Psycho-COVID: Long-term effects of COVID19 pandemic on brain and mental health
More infoEmerging evidence suggests that mental health symptoms in COVID-19 survivors are higher than expected, possibly indicating that such symptoms are more likely to develop post-infection than just persist as a residual component of the acute phase. It is thus imperative to investigate the potential development of a post-COVID mental health syndrome in the longer-term and identify its risk factors.
Material and methodsA prospective study investigated mental health symptoms associated with COVID-19 and its determinants over a 12-month period following the disease onset in all consecutive adult inpatients and outpatients with COVID-19 attending a tertiary referral hospital from March to May 2020.
ResultsA total of 479 patients (female, 52.6%) were followed-up for 12 months after COVID-19 onset. Of them, 47.2% were still presenting with at least one symptom. While most symptoms subsided as compared to COVID-19 onset (all p<0.001), a significant increase was observed only for symptoms of psychiatric disorders (10.2%) and lack of concentration and focus (20%; all p<0.001). Patients presenting with symptoms related to multiple body systems 12 months after contracting COVID-19 (all p≤0.034) were more likely to suffer from mental health domain-related symptoms at follow-up. Also, a higher risk of presenting with lack of concentration and focus 12 months post infection was found in those suffering of psychiatric symptoms at COVID-19 onset (p=0.005).
ConclusionsFindings of this study may have important public health implications, as they underlie the increased need for mental health support in COVID-19 survivors.
Nuevas evidencias sugieren que los síntomas de salud mental en los supervivientes de COVID-19 son mayores de lo esperado, lo que posiblemente indica que es más probable que dichos síntomas se desarrollen después de la infección en vez de sólo persistir como componente residual de la fase aguda. Por lo tanto, es imperativo investigar el posible desarrollo de un síndrome de salud mental post-COVID a largo plazo e identificar sus factores de riesgo.
Materiales y métodosUn estudio prospectivo investigó los síntomas de salud mental asociados a la COVID-19 y sus determinantes durante un periodo de 12 meses tras el inicio de la enfermedad en todos los pacientes adultos consecutivos con COVID-19, hospitalizados y ambulatorios, que acudieron a un hospital de tercer nivel entre marzo y mayo de 2020.
ResultadosUn total de 479 pacientes (mujeres, 52,6%) fueron seguidos durante 12 meses después del inicio de COVID-19. De ellos, el 47,2% seguía presentando al menos un síntoma. Mientras que la mayoría de los síntomas disminuyeron en comparación con el inicio de la COVID-19 (todos p<0,001), se observó un aumento significativo solamente de los síntomas de los trastornos psiquiátricos (10,2%) y la falta de concentración y enfoque (20%; todos p<0,001). Los pacientes que presentaban síntomas relacionados con múltiples sistemas del cuerpo 12 meses después de contraer la COVID-19 (todos p≤0,034) tenían más probabilidades de sufrir síntomas relacionados con el dominio de la salud mental en el seguimiento. Además, se encontró un mayor riesgo de presentar falta de concentración y enfoque 12 meses después de la infección en los que sufrían síntomas psiquiátricos al inicio de COVID-19 (p=0,005).
ConclusionesLos resultados de este estudio pueden tener importantes implicaciones para la salud pública, ya que subyacen a la mayor necesidad de apoyo a la salud mental de los supervivientes de COVID-19.
To date, a very large number of studies have been published on the effects of the COronaVIrus Disease 19 (COVID-19) pandemic on mental health. Most of these studies have focused on investigating the effects of the restrictions and social changes caused by the outbreak on the general population.1–3 A different issue concerns the persistence or onset of symptoms in survivors of severe COronaVIrus infection. The need to accurately describe the timing of symptom detection and their presumed attribution to COVID-19 has resulted in the formulation of several definitions. Shortly after the pandemic outbreak, the term ‘long COVID’ was introduced to refer to a wide range of symptoms persisting beyond the initial COVID-19 infection, with such umbrella term encompassing different types and combinations of health problems for various durations.4,5 This term has been used in thousands of publications to describe the presence of symptoms in the weeks and early months after the acute phase of COVID-19.6 As the observation period expanded, the term ‘post-COVID’, or ‘post-COVID syndrome’ began to be used, urging the World Health Organization (WHO) to formulate a standardized definition based upon international consensus.7 Common symptoms in COVID-19 survivors are fatigue, shortness of breath, cognitive dysfunction and other symptoms which generally have an impact on everyday functioning.8–11 They may be new following initial recovery from an acute COVID-19 episode or persist from the initial illness. Although psychiatric symptoms are not specifically mentioned in this definition, the risk of psychiatric symptoms associated with COVID-19 infection in various body districts has been widely reported.12
A recent meta-analysis included peer-reviewed studies reporting neuropsychiatric symptoms at post-acute or later time-points after COVID-19 infection and in control groups.13 Research evidence indicates that neuropsychiatric symptoms are common and persistent after COVID-19, with sleep disorders, fatigue, anxiety, and post-traumatic stress being experienced by up to one in four patients. The studies included in the meta-analysis had a follow-up duration of 77 days (range 14–182) and there was no evidence of difference between patients surveyed <12 weeks versus 12+ weeks after discharge in terms of symptoms prevalence. However, meta-analytic evidence from longer-term studies suggests increasing symptom prevalence over time,14 with higher anxiety and depressive symptoms in COVID-19 survivors 12 months after the hospital discharge as compared to patients admitted for reasons other than COVID-19.15
Risk factors associated with mental health problems have been investigated in patients recovering from COVID-19 acute phase.16,17 However, to the best of our knowledge no study has specifically assessed potential factors that might influence the occurrence of mental health symptoms in COVID-19 survivors in the longer-term. This is of crucial relevance for at least three lines of evidence. First, the most frequent psychiatric symptoms, that are depression, anxiety and insomnia, the so-called Common Mental Disorders (CMDs),18 with an estimated global lifetime prevalence between 25.9 and 32.6% account for a considering loss of health in the lifespan.19 Second, increasing evidence indicates that COVID-19 survivors may present with enduring cognitive impairments, possibly due to the experience of more severe disease as well as pre-existing cognitive frailty.20 Third, increasing evidence suggests the experience of poor sleep quality21 as well as psychiatric distress in the form of persistent somatic symptoms22 among COVID-19 survivors, with public health implications in terms of poorer quality of life.
Therefore, the aim of this study was twofold: (i) to analyze the course of mental health symptoms in COVID-19 survivors during the 12 months following the acute phase of the disease; and (ii) to identify sociodemographic and clinical predictors of post-COVID-19 mental health symptoms.
Material and methodsStudy design and sampleThis is a secondary analysis of data collected in a primary longitudinal study,23,24 where patients’ reported symptoms at 12 months after discharge have been documented.25 Briefly, the study employed a cohort study conducted at the University hospital of Udine, a tertiary referral hospital of 1000 beds serving approximately 350 000 inhabitants, which has been appointed as a regional hub for COVID-19 patients.
Case-tracing procedure and data collection at COVID-19 onsetThe recruitment started on the 1 March 2020, the day when the first COVID-19 patient was identified and ended on the 30 May 2020. All consecutive patients, aged 18 years or older, admitted or seen on an outpatient basis at the hospital Infectious Disease Department, with a confirmed diagnosis of COVID-19, were considered eligible for inclusion. For those patients expressing willingness to participate, sociodemographic, clinical, and laboratory data were collected at disease onset.
Acute COVID-19 definitionsDiagnosis of COVID-19 infection was established as confirmed or suspected. To confirm a COVID-19 case, a positive nucleic acid amplification test (NAAT) for SARS-CoV-2 in respiratory tract specimens was required. To identify a suspected COVID-19 case as having COVID-19, in the absence of SARS-CoV-2 NAAT positivity, laboratory or imaging findings suggestive of infection and/or positive serology, were required.23,24,26 Cycle threshold (Ct) values (i.e., the number of cycles needed to detect the virus) of the first positive NAAT were collected. Based on the COVID-19 disease severity scale, patients were classified into increasing severity groups, from asymptomatic to critical disease.27 Based on their disease management, patients were also classified as requiring Intensive Care Unit (ICU), hospital-ward, or outpatient-based treatment.23,24
Data collection at follow-up and post-COVID-19 syndrome definitionA twelve-month follow-up phone assessment, lasting between 10 and 30minutes, was carried out by trained nurses by using a standardized questionnaire, pilot-tested among ten patients, investigating specific persistent or emerging symptoms potentially associated with COVID-19. Participants’ narrative responses were treated according to the patient-reported outcomes (PROs)28 and subsequently categorized by four independent physicians into the presence of at least one of the following clinical presentations: dyspnoea, cough, fatigue (asthenia, myalgia), chest pain, anosmia/dysgeusia, headache, rheumatological involvement (back pain, arthralgia), neurological disorders (hypoesthesia, dizziness, hypoacusia), psychiatric/mood disorders (anxiety, sleep disorders, depression), inability to concentrate/brain fog, gastrointestinal disorders (diarrhoea, vomiting, nausea, epigastric pain, constipation and abdominal pain), skin lesions, hair loss, upper respiratory infection involvement (nose cold, sneezing, odynophagia) and ocular involvement (conjunctivitis, visual changes).29 In line with previous evidence,7 in order to be rated as ‘post-COVID’ symptoms, they had to have developed during or after COVID-19, and not to be explained by an alternative diagnosis.23,24
To study the post-COVID-19 mental health distress in a comprehensive perspective,19–22 symptoms of common psychiatric disorders (i.e., depression, anxiety, and insomnia), cognitive impairment (i.e., lack of concentration and focus), and somatic distress (i.e., fatigue) were gathered into a mental health domain.
Data analysisAbsolute values and percentages were reported. Medians and interquartile range (IQR) were calculated, according to the Shapiro–Wilk test establishing whether data were normally or non-normally distributed. Categorical variables between the two time-points (COVID-19 onset, 12-month follow-up) were compared using the McNemar test. Patients were dichotomized depending on whether they presented mental health domain-related post-COVID-19 symptomatology at the time of interview. Multivariable logistic regressions were performed to explore variables associated with post-COVID-19 mental health symptoms, estimating the odds ratios (OR; 95% confidence interval, CI). Variables were selected based on statistical significance in univariable analysis and clinical relevance. Analyses were performed by STATA 17.
EthicsAll study procedures were approved by the local Ethics Committee (CEUR-2020-OS-219, CEUR-2020-OS-205, CEUR-2021-OS-19). Procedures contributing to this work also complied with the ethical standards of the local health authority and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all subjects before data collection.
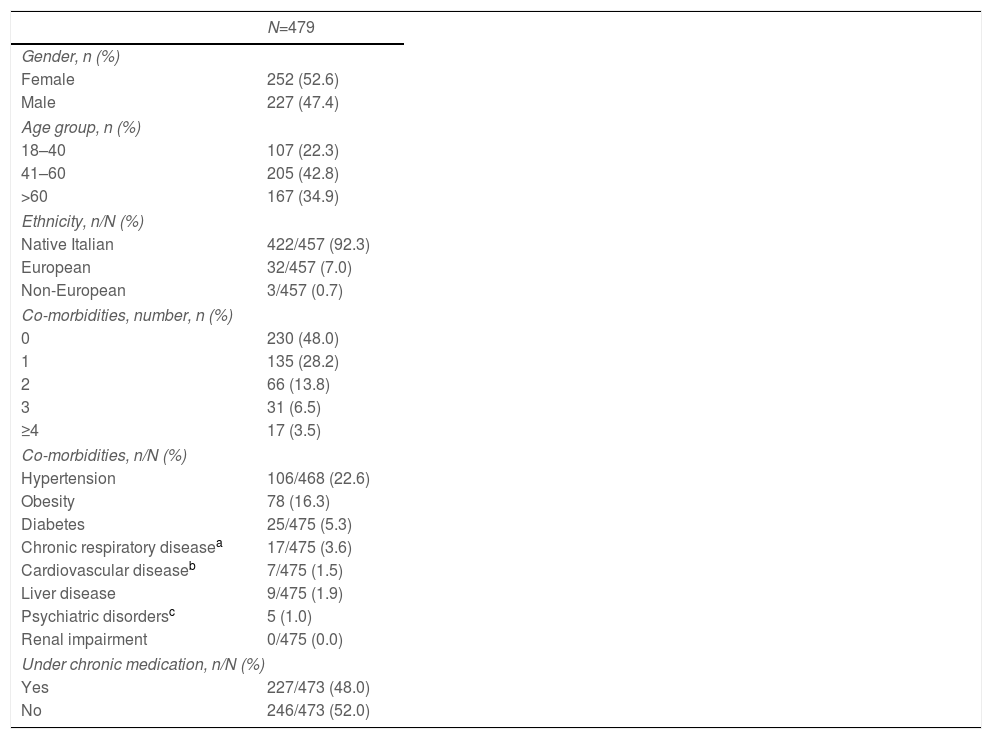
ResultsBaseline socio-demographic and clinical characteristicsData on study sample baseline characteristics have been reported elsewhere.24 Briefly, out of 1.067 patients with a COVID-19 diagnosis screened during the study period, 479 of them completed the 12-month follow-up assessment. The most represented age group was that the middle-aged one (41–60 years, 42.8%), with an overall median age of 54 years (IQR 43–65). A slight preponderance of female patients (52.6%) was observed, and most patients were native Italian (92.3%). About one in two patients presented with a medical comorbidity (52%) and was under chronic medication (48%) (Table 1).
Baseline socio-demographic and clinical characteristics.
| N=479 | |
|---|---|
| Gender, n (%) | |
| Female | 252 (52.6) |
| Male | 227 (47.4) |
| Age group, n (%) | |
| 18–40 | 107 (22.3) |
| 41–60 | 205 (42.8) |
| >60 | 167 (34.9) |
| Ethnicity, n/N (%) | |
| Native Italian | 422/457 (92.3) |
| European | 32/457 (7.0) |
| Non-European | 3/457 (0.7) |
| Co-morbidities, number, n (%) | |
| 0 | 230 (48.0) |
| 1 | 135 (28.2) |
| 2 | 66 (13.8) |
| 3 | 31 (6.5) |
| ≥4 | 17 (3.5) |
| Co-morbidities, n/N (%) | |
| Hypertension | 106/468 (22.6) |
| Obesity | 78 (16.3) |
| Diabetes | 25/475 (5.3) |
| Chronic respiratory diseasea | 17/475 (3.6) |
| Cardiovascular diseaseb | 7/475 (1.5) |
| Liver disease | 9/475 (1.9) |
| Psychiatric disordersc | 5 (1.0) |
| Renal impairment | 0/475 (0.0) |
| Under chronic medication, n/N (%) | |
| Yes | 227/473 (48.0) |
| No | 246/473 (52.0) |
n, number; N, number as a denominator.
Data on study sample acute COVID-19 presentation have been reported elsewhere.24 The large majority of COVID-19 patients were symptomatic (92%), with most of them presenting with mild symptoms of disease (67.7%) and a relatively lower proportion with moderate to critical severity (24.3%). Admission to hospital was required in almost one in three patients (29%), with Intensive Care Unit (ICU) being called in a relatively low proportion of patients (4.4%) and a median in-hospital stay of 7 days (IQR 3–11).
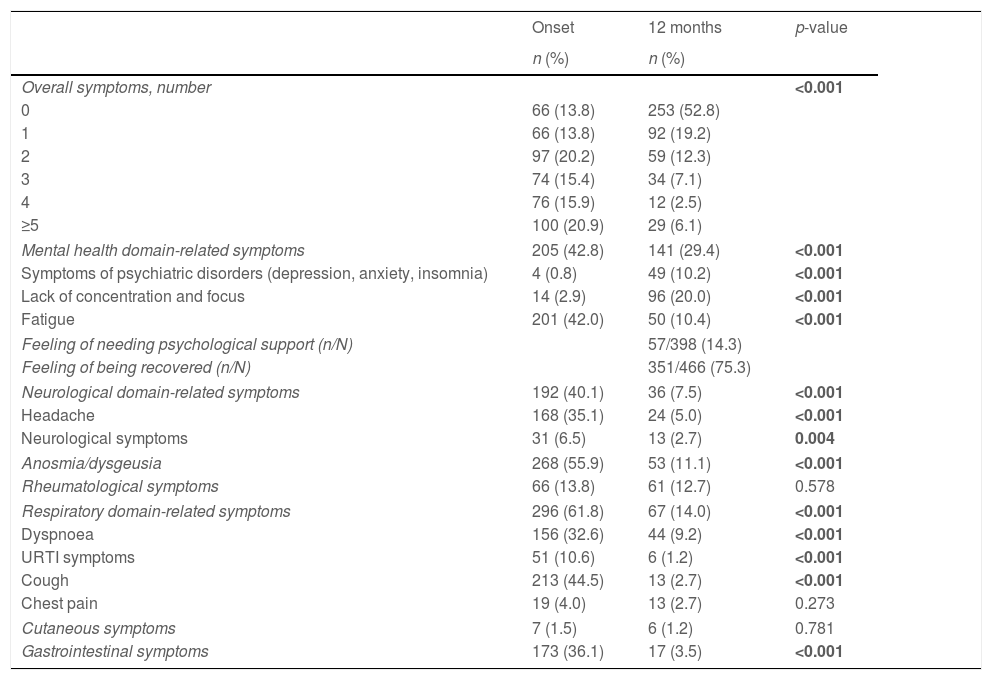
Symptom trajectory over the 12-month follow-up periodThe 12-month follow-up assessment was performed on day 410 (median, IQR 403–417). At that moment, despite the significant COVID-19 related symptom reduction (p<0.001), about half patients were still presenting with at least one symptom (47.2%). Most frequently reported symptoms at follow-up were within the mental health domain (symptoms of psychiatric disorders, 10.2%; lack of concentration and focus, 20%; and fatigue, 10.4%), affecting almost one third of patients (29.4%). While symptoms in most domains, including the neurological, respiratory, and gastrointestinal ones, improved as compared to the baseline assessment (all p<0.001), a significant increase was observed in the symptoms of psychiatric disorders (i.e., anxiety, depression, and insomnia; p<0.001) and lack of concentration and focus (p<0.001) within the mental health domain. Such symptoms were the only ones whose prevalence at follow-up was against the reducing trend (Table 2).
Clinical trajectory over the 12-month follow-up period.
| Onset | 12 months | p-value | |
|---|---|---|---|
| n (%) | n (%) | ||
| Overall symptoms, number | <0.001 | ||
| 0 | 66 (13.8) | 253 (52.8) | |
| 1 | 66 (13.8) | 92 (19.2) | |
| 2 | 97 (20.2) | 59 (12.3) | |
| 3 | 74 (15.4) | 34 (7.1) | |
| 4 | 76 (15.9) | 12 (2.5) | |
| ≥5 | 100 (20.9) | 29 (6.1) | |
| Mental health domain-related symptoms | 205 (42.8) | 141 (29.4) | <0.001 |
| Symptoms of psychiatric disorders (depression, anxiety, insomnia) | 4 (0.8) | 49 (10.2) | <0.001 |
| Lack of concentration and focus | 14 (2.9) | 96 (20.0) | <0.001 |
| Fatigue | 201 (42.0) | 50 (10.4) | <0.001 |
| Feeling of needing psychological support (n/N) | 57/398 (14.3) | ||
| Feeling of being recovered (n/N) | 351/466 (75.3) | ||
| Neurological domain-related symptoms | 192 (40.1) | 36 (7.5) | <0.001 |
| Headache | 168 (35.1) | 24 (5.0) | <0.001 |
| Neurological symptoms | 31 (6.5) | 13 (2.7) | 0.004 |
| Anosmia/dysgeusia | 268 (55.9) | 53 (11.1) | <0.001 |
| Rheumatological symptoms | 66 (13.8) | 61 (12.7) | 0.578 |
| Respiratory domain-related symptoms | 296 (61.8) | 67 (14.0) | <0.001 |
| Dyspnoea | 156 (32.6) | 44 (9.2) | <0.001 |
| URTI symptoms | 51 (10.6) | 6 (1.2) | <0.001 |
| Cough | 213 (44.5) | 13 (2.7) | <0.001 |
| Chest pain | 19 (4.0) | 13 (2.7) | 0.273 |
| Cutaneous symptoms | 7 (1.5) | 6 (1.2) | 0.781 |
| Gastrointestinal symptoms | 173 (36.1) | 17 (3.5) | <0.001 |
URTI, upper respiratory tract infection.
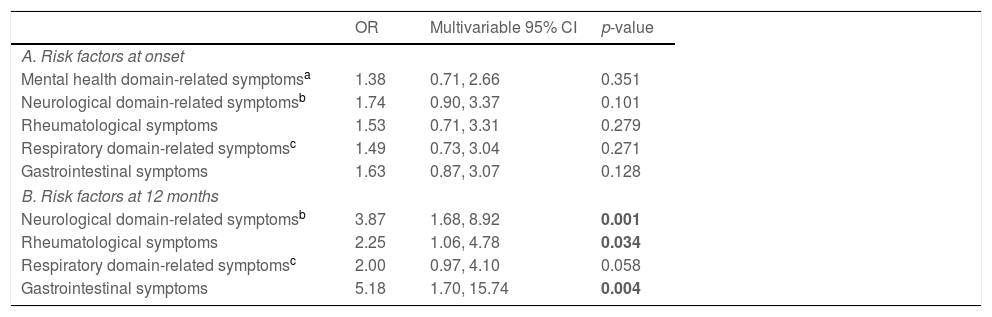
Based on univariate association with the risk of presenting with symptoms of psychiatric disorders at the 12-month follow-up (Supplementary Table 1), variables were added to a multivariate logistic model to analyze the influencing factors of long-term effects of COVID-19 on symptoms of psychiatric disorders. Presence of any symptom profile at onset, including the mental health domain-related ones, failed to reach statistical significance for an association with the development of symptoms of psychiatric disorders one year after contracting COVID-19. Instead, COVID-19 survivors presenting with neurological domain-related (OR=3.87, 95% CI=1.68–8.92, p<0.001), rheumatological (OR=2.25, 95% CI=1.06–4.78, p=0.034), and gastrointestinal (OR=5.18, 95% CI=1.70–15.74, p=0.004) symptoms at follow-up were more likely to suffer from symptoms of psychiatric disorders 12 months post infection. A weak association was also found for symptoms of the respiratory domain reported at follow-up (OR=2.00, 95% CI=0.97–4.10, p=0.058) (Table 3).
Symptoms of psychiatric disorders (depression, anxiety, insomnia) at 12-month follow-up.
| OR | Multivariable 95% CI | p-value | |
|---|---|---|---|
| A. Risk factors at onset | |||
| Mental health domain-related symptomsa | 1.38 | 0.71, 2.66 | 0.351 |
| Neurological domain-related symptomsb | 1.74 | 0.90, 3.37 | 0.101 |
| Rheumatological symptoms | 1.53 | 0.71, 3.31 | 0.279 |
| Respiratory domain-related symptomsc | 1.49 | 0.73, 3.04 | 0.271 |
| Gastrointestinal symptoms | 1.63 | 0.87, 3.07 | 0.128 |
| B. Risk factors at 12 months | |||
| Neurological domain-related symptomsb | 3.87 | 1.68, 8.92 | 0.001 |
| Rheumatological symptoms | 2.25 | 1.06, 4.78 | 0.034 |
| Respiratory domain-related symptomsc | 2.00 | 0.97, 4.10 | 0.058 |
| Gastrointestinal symptoms | 5.18 | 1.70, 15.74 | 0.004 |
Among COVID-19 survivors, a higher risk of symptoms of psychiatric disorders at 12-month follow-up was also observed in healthcare professionals as compared to workers in contact with public and in those reporting poor self-perceived recovery (Supplementary Table 1).
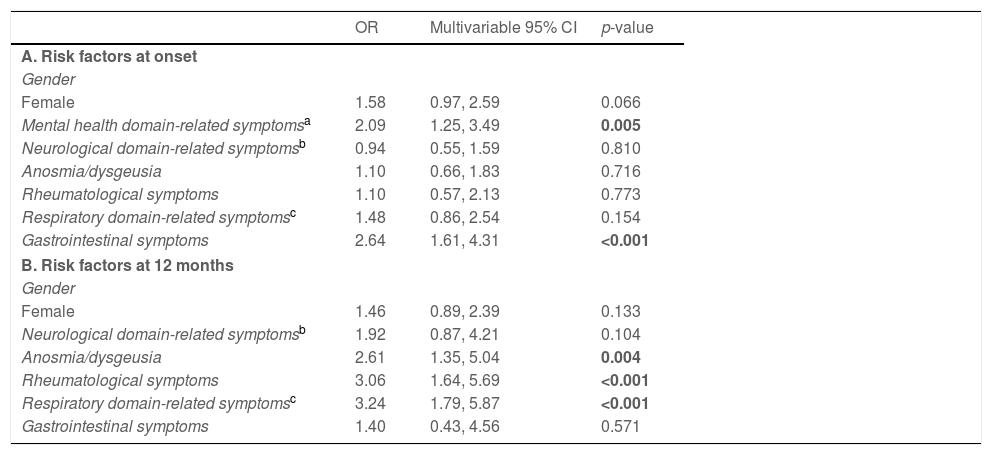
Lack of concentration and focus at 12-month follow-upBased on univariate association with the risk of presenting with lack of concentration and focus at the 12-month follow-up (Supplementary Table 2), variables were added to a multivariate logistic model to analyze the influencing factors of long-term effects of COVID-19 on lack of concentration and focus. Presence of mental health domain-related (OR=2.09, 95% CI=1.25–3.49, p=0.005) and gastrointestinal (OR=2.64, 95% CI=1.61–4.31, p<0.001) symptoms at onset increased the risk of developing lack of concentration and focus one year after contracting COVID-19. A weak association was also found for female gender (OR=1.58, 95% CI=0.97–2.59, p=0.066). Further, COVID-19 survivors presenting with rheumatological (OR=3.06, 95% CI=1.64–5.69, p<0.001) and respiratory domain-related (OR=3.24, 95% CI=1.79–5.87, p<0.001) symptoms as well as anosmia/dysgeusia (OR=2.61, 95% CI=1.35–5.04, p=0.004) at follow-up were more likely to suffer from lack of concentration and focus 12 months post infection (Table 4).
Lack of concentration and focus at 12-month follow-up.
| OR | Multivariable 95% CI | p-value | |
|---|---|---|---|
| A. Risk factors at onset | |||
| Gender | |||
| Female | 1.58 | 0.97, 2.59 | 0.066 |
| Mental health domain-related symptomsa | 2.09 | 1.25, 3.49 | 0.005 |
| Neurological domain-related symptomsb | 0.94 | 0.55, 1.59 | 0.810 |
| Anosmia/dysgeusia | 1.10 | 0.66, 1.83 | 0.716 |
| Rheumatological symptoms | 1.10 | 0.57, 2.13 | 0.773 |
| Respiratory domain-related symptomsc | 1.48 | 0.86, 2.54 | 0.154 |
| Gastrointestinal symptoms | 2.64 | 1.61, 4.31 | <0.001 |
| B. Risk factors at 12 months | |||
| Gender | |||
| Female | 1.46 | 0.89, 2.39 | 0.133 |
| Neurological domain-related symptomsb | 1.92 | 0.87, 4.21 | 0.104 |
| Anosmia/dysgeusia | 2.61 | 1.35, 5.04 | 0.004 |
| Rheumatological symptoms | 3.06 | 1.64, 5.69 | <0.001 |
| Respiratory domain-related symptomsc | 3.24 | 1.79, 5.87 | <0.001 |
| Gastrointestinal symptoms | 1.40 | 0.43, 4.56 | 0.571 |
A higher risk of lack of concentration and focus at 12-month follow-up was also observed in COVID-19 survivors reporting poor self-perceived recovery (Supplementary Table 2).
DiscussionVery recent systematic reviews and meta-analyses of studies investigating mid and long-term neuropsychiatric manifestations in COVID-19 survivors indicate that such symptoms are frequently reported.13,14 However, while generic (e.g., headache) and specific (e.g., anosmia, dysgeusia) neurological features of the disease are very common in the acute phase but generally resolve in a few weeks or months, cognitive and affective psychiatric features such as attentional deficits and anxious-depressive symptoms are reported to increase in prevalence over time. Such peculiar pattern of symptom trajectory has been suggested to indicate that psychiatric symptoms are more likely to develop post-infection than just persist as a residual component of the acute phase.13,14 However, while data are rapidly converging on the evidence of greater psychiatric distress six or more months post infection as compared to the weeks immediately following viral positivity, it is still unclear when and whether to expect such symptoms to plateau and start to reduce. Also, literature is scant of studies addressing risk factors of post-COVID psychiatric syndrome. Thus, efforts are needed to increase knowledge about determinants of post-COVID-19 syndrome, especially in terms of psychiatric manifestations, to support restitutio at integrum and mitigate the risk of potentially irreversible low adjustment, poor quality of life, and decreased overall well-being.
By accessing a prospective design, the current secondary analysis was aimed at investigating the mental health status of patients who have developed COVID-19 disease, focusing on a wide range of symptoms including Common Mental Disorders (CMDs)-related symptoms (i.e., anxiety, depression, and insomnia), cognitive impairment (i.e., lack of concentration and focus), and somatic distress (i.e., fatigue) features, over a 12-month period. Further, the study systematically examined the association between several socio-demographic and clinical characteristics collected at both COVID-19 onset and after 12-month, and mental health outcome at follow-up. Results herein reported support previous evidence of increasing long-term affective and cognitive symptoms in COVID-19 survivors, also confirming that additional symptoms, including anosmia and dysgeusia, are less preponderant in the longer-term, being characteristic of the acute COVID-19 phase. Findings of this study are novel in demonstrating the association between persisting symptoms related to multiple body systems and increasing affective and cognitive symptoms at follow-up as well as between psychiatric symptoms at onset and cognitive symptoms at follow-up. Also, female COVID-19 survivors tend to be at higher risk of presenting with cognitive difficulties in the long-term.
The high prevalence of mental health symptoms 12 months after developing the COVID-19 disease is likely due to both direct (e.g., biological mechanisms) and indirect (e.g., confinement and social isolation) effects. There is evidence of postmortem neuropathological hallmarks in individuals who have suffered from COVID-19,30 consistent with implication of the pathognomonic smell and taste dysfunctions for brain infection through the olfactory epithelium.31 Along with CNS infection, COVID-19 induced systemic inflammation may trigger massive neuroinflammatory responses involving reactive astrogliosis and microglia activation.32 In turn, such increased neuroinflammatory response may result in a disruption of glutamate signalling, provoking new-onset or re-exacerbation of preexisting psychiatric vulnerabilities.33 It is thus not surprisingly the involvement of multisystem symptoms at follow-up and psychiatric symptoms at onset in increasing the risk for psychiatric distress in the long-term. On the other hand, independent evidence indicates that COVID-19-associated dramatic social changes may have elicited strong fear reactions and preoccupations with downstream effects on mental health, in both individuals with pre-existing mental difficulties34 and otherwise healthy people,35 with consequences in terms of higher and more severe mental health-related emergencies since the COVID-19 outbreak.36 Despite not designed to address such an issue, results of the current study do not disconfirm an indirect effect of COVID-19 on mental well-being. In fact, in line with previous research evidence, they suggest a higher risk of psychiatric symptoms in healthcare professionals as compared to other work sectors, possibly as a consequence of the stressful experience of being first-line responders dealing with the pandemic,37 as well as in those reporting poor self-perceived recovery (Supplementary Tables 1 and 2).38
Surprisingly, neither hospitalization status or management (ward vs outpatient clinic) did modify the risk of presenting with post-COVID-19 mental health syndrome (Supplementary Tables 1 and 2). Hospitalized patients have been found to be more likely to present with long-term symptoms that are related to the respiratory tract, including cough, dyspnoea, and chest pain, possibly due to the underlying respiratory conditions such as pneumonia, respiratory distress, and lung damage, increasing the need for COVID-19 management in hospital. Consistent, risk factors for respiratory distress including history of Chronic Pulmonary Obstructive Disease and tobacco use have also been associated with the persistence of respiratory symptoms 6 month after COVID-19 diagnosis.39,40 Instead, such disease severity parameters have not been confirmed as risk predictors of post-COVID-19 psychiatric syndrome by recent meta-analytic evidence,13,14 urging for further investigations to establish a standard severity score that can predict the subsequent development of post-COVID-19 psychiatric syndrome.41
Results from this study must be seen considering some limitations. In the absence of a standardized approach to diagnosis and treatment of post-COVID-19 mental health syndrome,42 this study may have suffered the difficulty to comprehensively account for the complex clinical picture of the condition. In fact, a recent review has suggested that the lack of a unifying paradigm in the field has led to divergent post-COVID-19 syndrome definitional criteria across studies, potentially jeopardizing treatment success of the condition.43 Also, based on the evidence of negative mental health effects of the pandemic outbreak in terms of forced changes in daily routine and habits, social isolation, and fear,34–36 lacking a control group of non-COVID-19 patients, the current study does not allow drawing conclusions whether the observed post-COVID-19 mental health syndrome results from such indirect consequences of the pandemic rather than direct effects of the virus at multiple body levels. Moreover, whether such effects are COVID-19 specific or would have occurred in the context of other medical conditions requiring hospitalization in hospital Infectious Disease Department or Intensive Care Unit (ICU) remains to be tested. Similarly, the current study did not investigate the effects of the variants of concern more recently emerged. Further, despite the potential generalizability of the findings to both inpatient and outpatient services, the investigation was performed in a single recruiting hub, introducing a potential selection bias. Also, assessments were based on patient self-reported symptoms44 which may lack of standardization as compared to objective assessments.45–47 The study did not investigate whether presenting with symptoms of post-COVID-19 psychiatric syndrome may result in specific impairments in function, as emphasized in the updated DSM-5-related disability model.48 Finally, the study did not assess brain function, that may offer important insight into the neurophysiological correlates of post-COVID-19 symptoms.49
In conclusion, findings of this study may have important public health implications, as they underlie the increased need for mental health support in COVID-19 survivors. While the long-term ramifications of COVID-19 in terms of mental distress are still largely unknown, accumulating data begin to suggest that psychiatric disorders may be on a rise. There is a pressing need to better understand the risk factors for poor neuropsychological outcomes as well as best treatment strategies in COVID-19 survivors.
FundingThis research was funded by PRIN 2017 n. 20178S4EK9 – “Innovative statistical methods in biomedical research on biomarkers: from their identification to their use in clinical practice”.
Conflicts of interestMarco Colizzi has been a consultant/advisor to GW Pharma Limited, GW Pharma Italy SRL, and F. Hoffmann-La Roche Limited, outside of this work. Maddalena Peghin reports receiving grants and personal fees from Pfizer, MSD, Menarini and Dia Sorin, outside of this work. Carlo Tascini has received grants in the last two years from Correvio, Biotest, Biomerieux, Gilead, Angelini, MSD, Pfizer, Thermofisher, Zambon, Shionogi, Avir Pharma and Hikma, outside of this work. All the other authors declare no conflict of interest.
The authors would like to thank all clinical and nursing staff who cared for the patients at Udine Infectious Disease Clinic during hospitalization and ambulatory management. The authors are grateful to all patients for their collaboration.