sus tipos de administración. Se obtuvieron 10 artículos relevantes que cumplían con los criterios del estudio: un ensayo clínico, 5 estudios de cohorte y 4 series de casos. La forma de administración utilizada en el 60% de los trabajos fue la infusión intravenosa. La ketamina parece ser un tratamiento seguro y eficaz para la depresión bipolar, aunque la duración de la acción es breve. Los efectos adversos que se observaron se produjeron generalmente en el momento de la infusión y tendieron a desaparecer completamente al cabo de 1-2h. Por tanto, es necesario realizar más estudios para explorar nuevas vías de administración, así como su seguridad y efectos adversos.
Bipolar disorder (BD) is one of the most incapacitating medical diseases and it is also one of the most costly for the healthcare system.1 Depression, dysthimia and mixed states as a whole are the most prevalent components of BD.2 Although effective treatments for bipolar depression exist,3–6 a significant number of cases are resistant due to lack of efficacy or intolerance of side effects. There is therefore a major need to develop new drugs with a rapid antidepressive effect, given the risk of suicide among these patients.7
During the last decade, several studies have shown that the glutamatergic system and most particularly the N-methyl-d-aspartate receptor (NMDA) are involved in the efficacy of antidepressive treatments, which makes it a target for the development of new treatments.7 Ketamine is a phencyclidine derivate and NMDA receptor antagonist which has been shown to have a swift and potent effect against depressive episodes at sub-anaesthetic doses.8,9 In the case of unipolar depression at least 8 clinical trials have been published that showed ketamine to be fast-acting and effective in this diagnostic group.10–17
This review has the aim of systematically analysing and summarising the scientific evidence of the most recent works on the efficacy and safety of using ketamine to treat bipolar depression, as well as its different forms of administration.
Material and methodsThe PubMed and Embase databases were searched electronically. The search was restricted to prospective works, series of cases or clinical trials published in English or Spanish from January 2012 to October 2015. The following MESH terms were used: “ketamine”, “bipolar disorder” and “bipolar depression”. The strategy was restricted to studies undertaken in human beings. A manual search was subsequently performed together with a reverse search based on the works selected. The publications which included patients diagnosed BD were included. Works with samples containing patients with a different diagnosis were excluded from the study, as were those which did not evaluate the efficacy of ketamine in cases of depression.
The papers were summarised and evaluated using the on-line tool “Osteba Fichas de lectura crítica”(FLC).18 This tool makes it possible to read papers in detail and determine their methodological quality. This selection and data extraction process was undertaken by 2 reviewers, who in case of disagreement met to decide which works to include or exclude from the review, or regarding the quality of a paper.
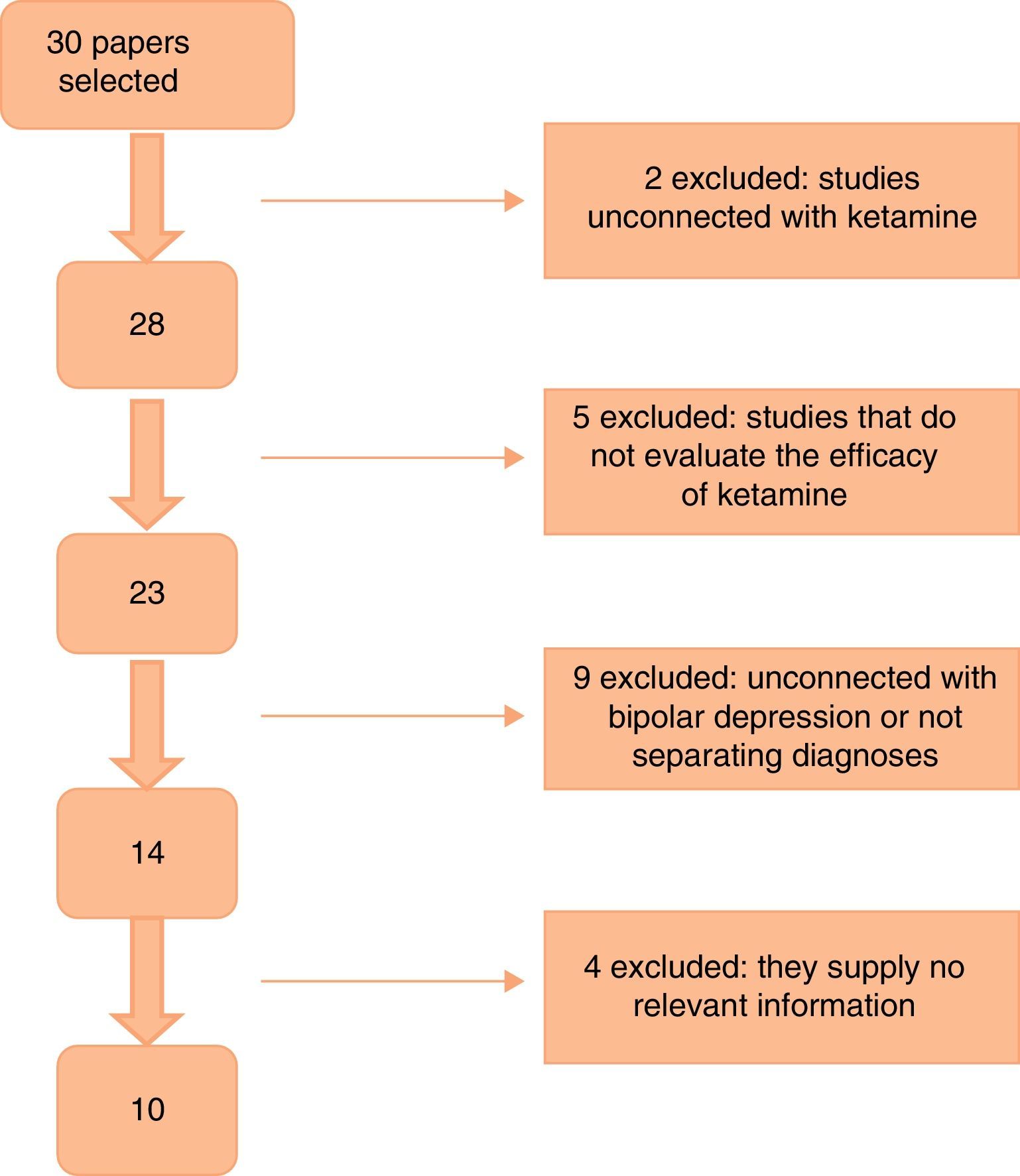
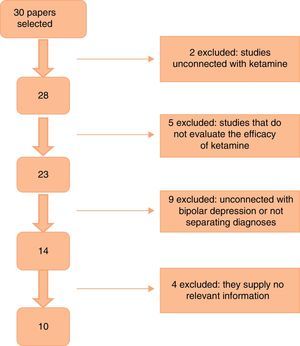
Fig. 1 shows the paper selection flow based on PRISMA19 methodology.
ResultsThe first search found a total of 30 papers, of which 10 that fulfilled inclusion criteria and contained relevant information were selected. No work selected by manual or reverse search was selected. Of the works selected, one is a clinical trial, 5 are cohort studies and 4 are case series (as shown in Table 1). The majority of the studies used a 0.5mg/kg dose of ketamine administered by intravenous (i.v.) infusion. Two studies used intramuscular (i.m.) doses of 50–100mg ketamine,20,21 while one used intranasal (i.n.) ketamine at a dose of 10mg/administration22 and another one used a 10mg dose of sublingual (s.l.) ketamine.23 All of the papers specified a response criterion, and this was defined as a reduction of ≥50% in the score on one of the scales used to measure depression (the Hamilton Rating Scale for Depression [HDRS] or the Montgomery-Asberg Depression Rating Scale [MADRS]).
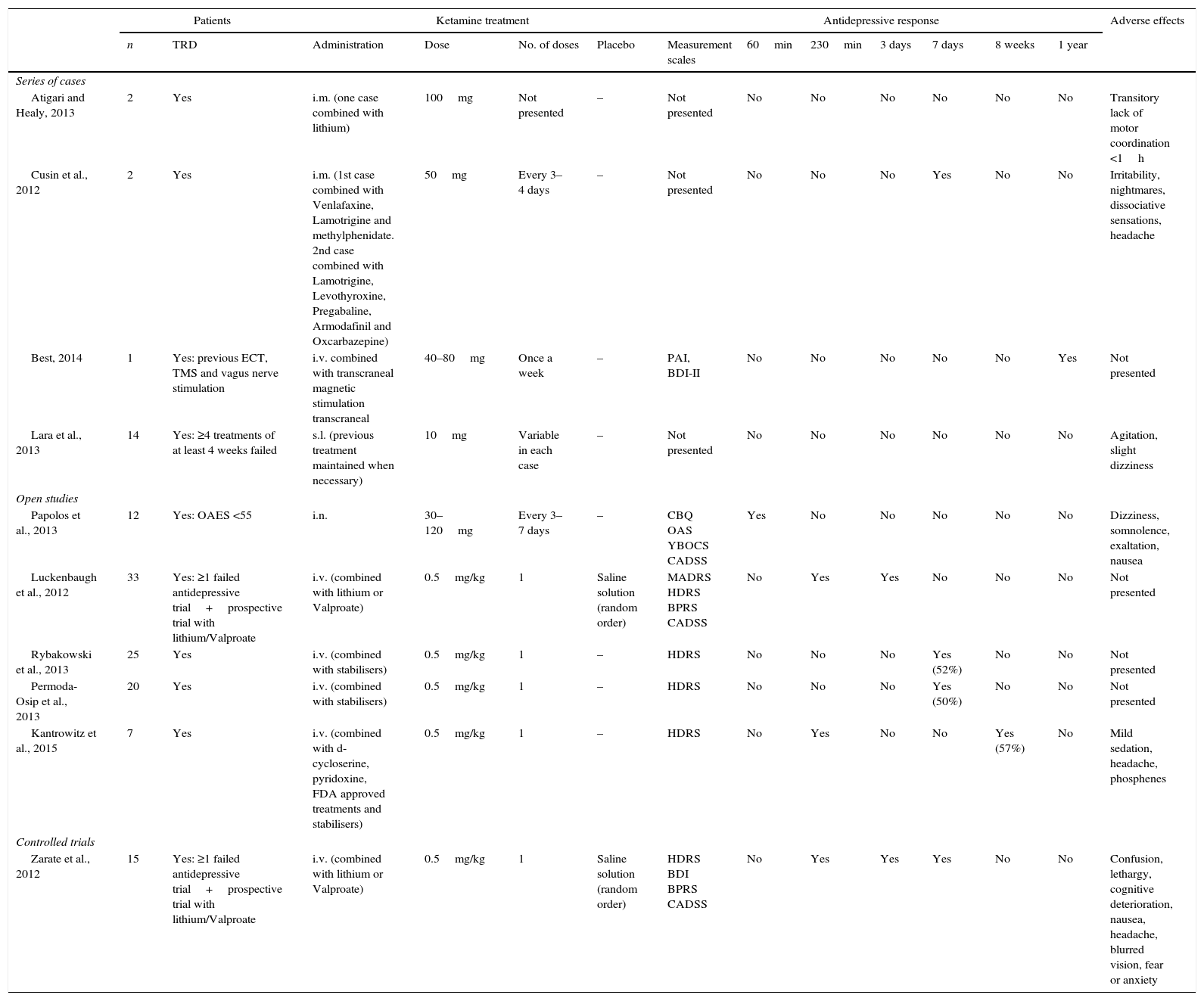
Summary of the case series, controlled and open randomised trials on the use of ketamine as a treatment for bipolar depression.
| Patients | Ketamine treatment | Antidepressive response | Adverse effects | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | TRD | Administration | Dose | No. of doses | Placebo | Measurement scales | 60min | 230min | 3 days | 7 days | 8 weeks | 1 year | ||
| Series of cases | ||||||||||||||
| Atigari and Healy, 2013 | 2 | Yes | i.m. (one case combined with lithium) | 100mg | Not presented | – | Not presented | No | No | No | No | No | No | Transitory lack of motor coordination <1h |
| Cusin et al., 2012 | 2 | Yes | i.m. (1st case combined with Venlafaxine, Lamotrigine and methylphenidate. 2nd case combined with Lamotrigine, Levothyroxine, Pregabaline, Armodafinil and Oxcarbazepine) | 50mg | Every 3–4 days | – | Not presented | No | No | No | Yes | No | No | Irritability, nightmares, dissociative sensations, headache |
| Best, 2014 | 1 | Yes: previous ECT, TMS and vagus nerve stimulation | i.v. combined with transcraneal magnetic stimulation transcraneal | 40–80mg | Once a week | – | PAI, BDI-II | No | No | No | No | No | Yes | Not presented |
| Lara et al., 2013 | 14 | Yes: ≥4 treatments of at least 4 weeks failed | s.l. (previous treatment maintained when necessary) | 10mg | Variable in each case | – | Not presented | No | No | No | No | No | No | Agitation, slight dizziness |
| Open studies | ||||||||||||||
| Papolos et al., 2013 | 12 | Yes: OAES <55 | i.n. | 30–120mg | Every 3–7 days | – | CBQ OAS YBOCS CADSS | Yes | No | No | No | No | No | Dizziness, somnolence, exaltation, nausea |
| Luckenbaugh et al., 2012 | 33 | Yes: ≥1 failed antidepressive trial+prospective trial with lithium/Valproate | i.v. (combined with lithium or Valproate) | 0.5mg/kg | 1 | Saline solution (random order) | MADRS HDRS BPRS CADSS | No | Yes | Yes | No | No | No | Not presented |
| Rybakowski et al., 2013 | 25 | Yes | i.v. (combined with stabilisers) | 0.5mg/kg | 1 | – | HDRS | No | No | No | Yes (52%) | No | No | Not presented |
| Permoda-Osip et al., 2013 | 20 | Yes | i.v. (combined with stabilisers) | 0.5mg/kg | 1 | – | HDRS | No | No | No | Yes (50%) | No | No | Not presented |
| Kantrowitz et al., 2015 | 7 | Yes | i.v. (combined with d-cycloserine, pyridoxine, FDA approved treatments and stabilisers) | 0.5mg/kg | 1 | – | HDRS | No | Yes | No | No | Yes (57%) | No | Mild sedation, headache, phosphenes |
| Controlled trials | ||||||||||||||
| Zarate et al., 2012 | 15 | Yes: ≥1 failed antidepressive trial+prospective trial with lithium/Valproate | i.v. (combined with lithium or Valproate) | 0.5mg/kg | 1 | Saline solution (random order) | HDRS BDI BPRS CADSS | No | Yes | Yes | Yes | No | No | Confusion, lethargy, cognitive deterioration, nausea, headache, blurred vision, fear or anxiety |
BDI II: Beck Depression Inventory II; BPRS: Brief Psychiatric Rating Scale; CADDS: Clinician Administered Dissociative States Scale; CBQ: Child Bipolar Questionnaire; TRD: treatment resistant depression; OAES: Overall Activity Evaluation Scale; FDA: Food and Drug Administration; HDRS: Hamilton Rating Scale for Depression; i.m.: intramuscular; i.n.: intranasal; i.v.: intravenous; s.l.: sublingual; MADRS: Montgomery-Asberg Depression Rating Scale; OAS: Overt Aggression Scale; PAI: Personality Assessment Inventory; ECT: electroconvulsive therapy; TMS: transcraneal magnetic stimulation; YBOCS: Yale Brown Obsessive-compulsive Scale.
The aim of the work undertaken by Zarate et al.9 was to verify the result obtained beforehand by Diazgranados et al.8 that ketamine has a swift antidepressive effect in patients with bipolar depression. A double blind, randomised, crossed and controlled study was performed for this in which the patients received i.v. ketamine (0.5mg/kg) or a placebo 2 weeks apart. The symptoms of anxiety and depression improved more in the patients who received ketamine vs. the placebo in the 40min after the infusion (HDRS: F=3.08, P=.001; VAS-Anxiety: F=2.12, P=.030). The response rate 40min after the infusion (reduction in MADRS ≥50%) was 64% for the patients taking ketamine. During the first day the response rate was 43%, and approximately 30% were in remission (MADRS <10). The duration of the antidepressive effect was 3 days as measured by the MADRS and HDRS, but the improvement in depressive symptoms on the Beck Depression Inventory (BDI) was longer lasting (14 days vs. 3 days). The most interesting result of this study was that ketamine has a swift and continuous anti-suicide effect (in the first 40min).
Luckenbaugh et al.24 carried out a study to determine the degree to which the antidepressive effect of ketamine is altered in patients with bipolar depression who have a family history of alcohol dependency (FHAP). The study undertook a post hoc analysis by combining the samples from 2 previous independent double-blind, crossed and controlled studies in which i.v. ketamine was administered (0.5mg/kg) combined with lithium or Valproate.8,9 The main result was that the individuals with a FHAP showed better and longer-lasting improvements to their depressive symptoms compared to those without a FHAP (MADRS: P=.030, d=0.9, IC 95%: 0.02–0.37; HDRS: P=.010, d=0.25, IC 95%: 0.05–0.45). The subjects with a FHAP also displayed less perceptive distortion due to ketamine. When the patients with a personal history of alcoholism were analysed, they had lower scores on the Clinician-Administered Dissociative States Scale ([CADSS]: F=2.33, P=.010), Brief Psychiatric Rating Scale Positive ([BPRS] Positive: F=5.04, P=.030) and BPRS Dysphoria (F=9.17, P=.003).
The work published by Atigari and Healy20 describes the cases of 2 individuals with a long history of resistant BD who took part in ketamine studies during a depressive phase. For one of them the only effective treatment was electroconvulsive therapy (ECT). Although the other patient responded to lithium, the response was different in each episode. Both cases experienced an immediate response to treatment with ketamine and remained in remission several months later. Both of these cases had responded previously to treatments with convulsive properties. Due to this the authors underline the need to research the convulsive properties of treatments such as ketamine or ECT as an additional mechanism to their antidepressive action.
The paper published by Best25 describes a clinical case of severe depression within ECT-resistant BD I, transcraneal magnetic stimulation (TMS) or stimulation of the vagus nerve. After these different strategies the subject accepted combined treatment of TMS and ketamine (40–80mg) administered weekly for 3 years. One year after the start of treatment remission of the symptoms occurred, with partial improvement in functioning. More specifically, depression, anxiety, attention, concentration and clear thinking all improved. The patient was also in a better mood and without suicidal ideas.
The study carried out by Kantrowitz et al.26 is the first one to investigate the efficacy of ketamine (0.5mg/kg) in resistant bipolar depression, followed by daily doses of d-cycloserine during 8 weeks (an initial dose of 250mg raised to 1000mg in 3 weeks). All of this was supported using pyridoxine, a treatment approved by the FDA, together with mood stabilisers. Seven subjects completed the study, 4 of whom achieved remission (HDRS <7). A significant response was obtained from the basal moment to all of the moments measured except at 2 weeks (F=161.8, P<.001), with a major effect on the first day (d cohen=2) and at 8 weeks (d cohen=1.1). The authors conclude that it would be recommendable to perform studies to analyse the efficacy and safety of d-cycloserine as an independent treatment for bipolar depression.
The work by Rybakowski et al.27 included 25 patients with a bipolar depressive episode who were given a single i.v. dose of ketamine (0.5mg/kg). All of the patients were given stabilisers and had been resistant to treatment with antidepressives. One patient responded to the treatment 6h after the infusion (a fall in the HDRS ≥50%). At 24h 24% of the patients had responded, while at 7 days 52% showed a good response and maintained this until day 14. Remission (HDRS <7) was achieved by 4 patients after 24h, while 8 patients achieved this at 7 days. After 14 days 12 patients experienced remission of their depressive symptoms.
Permoda-Osip et al.28 analysed the efficacy of ketamine and its relationship with B group vitamin deficit in 20 patients with a bipolar depressive episode. For this they were given a single dose of i.v. ketamine (0.5mg/kg). 7 days after the infusion 10 patients responded to the treatment (with a fall in the HDRS ≥50%). Additionally, it was found that B12 vitamin levels in these patients had increased in comparison with the subjects who had not responded (P=.047), although the authors underline that these results may be affected by patient sex, given that 90% of them were women. Nevertheless, they found no significant results for folic acid or homocysteine.
Types of administrationPapolos et al.22 analysed the efficacy and safety of i.n. ketamine in 12 children (6–19 years old) diagnosed BD with phobic symptoms (BD-FOH). The i.n. ketamine was tolerated well with minimum side effects, which were observed to be dose-dependent. The therapeutic effect lasted from 36 to 60h. Swift improvement was found in hyperactivity, aggressive behaviour and anxiety. In the majority of cases mania and depressive symptoms were found to disappear completely. Once remission had been achieved this was maintained for 3–7 days without side effects. Nevertheless, once the effect of the ketamine had disappeared, the symptoms returned quite quickly and gradually.
The work published by Cusin et al.21 presents 2 cases treated with i.m. ketamine. The oral and i.v. administration of ketamine were not effective, although the preparation of 50mg of i.m. ketamine every 3–4 days remained effective during several months and was tolerated well.
Lara et al.23 analysed the efficacy and tolerability of very low s.l. doses of ketamine (10mg). The dosage varied from one patient to another, and it may have been a single dose or one repeated every 2–7 days. Of the 14 patients diagnosed BD in depressive phase, 4 of them achieved a remission of depressive symptoms and 5 of them maintained the response after treatment with ketamine. The authors observed good tolerability in the majority of cases and a swift and powerful action of the ketamine on mood and cognition. The therapeutic benefits of this type of s.l. administration are similar to those obtained with i.v. administration, but tolerability is better, it is easier to use and it is also safer in terms of adverse effects.
Side effectsThe work published by Zarate et al.9 found that during infusion of ketamine or a placebo about 10% of subjects felt dizziness, somnolence, cognitive deterioration, anxiety, nausea, blurred vision or headache. There were no significant differences in side effects in comparison with the placebo after 80min. During the infusion phase the patients experienced dizziness, difficulty in falling asleep, dry mouth and flatulence. There were no significant changes in heart rate, respiratory rate or laboratory values during the study. These effects were also observed in the work by Cusin et al.21
Papolos et al.22 rarely found any side effects after more than 1h after administration. The dissociative effects experienced were: distortion of reality, the feeling of bodily changes, acting as if dreaming, the feeling of unreality or identity confusion. These effects generally were mild or moderate in intensity and always lasted for less than 60min after administration. Once tolerance had been developed (4–5 administrations of the final effective dose) these effects gradually declined and were hardly observed.
In the study by Kantrowitz et al.,26 3 subjects showed mild sedation, 2 had headaches and one experienced phosphenes.
In the work by Lara et al.,23 2 bipolar subjects presented agitation during several hours. It was common to feel slightly dazed, which in the majority of cases disappeared in less than 30min.
In another report of cases,20 one of the patients experienced transitory lack of motor coordination that disappeared one hour after administration.
ConclusionsAccording to the studies analysed in this review (one clinical trial, 5 cohort studies and 4 series of cases), the results suggest that ketamine is an NMDA receptor antagonist that has been shown to be swiftly effective in reducing depressive symptoms and attempted suicides in depressed subjects with BD, although this effect has not been shown to last over time.8,9 These data agree with the results obtained in different reviews published recently on the use and efficacy of ketamine in depression.29–31 Moreover, based on the results obtained, ketamine may be recommendable as an adjuvant treatment for patients taking antidepressive treatment or mood stabilisers with an incomplete response, for those who need to have a swift response or in resistant patients.31 However, we believe that more studies are necessary to support this statement. The duration of the antidepressive effect varies from 3 to 14 days, depending on the scale used to measure symptoms. In any case, it seems necessary to commence maintenance treatment after the initial approach, given that following discontinuation the symptoms reappear relatively quickly. For patients with bipolar depression the administration of ketamine seems to be indicated in association with mood stabilisers, or as a second-line treatment for certain clinical subgroups of patients (non-responding patients), or in specific disease endophenotypes such as patients with FHAP. ECT is a highly effective treatment for depression5 and it has been suggested that its efficacy could increase if ketamine is administered when the test is performed.32 Nevertheless, this has not been clearly proven, and the association has no clear advantages compared to ECT alone.29 Loo et al.33 evaluated the neuroprotector effect and efficacy of ketamine complementary to thiopental during ECT anaesthesia for patients with severe depressive disorder. Contrary to the main hypothesis, it has not been found that administering ketamine during ECT reduces cognitive side effects. An improvement was only detected during the first week of treatment (F=4.56, P=.039, η2=0.102). These results agree with those obtained in other studies that did not separate cases of severe depression from BD.34 However, the authors warn that this study used a subanaesthetic dose of ketamine (0.5mg/kg) in combination with thiopental. This leaves a lot of room for future research using different doses of ketamine, in combination with other anaesthetics or other types of ECT.
The risk factors for depression include low levels of vitamin B12 and folic acid, as well as high levels of homocysteine. A relationship has been found between these factors in depressed patients.35 Additionally, vitamin B12 has been found to influence the mechanism of action of ketamine.28 It would therefore be of interest to monitor the levels of the said vitamin when prescribing ketamine, as both molecules have been found to have a synergic effect.36 The important role of vitamin B12 may be due to the fact that the said vitamin is essential for the correct working of methionine synthase and methylmalonyl-coA mutase. These enzymes work as catalysers in the conversion of homocysteine into methionine. Methionine is an essential amino acid for the synthesis of S-adenosylmethionine (SAMe), which has a stimulating effect on the central monoaminergic neurotransmitters. It would be recommendable in subsequent studies to analyse the effect of ketamine after a vitamin B12 supplement in patients with low levels of the same. Along the same lines, interest has also been aroused in vitamin B6 (pyridoxine), which raises the level of serotonin in the blood, and as a supplement to the treatment could help to reduce the symptoms of depression. Additionally, d-cycloserine, which at high doses (>750mg) acts as an NMDA receptor antagonist, may improve the safety of treatment using ketamine.26 It would also be highly interesting to have biomarkers that predict which patients will respond to ketamine or not. Hashimoto37 has found that levels of the inflammatory biomarker IL-6 are higher in the group of patients who went on to respond to ketamine. It would therefore be of interest to explore the relationship between the rapid antidepressive effect of ketamine and the expression of inflammatory routes in depression.
The results obtained by Luckenbaugh et al.24 confirm those of other studies that show a greater improvement in patients with alcohol dependency and those with FHAP treated with ketamine.38 The authors suggest that a genetic variation in gene NR2A that expresses NMDA receptor could be involved in the susceptibility to alcohol dependency. This variation may make patients with FHAP more sensitive to the effect of ketamine.39 This would indicate that NMDA receptor alterations in patients with genetic alcoholism heritability could be a different neurobiological subtype that leads to altered responses to ketamine.
Although ketamine is usually administered by i.v. infusion, new formats have been studied recently such as oral, i.n., i.m. or s.l. When administered orally ketamine undergoes a metabolic transformation the first stage of which takes place in the liver and converts it into norketamine.40 Although norketamine is biologically weaker than unmetabolised ketamine, it has plasma levels that are 3 times higher and a longer half-life (12h vs. 2h). The maximum concentrations in plasma occurs in less than 1min with i.v. ketamine, while with i.m. ketamine this takes from 5 to 15min and with orally administered ketamine it takes 30–60min. Parenteral preparations have a high level of bioavailability (i.m. 93%, i.v. 100%), while i.n. administrated achieves a bioavailability of 25–50%, and the oral preparation is poorly absorbed gastrointestinally (20% bioavailability).40 With oral ketamine oral we found cases in which it did not seem to be effective,21 while in other studies it did bring about a reduction in symptoms.41 A series of studies have pointed out that i.m. administration produces a rapid and sustained effect on depressive symptoms in bipolar depression as well as in other types of disorder (obsessive-compulsive disorder or severe depression).20,21 The i.n. and s.l. routes have also proven effective in different diagnoses with greater tolerability, use and safety than the i.v. route, so that although the results are still preliminary they seem to be strong alternatives.22,23 The interest in developing new ways of administration is due above all to facilitate use for maintenance doses with less invasive routes, thereby improving adherence. For repeated doses of ketamine i.n. or i.m. administration would be more practical. The oral preparation should be considered as a maintenance treatment once the patient has responded to an initial i.v. dose,42 although due to the addictive potential of ketamine and ease of access to preparations of this type it may increase the risk of abuse and should be administered with care.29
Due to the growing interest in the use of ketamine as an antidepressive treatment, it is important to evaluate the side effects that may arise from using it. The administration of subanaesthetic doses of ketamine may cause adverse effects, physical as well as psychomimetic and neuropsychological. In general, positive, negative, dissociative and maniac symptoms have been described.8,9,42 Effects of this type usually arise at the moment ketamine is infused, and they tend to disappear completely after 60min. Other studies too have observed perceptive alterations that lasted no longer than 2h.9 The severity of these adverse effects has not been found to vary depending on the dose or form of administration.35 The most frequently observed physical adverse effects are headache, nausea, feeling slightly dazed, sleepiness and dizziness.9,21–23,26,30 Symptoms of this type tend to be dose-dependent, although once again they only occur at the moment of infusion and generally last for a short period of time.43
The synaptogenic and behavioural effects of ketamine depend on stimulation of the mammalian target of rapacymin receptor (mTOR).44 Studies point out that over-regulation of mTOR may cause an acceleration in the growth of tumours,45 so that it does not seem indicated to use ketamine in depressed bipolar patients with a current or past history of neoplasia. Moreover, ketamine must be administered with caution to patients with cardiovascular diseases (hypertension or ischaemic heart disease), as it stimulate the cardiovascular system, increasing cardiac effort and frequency, as well as arterial pressure.31,46 On the other hand, it is usual for subjects with a history of ketamine abuse to receive larger doses than the recommended ones to treat depressive symptoms, and they may even receive them more often than is necessary. Additionally, in such cases other types of substances are usually consumed,46 so that treatment of these patients with ketamine must take place with extreme precaution. Several studies have linked ketamine abuse with the development of cystitis or biliary dilation.47,48 It is therefore necessary to undertake studies of the safety of ketamine, to develop long-term treatments using it.
To conclude, we can say that ketamine may be considered to be a safe treatment that would be effective in treating some cases of bipolar depression, although it has a short duration of action, in the absence of confirming studies designed ad hoc for bipolar depression. More studies are necessary to explore new routes of administration as well as their safety and adverse effects, with the aim of improving compliance, given that treatment must be maintained over time to prevent relapse. The efficacy of this drug opens up the possibility of exploring alternative ways of treating bipolar depression, giving greater hope now for psychopharmacological innovation.49,50
Conflict of interestsDr. Gonzalez-Pinto has received grants and has worked as an advisor, tutor or expert speaker for the following entities: Almirall, AstraZeneca, Bristol-Myers Squibb, Cephalon, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Otsuka, Pfizer, Sanofi-Aventis, Servier, Shering-Plough, Solvay, the Spanish Ministry for Science and Innovation (CIBERSAM), the Science Ministry (Carlos III institute), the Basque government, the Stanley Medical Research Institute, and Wyeth.
Dr. Vieta has received grants and has worked as an advisor, tutor or expert speaker for the following entities: AB-Biotics, Actavis, Allergen, AstraZeneca, Bristol-Myers Squibb, Ferrer, Forest Research Institute, Gedeon Richter, Glaxo-Smith-Kline, Janssen, Lundbeck, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, Sunovion, Takeda, Telefónica, Fundación del Cerebro y el Comportamiento, the Spanish Ministry of Economy and Competitiveness (CIBERSAM and PI 12/00910), 7th European Framework Program (ENBREC), the Stanley Medical Research Institute and in the Comissionat per a Universitats i Recerca del DIUE, of the Generalitat de Catalunya (2014 SGR 398).
The other authors have no conflict of interests to declare.
This study was supported by Spanish Government funds “Fondo de Investigación Sanitaria” FEDER (PI12/02077); the European Fund for Regional Development: “Mastermind” (partially financed by the Policy Support Program ICT [ICT PSP] as part of the European Community Innovation and Competitiveness Framework Program), the Basque Foundation for Healthcare Innovation and Research (BIOEF); the Mental Health Research Network Centre (CIBERSAM) and the Basque Country University (GIC12/84). The Psychiatric Research Unit of Araba Teaching Hospital is supported by the Stanley Research Foundation (03-RC-003). Carlos III Institute (ISCIII).
Please cite this article as: Alberich S, Martínez-Cengotitabengoa M, López P, Zorrilla I, Núñez N, Vieta E, et al. Eficacia y seguridad de la ketamina en depresión bipolar: una revisión sistemática. Rev Psiquiatr Salud Ment (Barc). 2017;10:104–112