Switching antipsychotics is common in the clinical practice setting and is associated with potential clinically relevant complications. An expert group selected by Spanish Society of Psychiatry and the Spanish Society of Biological Psychiatry has reviewed the evidence provided by randomised clinical trials and other relevant information to reach consensus recommendations for switching antipsychotics. In this article, we will review all the information that has led to those recommendations and which includes: indications and contraindications for switching antipsychotics, pharmacological issues, switching strategies, switching antipsychotics due to efficacy problems, switching antipsychotics due to tolerability issues (including extrapyramidal symptoms and tardive dyskinesia, weight gain, metabolic disorders, hyperprolactinemia, sexual dysfunction, persistent sedation, and QT prolongation), switching antipsychotics due to lack of treatment compliance, and switching antipsychotics in patients with bipolar disorders.
El cambio de antipsicóticos es un hecho frecuente en la práctica clínica y está sujeto a potenciales complicaciones clínicamente relevantes. Un grupo de expertos seleccionados por la Sociedad Española de Psiquiatría y la Sociedad Española de Psiquiatría Biológica ha revisado y discutido las pruebas provenientes de los ensayos clínicos y otros artículos relevantes para llegar a unas recomendaciones de consenso sobre el cambio de antipsicóticos. En este artículo se revisa toda la información que ha dado lugar a esas recomendaciones y que incluye: indicaciones y contraindicaciones del cambio de antipsicóticos, aspectos farmacológicos, estrategias de cambio, el cambio por motivos de eficacia, el cambio por motivos de tolerabilidad (incluyendo los síntomas extrapiramidales y la discinesia tardía, el aumento de peso, los trastornos metabólicos, la hiperprolactinemia, la disfunción sexual, la sedación persistente y la prolongación del QT), el cambio por problemas de cumplimiento y el cambio de antipsicóticos en el trastorno bipolar.
The need to switch antipsychotics during treatment of a patient with a psychiatric disorder is a common occurrence in daily clinical practice—whether because of efficacy or tolerability limitations or due to the interindividual variability in response to these drugs. There are difficulties and potential complications inherent to the switching process that are clinically significant and could be prevented or at least minimized with proper clinical management.
Despite its prevalence and importance, however, we have very little evidence available from clinical trials to guide us in the process of switching antipsychotics. Many aspects of this process—switching due to efficacy issues with the previous antipsychotic or due to the appearance of metabolic disorders, for example—have scarcely been evaluated in clinical trials. Moreover, the clinical trials that do evaluate aspects of switching antipsychotics, either primarily or secondarily, are fraught with limitations:
- -
Generally speaking, they are clinical trials with a small sample size.
- -
The vast majority of these studies were conducted in adult patients with schizophrenia, whereas these drugs are commonly used for other indications and in other populations—a situation that could also influence the results of the switch.
- -
In existing clinical trials, the comparator chosen seldom yielded good information, the comparison being limited to switching antipsychotics vs maintaining the previous therapy, which favours the drugs being studied.
Several good, narrative reviews on switching antipsychotics have been published1–9 as well as 2 systematic reviews: one on the switching technique10 and the other on how switching antipsychotics impacts weight and metabolic parameters.11 However, this article's authors are of the opinion that none of these reviews adequately covers the multiple aspects to be taken into account in caring for a patient who requires a change of antipsychotic; these include not only the pharmacokinetic and pharmacodynamic aspects of the drugs involved but also the clinical features of the patient undergoing this therapeutic intervention.
In view of all of the above, the Sociedad Española de Psiquiatría (SEP) and the Sociedad Española de Psiquiatría Biológica (SEPB) thought it vital to clinical practice that a document can be drawn up reviewing all those aspects and providing practical recommendations, as specific as possible, as to when and how to switch antipsychotics. In this article, we explain how these recommendations were developed and present a summary of the information on which they were based. At the end, we attempt to synthesize it all in a “decalogue” of practical recommendations.
MethodologyA small group of experts in psychiatry, psychopharmacology, and pharmacoepidemiology established the working methodology for developing these recommendations by consensus. First, through a bibliographic search in Medline up to January of 2010 (subsequently updated to November of 2010), the literature on clinical trials or subanalyses of clinical trials on switching antipsychotics was reviewed. The details of this search and the articles included in the review will be the objective of a different article.
The information derived from the search was reviewed and discussed by this group of experts at a second meeting. They developed an initial table of contents that this review should cover and a first draft of the recommendations for switching antipsychotics. This group of experts refined the draft in 2 subsequent teleconferences. Once the group of experts had reached a consensus on the recommendations; these and the information on which they were based were reviewed and discussed in a meeting with a wider group of 34 psychiatrists (the RECAP group), who also were selected by the SEP and the SEPB. The suggestions and modifications proposed at this meeting were used to develop a second draft of the recommendations; this was distributed to all members of the group for comments, which were then incorporated into new versions. The draft recommendations finally agreed upon by this wider group of psychiatrists are the one presented in paragraph Decalogue for Switching Antipsychotics of this review. In the remaining sections of this review, an attempt is made to summarize the information discussed in the above-mentioned meetings on which these recommendations were based.
Frequency of and reasons for switching antipsychoticsClinical trial results suggest that, over a period of 1 year, approximately 30% of patients with schizophrenia switch antipsychotics.12,13 These data are backed by data from observational studies.14,15 It appears that, when antipsychotics must be switched, the risk is greater for patients taking conventional antipsychotics than for patients being treated with second-generation antipsychotics.12,14 The frequency of switching increases over time. Analysing medication records for a random sampling of 400 outpatients with schizophrenia, Covell et al. found that the incidence of antipsychotic switching was 21% at 6 months, 33% at 1 year, and 45% at 2 years14; in addition, 40% of those who had switched antipsychotics at least once switched antipsychotics a second time within the first 2 years they were followed.14 As brought to light by these results, this situation is not only quite common in clinical practice but also quite limited in its success—all the more so if we take into account, as one study suggests that patients quite often go back to taking the previous antipsychotic.16
Generally speaking, the reasons for switching—even when the switch is to a depot antipsychotic—are a lack of efficacy as well as tolerability issues and, to a lesser extent, compliance issues.12,17,18 Naturally, these reasons would depend on what antipsychotic the patient was taking and the one he/she would be taking after the switch. In a study on clinical practice, it was observed that, when patients were switched to a conventional antipsychotic, it was primarily because of efficacy issues (especially if switching from an atypical antipsychotic), while if patients were switched to an atypical, it was primarily because of tolerability issues (especially if switching from a conventional antipsychotic).17As for more specific reasons under lack of efficacy or poor tolerability, the information available is much more limited. In a post hoc analysis of a pragmatic clinical trial in which 191 out of 648 patients with schizophrenia required a switch and were randomised to receive olanzapine, risperidone, or a conventional antipsychotic, Nyhuis et al. identified the following predictors of antipsychotic switching: no antipsychotic therapy in the year prior to the randomisation, pre-existing depression, being female, no concomitant substance abuse disorder, exacerbation of akathisia, and exacerbation of depression/anxiety symptoms.13 The following side effects are those most annoying to patients or most clinically significant to the psychiatrist: extrapyramidal symptoms, weight gain, cognitive disorders, sedation, disorders related to hyperprolactinemia, sexual dysfunction, depression, agranulocytosis, and metabolic disorders.19–21
Side effects are a prominent reason for switching in the childhood-youth population where such effects are more marked than in adults.22 In this population, the side effects stemming from hyperprolactinemia and weight gain are particularly significant.22,23
Indications and contraindications for switchingAs stated above, there are various possible indications for switching antipsychotics; those according to McEvoy et al.24 are summarized in Table 1.
Possible indications for switching antipsychotics.
| Efficacy issues |
| • Persistent negative or positive symptoms |
| • Relapse despite good compliance |
| • To improve level of functioning |
| Tolerability issues |
| • Extrapyramidal side effects not responsive to antiparkinsonian therapy |
| • Tardive dyskinesia |
| • Persistent cognitive problems |
| • Persistent sedation |
| • Metabolic disorders |
| • Increased QT |
| • Other annoying side effects such as sexual dysfunction, for example |
| Other issues |
| • Therapeutic non-compliance |
| • Preference of patient or family members |
Based on McEvoy et al.24
As was also stated, however, besides the problems that may arise with switching antipsychotics, quite often switching does not produce the expected results, and a significant number of patients switch back to the previous antipsychotic. In a post hoc analysis of Phase 1 of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), a pragmatic clinical trial on patients with schizophrenia, the study's primary result—treatment interruption for any cause—was analysed comparing (1) patients who were randomised to olanzapine and risperidone, meaning their antipsychotic was switched to one of these two drugs, and (2) patients for whom randomisation meant continuing the antipsychotic they had been taking before.25 The objective was to evaluate the effectiveness of switching antipsychotics compared to continuing the treatment, and the results showed that the incidence of treatment interruption was lower in patients for whom randomisation meant continuing olanzapine or risperidone therapy than in patients who had changed to one of these two antipsychotics.25 This being a post hoc and, therefore, exploratory analysis, it is conditioned by the study's overall results, which favoured olanzapine.26 However, along with the results mentioned above showing the high incidence of a second antipsychotic switch,16 these results suggest that the benefits of the previous antipsychotic should be maximized prior to making the decision to switch antipsychotics.
Besides this precaution, there are some general recommendations that could be thought of as possible contraindications to switching antipsychotics (Table 2) and should be considered before making this decision.1,24
Possible contraindications to switching antipsychotics.
| • Patients who have just recovered from an acute psychotic episode and are taking the medication that was successful in treating this episode |
| - It is recommended that, having recovered, the patient be stable for 3–6 months before switching. |
| - This is the period of time used in the majority of clinical trials on switching APS. |
| • Patients receiving and compliant with a depot APS who have a history of non-compliance with oral APS, especially if this non-compliance is recent |
| • Excellent response to previous APS therapy |
| • Patient or family rejects the recommendation |
| • Outpatients for whom an exacerbation in the near future would mean an unacceptable risk for self-harm behaviour or behaviour that is a danger to others |
| • Inpatients whose psychotic process requires immediate stabilization because they are a danger to themselves or to others |
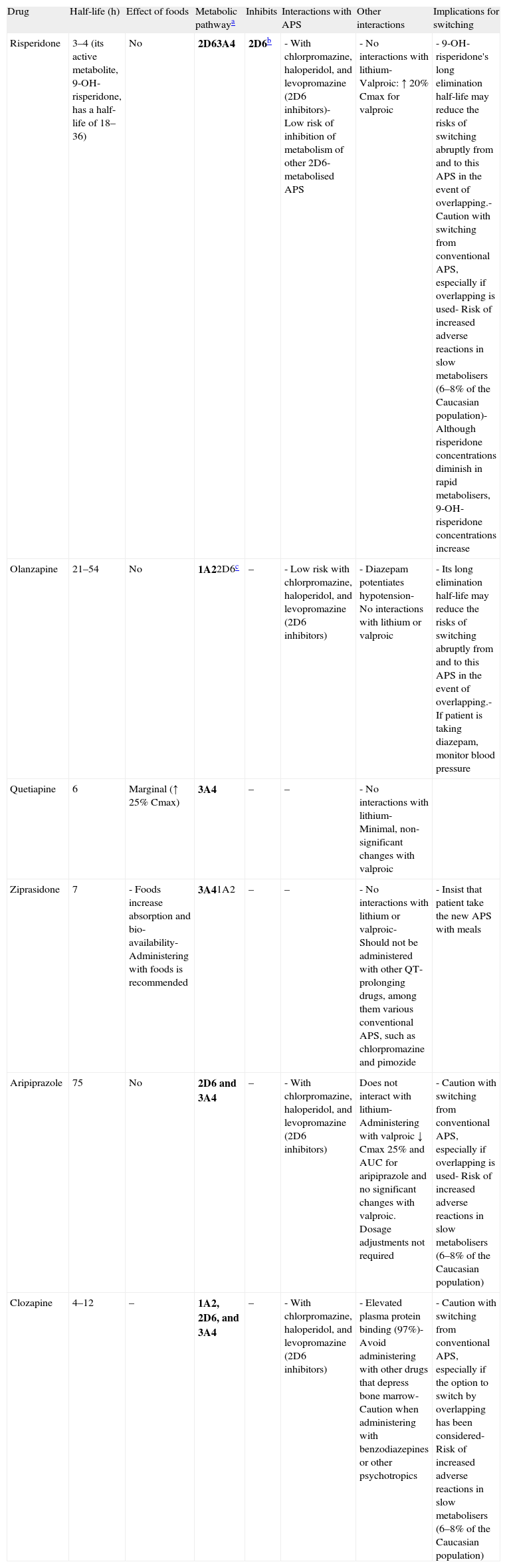
The pharmacokinetic and pharmacodynamic characteristics of the antipsychotics involved are very important in terms of establishing individual recommendations for switching between the different antipsychotics. Among the most important pharmacokinetic characteristics to keep in mind are the elimination half-life and the metabolism of these drugs. Antipsychotics that have a long elimination half-life—risperidone (due to the long elimination half-life of its active metabolite), olanzapine, or aripiprazole, for example—may present fewer problems when discontinued, if an abrupt or immediate change of antipsychotic is required; however, they may present a greater risk of pharmacodynamic interactions with other antipsychotics that are similar in terms of their affinity for certain receptors (see below). The antipsychotics are metabolised mainly in the liver by the cytochrome p450 system, so they could interact with other drugs that behave as inhibitors of their primary metabolic pathway or are themselves its substrates because an interaction due to competitive inhibition may occur. The isoenzyme 2D6 pathway is particularly important in the case of the antipsychotics because there are many psycho-active drugs that behave as inhibitors of that metabolic pathway; antipsychotics (chlorpromazine, haloperidol, and levopromazine, for example), antidepressants (paroxetine, duloxetine, and fluoxetine, for example), and a large portion of the antidepressants and antipsychotics (among them chlorpromazine, haloperidol, perphenazine, aripiprazole, clozapine, risperidone, sertindole and, to a lesser degree, olanzapine) are also metabolised by that pathway. Other antipsychotics are metabolised primarily by the 3A4 pathway (quetiapine and ziprasidone, for example) or also use this pathway (risperidone, aripiprazole, and clozapine, for example) or the isoenzyme 1A2 pathway (clozapine and olanzapine, for example). The only atypical antipsychotic that would not be expected to present significant interaction problems at the cytochrome p450 level is paliperidone. This drug has demonstrated in vitro that it does not substantially inhibit isoenzymes CYP1A2, CYP2A6, CYP2C8/9/10, CYP2D6, CYP2E1, CYP3A4, and CYP3A5. Paliperidone is also not a CYP1A2, CYP2A6, CYP2C9, or CYP2C19 substrate, and it appears that CYP2D6 and CYP3A4 are only minimally involved in its metabolism. Table 3 gives a summary of the key pharmacokinetic characteristics for the most commonly used atypical antipsychotics and the implications these characteristics have in switching antipsychotics. The reader may find more detailed information on the pharmacokinetics of these drugs in a review on the subject27,28 and in the technical data sheets for the different antipsychotics.
Pharmacokinetic aspects in switching between antipsychotics.
| Drug | Half-life (h) | Effect of foods | Metabolic pathwaya | Inhibits | Interactions with APS | Other interactions | Implications for switching |
| Risperidone | 3–4 (its active metabolite, 9-OH-risperidone, has a half-life of 18–36) | No | 2D63A4 | 2D6b | - With chlorpromazine, haloperidol, and levopromazine (2D6 inhibitors)- Low risk of inhibition of metabolism of other 2D6-metabolised APS | - No interactions with lithium- Valproic: ↑ 20% Cmax for valproic | - 9-OH-risperidone's long elimination half-life may reduce the risks of switching abruptly from and to this APS in the event of overlapping.- Caution with switching from conventional APS, especially if overlapping is used- Risk of increased adverse reactions in slow metabolisers (6–8% of the Caucasian population)- Although risperidone concentrations diminish in rapid metabolisers, 9-OH-risperidone concentrations increase |
| Olanzapine | 21–54 | No | 1A22D6c | – | - Low risk with chlorpromazine, haloperidol, and levopromazine (2D6 inhibitors) | - Diazepam potentiates hypotension- No interactions with lithium or valproic | - Its long elimination half-life may reduce the risks of switching abruptly from and to this APS in the event of overlapping.- If patient is taking diazepam, monitor blood pressure |
| Quetiapine | 6 | Marginal (↑ 25% Cmax) | 3A4 | – | – | - No interactions with lithium- Minimal, non-significant changes with valproic | |
| Ziprasidone | 7 | - Foods increase absorption and bio-availability- Administering with foods is recommended | 3A41A2 | – | – | - No interactions with lithium or valproic- Should not be administered with other QT-prolonging drugs, among them various conventional APS, such as chlorpromazine and pimozide | - Insist that patient take the new APS with meals |
| Aripiprazole | 75 | No | 2D6 and 3A4 | – | - With chlorpromazine, haloperidol, and levopromazine (2D6 inhibitors) | Does not interact with lithium- Administering with valproic ↓ Cmax 25% and AUC for aripiprazole and no significant changes with valproic. Dosage adjustments not required | - Caution with switching from conventional APS, especially if overlapping is used- Risk of increased adverse reactions in slow metabolisers (6–8% of the Caucasian population) |
| Clozapine | 4–12 | – | 1A2, 2D6, and 3A4 | – | - With chlorpromazine, haloperidol, and levopromazine (2D6 inhibitors) | - Elevated plasma protein binding (97%)- Avoid administering with other drugs that depress bone marrow- Caution when administering with benzodiazepines or other psychotropics | - Caution with switching from conventional APS, especially if the option to switch by overlapping has been considered- Risk of increased adverse reactions in slow metabolisers (6–8% of the Caucasian population) |
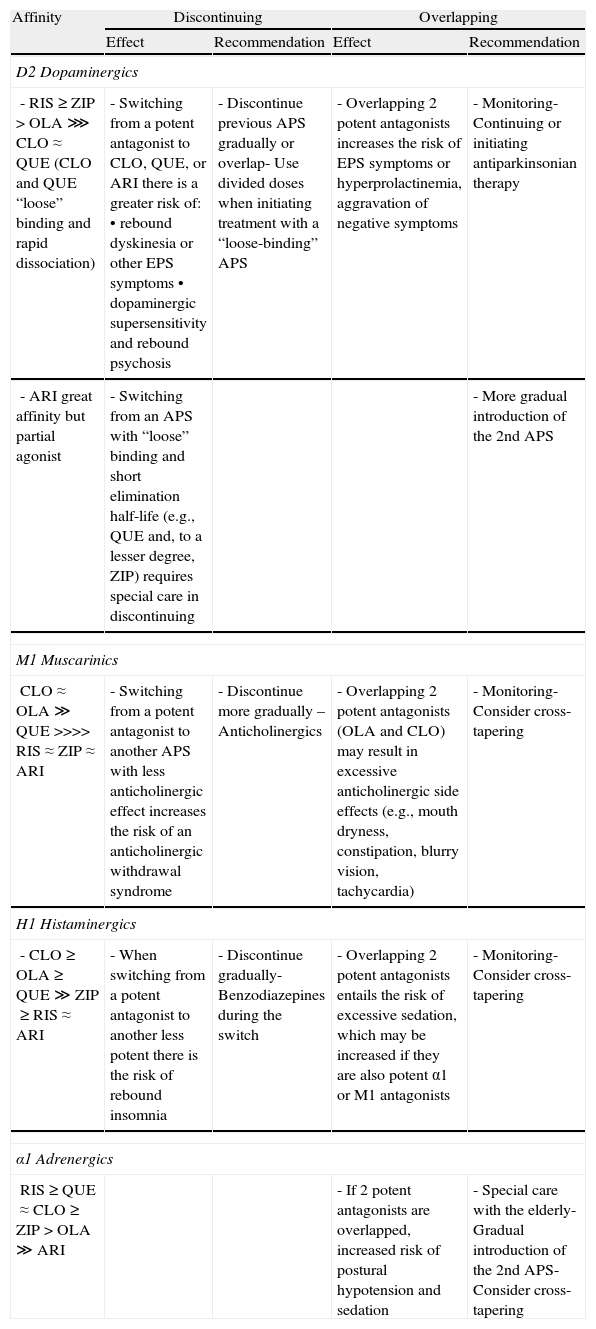
From a pharmacodynamic standpoint, the affinity of the different antipsychotics for D2 dopaminergic, muscarinic cholinergic, histaminergic, and adrenergic receptors29–31 may have implications for the switch in terms of both discontinuing the previous antipsychotic and introducing the new one,32 especially if the withdrawal is abrupt or the introduction involves what has been referred to as overlapping. In Table 4 we present a summary of the impact these pharmacodynamic aspects have on the treatment switching process for the most commonly used atypical antipsychotics.
Pharmacodynamic aspects in switching between antipsychotics.
| Affinity | Discontinuing | Overlapping | ||
| Effect | Recommendation | Effect | Recommendation | |
| D2 Dopaminergics | ||||
| - RIS≥ZIP>OLA⋙CLO≈QUE (CLO and QUE “loose” binding and rapid dissociation) | - Switching from a potent antagonist to CLO, QUE, or ARI there is a greater risk of:• rebound dyskinesia or other EPS symptoms• dopaminergic supersensitivity and rebound psychosis | - Discontinue previous APS gradually or overlap- Use divided doses when initiating treatment with a “loose-binding” APS | - Overlapping 2 potent antagonists increases the risk of EPS symptoms or hyperprolactinemia, aggravation of negative symptoms | - Monitoring- Continuing or initiating antiparkinsonian therapy |
| - ARI great affinity but partial agonist | - Switching from an APS with “loose” binding and short elimination half-life (e.g., QUE and, to a lesser degree, ZIP) requires special care in discontinuing | - More gradual introduction of the 2nd APS | ||
| M1 Muscarinics | ||||
| CLO≈OLA≫QUE>>>>RIS≈ZIP≈ARI | - Switching from a potent antagonist to another APS with less anticholinergic effect increases the risk of an anticholinergic withdrawal syndrome | - Discontinue more gradually – Anticholinergics | - Overlapping 2 potent antagonists (OLA and CLO) may result in excessive anticholinergic side effects (e.g., mouth dryness, constipation, blurry vision, tachycardia) | - Monitoring- Consider cross-tapering |
| H1 Histaminergics | ||||
| - CLO≥OLA≥QUE≫ZIP≥RIS≈ARI | - When switching from a potent antagonist to another less potent there is the risk of rebound insomnia | - Discontinue gradually- Benzodiazepines during the switch | - Overlapping 2 potent antagonists entails the risk of excessive sedation, which may be increased if they are also potent α1 or M1 antagonists | - Monitoring- Consider cross-tapering |
| α1 Adrenergics | ||||
| RIS≥QUE≈CLO≥ZIP>OLA≫ARI | - If 2 potent antagonists are overlapped, increased risk of postural hypotension and sedation | - Special care with the elderly- Gradual introduction of the 2nd APS- Consider cross-tapering | ||
APS: antipsychotic; ARI: aripiprazole; CLO: clozapine; EPS: extrapyramidals; OLA: olanzapine; QUE: quetiapine; RIS: risperidone; ZIP: ziprasidone.
Despite the difficulties of using clozapine because of the monitoring required, this drug continues to play an essential role in treating schizophrenia because it is superior to other antipsychotics in resistant schizophrenia33–36 and because of its role with patients at risk for suicidal behaviour.37,38 Given that clozapine use is often limited to those populations, there must be a very sound basis for replacing it with another antipsychotic. It also appears that clozapine may cause more problems when discontinued than other antipsychotics, such as increased risk of a withdrawal syndrome, a psychotic exacerbation, extrapyramidal symptoms, and even interference afterwards with the response to a different antipsychotic.1,39–41
The recommendations for switching from clozapine include:
- -
If possible, clozapine should be discontinued gradually by reducing the dosage 50mg/week.1 Other authors recommend that it could be reduced even more gradually (25mg/week) and that a benzodiazepine could be added.42
- -
The anticholinergics may prevent or alleviate clozapine withdrawal symptoms.43
- -
Some case reports suggest that, if agranulocytosis is the reason for switching from clozapine, it is preferable to switch to an antipsychotic with a different receptor affinity profile—avoid switching to olanzapine or quetiapine, for example.4 If the reason for switching is not agranulocytosis, it is preferable to switch to an antipsychotic with a similar receptor affinity profile.
- -
Haematological controls should continue until at least 3–4 weeks after the clozapine was suspended.
- -
Overlapping should be used with caution in a switch involving subsequent treatment with chlorpromazine, haloperidol, or levopromazine because these drugs inhibit isoenzyme 2D6, one of clozapine's metabolic pathways.
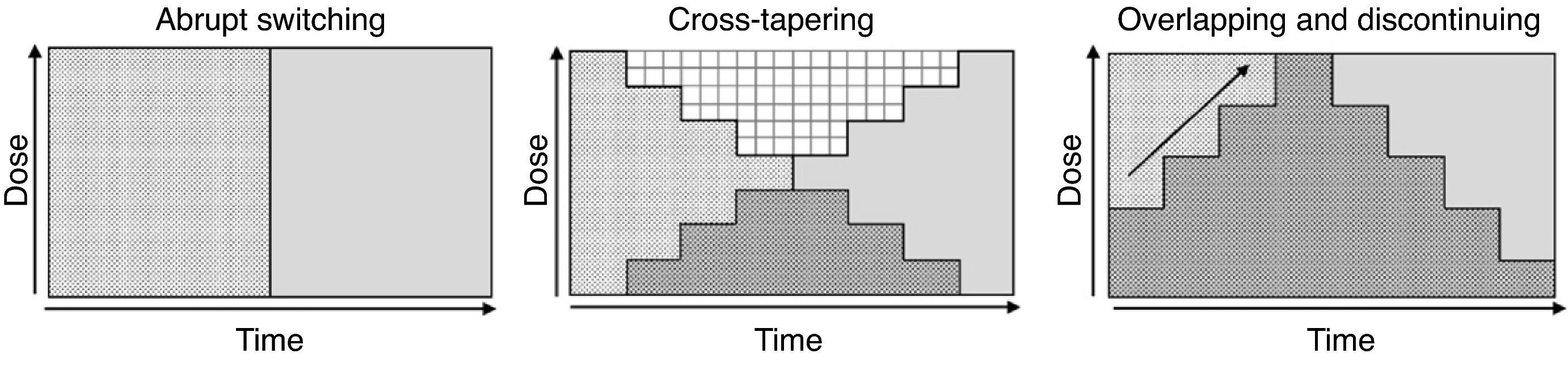
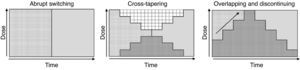
Although various switching strategy categorizations have been proposed,44–46 from this point forward, we will refer to 3 general types (Fig. 1) that, in our opinion, synthesize the proposed strategies. These are also the ones found in some reviews2 and the ones that have been evaluated in an expert consensus24:
- -
Abrupt switching; discontinuing the previous antipsychotic immediately and initiating the new antipsychotic at customary dosages.
- -
Cross-tapering; initiating the gradual increase in dosage of the new antipsychotic while, at the same time, gradually discontinuing the previous antipsychotic.
- -
Overlapping and discontinuing; maintaining the dosage of the previous antipsychotic and, at the same time, gradually initiating the new antipsychotic; once an effective dosage of the new antipsychotic is reached, the previous antipsychotic is gradually discontinued.
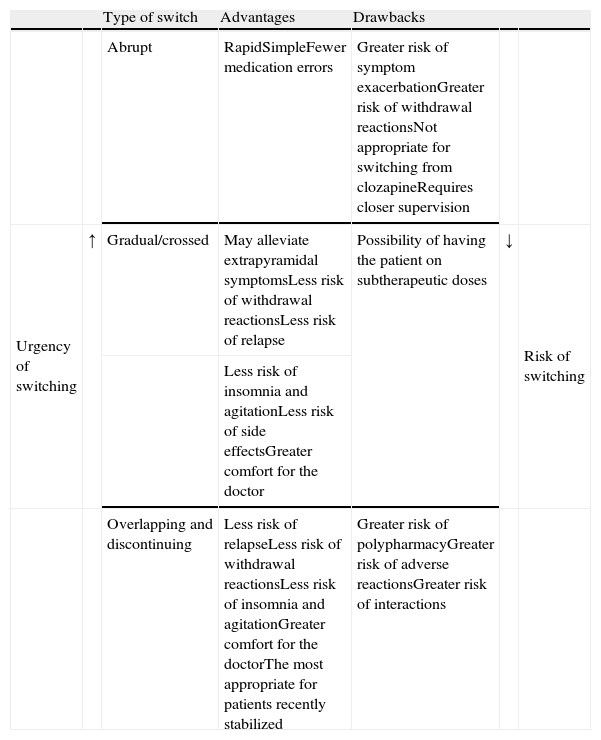
We do not have reliable data on which of the strategies is most commonly used. However, even though experts favour the more conservative overlapping or cross-tapering strategies,24 some data suggest that abrupt switching is still quite commonly used in clinical practice.47–49 One or the other type of switching strategy should be chosen on a case-by-case basis and will depend, among other factors, on (1) the above-mentioned pharmacological aspects, (2) the reason for the switch (for example, the appearance of a serious adverse effect, such as agranulocytosis, requiring that the antipsychotic be promptly discontinued), and (3) the therapeutic context (for example, abrupt switching of antipsychotics is more feasible with inpatients than with outpatients). Table 5 shows the advantages and drawbacks for each switching strategy. However, randomised clinical trials have been conducted to evaluate different switching strategies with some antipsychotics; we will describe these trials in greater detail in the next section.
Advantages and drawbacks of the different types of switching strategies.
| Type of switch | Advantages | Drawbacks | ||||
| Abrupt | RapidSimpleFewer medication errors | Greater risk of symptom exacerbationGreater risk of withdrawal reactionsNot appropriate for switching from clozapineRequires closer supervision | ||||
| Urgency of switching | ↑ | Gradual/crossed | May alleviate extrapyramidal symptomsLess risk of withdrawal reactionsLess risk of relapse | Possibility of having the patient on subtherapeutic doses | ↓ | Risk of switching |
| Less risk of insomnia and agitationLess risk of side effectsGreater comfort for the doctor | ||||||
| Overlapping and discontinuing | Less risk of relapseLess risk of withdrawal reactionsLess risk of insomnia and agitationGreater comfort for the doctorThe most appropriate for patients recently stabilized | Greater risk of polypharmacyGreater risk of adverse reactionsGreater risk of interactions |
Based on Refs. [1,2,4,24].
Eight clinical trials evaluating strategies for switching to different antipsychotics have been published: 1 with risperidone,50 2 with olanzapine,51,52 2 with ziprasidone,53,54 and 3 with aripiprazole.55–57 All were short-term trials conducted in patients with schizophrenia.
Besides these trials, there is the meta-analysis Remington et al. conducted on 4 of them,51–53,55 concluding that the gradual strategies show no advantage over abrupt switching.10 Regardless of the results of this meta-analysis and the conclusions of any of the individual studies, in our opinion, the results of the 8 trials published to date suggest that:
- -
Switching antipsychotics with immediate replacement of the previous antipsychotic is generally associated with a higher incidence of abandonment for any cause.50,55–57
- -
Whether an immediate or more gradual initiation of the new antipsychotic is indicated depends on the drug. Thus, it appears that, with olanzapine, results would be better if it were introduced immediately at therapeutic dosages51 while, in contrast, aripiprazole would require a more gradual initiation.55
- -
Generally speaking, it appears that the overlapping strategy is the one that affords better results overall in that there are fewer treatment interruptions and better control of the symptomatology.50,51,54,56,57
Specific recommendations for switching to certain antipsychotics have been published; the interested reader may consult these for switching to risperidone, both the standard formulation58,59 and the long-acting formulation,60,61 olanzapine,62,63 quetiapine,64,65 ziprasidone,66–68 aripiprazole,69–71 sertindole,72 amisulpride,47,73 and clozapine.62 These are interesting articles, of course, but this article's authors are of the opinion that none of them covers the wide array of nuances that must be taken into account when switching antipsychotics.
Switching antipsychotics in different clinical situationsNext, we will review the results available from clinical trials that evaluated switching antipsychotics as a therapeutic strategy in particular situations: lack of efficacy, tolerability issues, or therapeutic non-compliance with another antipsychotic. This information is evaluated in the context of the most important data we have available on efficacy and tolerability for the different antipsychotics. A word of warning: the fact that we are discussing these clinical situations in the context of the switch does not necessarily mean that we believe switching to be the best or only therapeutic option in that situation.
Switching because of limited efficacy of the previous antipsychoticThe most important information on switching antipsychotics in patients with inadequate response to the previous antipsychotic comes from 2 pragmatic clinical trials, basically: the Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS), which included 2 trials, and the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), which also included various study phases.
In CUtLASS, 227 patients who had an inadequate response to or intolerance of the previous antipsychotic were randomised to receive either a first-generation or a second-generation antipsychotic; in both cases, the psychiatrist chose the individual antipsychotic within each class.35 After 1 year of treatment, the patients treated with first-generation antipsychotics showed no significant differences in terms of quality of life (the study's primary variable), efficacy, or tolerability, compared to those treated with second-generation antipsychotics.35
In Phase 2T of CATIE, 444 patients who had interrupted the antipsychotic treatment from Phase 1 of the study for different reasons, including lack of efficacy, received olanzapine, quetiapine, risperidone, or ziprasidone on a randomised basis.74 In this trial, olanzapine and risperidone showed efficacy superior to that of quetiapine and ziprasidone, as evaluated by time until treatment interruption.74 When efficacy is evaluated as risk of treatment interruption, some meta-analyses on patients with schizophrenia would support these results for olanzapine75 and, to a lesser degree, for risperidone.76
The results of 3 other studies77–79 complete the information on switching to these second-generation antipsychotics in patients with inadequate response to other antipsychotics; in our opinion; however, these studies are of much less importance than CUtLASS and CATIE. In 2 clinical trials with a small sample size, olanzapine and risperidone showed similar results.77,78 In addition, with patients not responding after 2 weeks of risperidone therapy, and using a more specific design, Kinon et al. found a slightly superior but significant improvement in psychotic symptomatology in patients randomised to switch to olanzapine compared to those randomised to continue risperidone.79 Consistent with the first 2 clinical trials on switching mentioned, however, in a meta-analysis of clinical trials directly comparing olanzapine and risperidone in patients with schizophrenia, both drugs showed similar efficacy in terms of their impact on mental status.80 In this review, we have not included randomised clinical trials on patients with refractory schizophrenia that, as part of their design, incorporate a period of treatment with a different antipsychotic to confirm the lack of response prior to randomising the treatments they wish to compare81–86 and that, to a greater or lesser extent, attempt to copy the original design proposed by Kane et al.33
The results for clozapine in patients who switch due to a lack of efficacy also come from the CUtLASS 2 and CATIE 2E studies. In these 2 trials, clozapine showed efficacy superior to that of other atypical antipsychotics as the comparison group, evaluated by the change in psychotic symptomatology87; it was also superior to that of quetiapine and risperidone but not to that of olanzapine, as evaluated by time until treatment interruption.36
It is important to highlight some key aspects of clozapine. Despite the fact that, for switching in patients with efficacy issues, these results are good and also consistent with the above-mentioned comparative trials on patients with resistant schizophrenia,33,34 this is an option that is under-utilised in clinical practice.88,89 In Phase 2E of CATIE, only 27% of the patients who had interrupted the Phase 1 treatment for lack of efficacy were willing to participate.36 Remembering that, in patients who have not responded to 2 antipsychotic therapy attempts, the response to a third attempt is vastly inferior to that seen with the first 2 attempts78 and that, thanks to haematological controls, treatment-induced agranulocytosis and its associated deaths are quite rare,89 it seems prudent not to delay the use of clozapine in this patient subgoup.89 However, clozapine's superior efficacy compared to other atypical antipsychotics does not extend to patient subgroups other than those who have shown a lack of efficacy.90
Except for a study in patients with schizophrenia and depression,91 we have no randomised trials that evaluate switching antipsychotics in situations where there are specific efficacy issues, such as the persistence of positive or negative symptoms or where depressive symptoms are present; therefore, if switching is thought to be appropriate in a situation of this type, our choice should be guided by the already known profiles of these drugs. In the only randomised clinical trial available, in patients with schizophrenia and a major or minor depressive episode, there was a significantly greater reduction in depressive symptomatology associated with switching to amisulpride than with continuing risperidone.91 The results of a meta-analysis of clinical trials on patients with schizophrenia indicate that not only amisulpride but also aripiprazole, clozapine, olanzapine, and quetiapine have an effect on depressive symptoms that is significantly superior to that of the conventional antipsychotics.92 In that meta-analysis, ziprasidone and risperidone showed no superiority over haloperidol for depressive symptoms.92 However, ziprasidone has an attractive pharmacological profile for treating affective disorders and has demonstrated its efficacy in treating patients with bipolar I disorder and manic or mixed episodes.93 In bipolar depression, quetiapine and the olanzapine–fluoxetine combination are the ones that offer better results.94
Although the atypical antipsychotics were presented as promising treatment for the negative symptoms of schizophrenia, their effect on these symptoms is modest, at best—especially on primary negative symptoms.95 There is no basis, then, on which any specific recommendation can be made regarding switching antipsychotics in this situation. The atypical antipsychotic that has best demonstrated its efficacy in primary or predominating negative symptoms is amisulpride at low dosages (50–300mg/day), and it has been shown to be superior to placebo in several clinical trials.96
Switching because of tolerability issues with the previous antipsychoticSwitching because of extrapyramidal symptoms or tardive dyskinesiaSwitching as a therapeutic strategy in the presence of extrapyramidal symptoms associated with antipsychotic therapy has been evaluated in 3 randomised clinical trials.97–99 Cortese et al. found that switching to quetiapine significantly improved Parkinsonism and akathisia but not tardive dyskinesia symptoms.97 The other 2 trials evaluated switching to olanzapine or risperidone in patients with extrapyramidal symptoms98 or tardive dyskinesia99; both drugs improved Parkinsonism and tardive dyskinesia but not akathisia, and no differences were found between the 2 drugs in terms of their impact on the extrapyramidal symptoms evaluated. Lastly, a 24-week trial compared the effect of switching to risperidone or olanzapine in 60 patients with schizophrenia and diagnosed with neuroleptic-induced tardive dyskinesia.99 At the end of treatment, there were no differences between the 2 drugs in terms of improving the tardive dyskinesia, as evaluated by the score on the Abnormal Involuntary Movements Scale (AIMS).99
The results of the 2 studies with olanzapine and risperidone98–100—in particular, the fact that (1) there was no difference in results between these drugs, (2) both drugs cause dose-dependent extrapyramidal side effects,101,102 and (3) that risperidone has more extrapyramidal side effects than various atypicals76 and olanzapine more than quetiapine75—suggest that switching to these 2 antipsychotics as a therapeutic strategy in a patient with extrapyramidal symptoms should be reserved for very select cases. On the other hand, the improvement observed in the clinical trial on switching with quetiapine97 is consistent with its favourable profile in terms of undesirable extrapyramidal effects103 and supports its use in these cases. In addition, the results of 2 meta-analyses indicate that the use of antiparkinsonian medication is associated significantly less with quetiapine than with risperidone, olanzapine, and ziprasidone.104,105 In the trial on switching, however, quetiapine did not significantly improve the score for tardive dyskinesia,97 but this could be due to the study's small sample size. Another switching option in patients with tardive dyskinesia would be clozapine, which has proven to be effective,106 despite the lack of randomised clinical trials in this area, and some authors consider it as the treatment of choice in these cases.107
Switching because of weight gainIn 2 randomised clinical trials of 16 and 26 weeks, respectively, patients who switched to aripiprazole lost 1.8kg,108 and those who switched to quetiapine lost 0.82kg,109 compared to the patients in both studies who, continuing to take olanzapine, gained weight. In a CATIE subanalysis, patients who were taking olanzapine prior to randomisation and continued this therapy after randomisation were compared with patients who were randomised to switching to quetiapine, risperidone, perphenazine, or ziprasidone.110 During the 18-month follow-up period, patients who continued on olanzapine were found to have had a significant weight gain; however, except for those on risperidone, who had no change in weight, patients on the other treatments lost weight, those taking ziprasidone or perphenazine being the ones who lost the most weight.110
Despite the results of this CATIE analysis, there are data suggesting that quetiapine and perphenazine are associated with clinically significant weight gain, even though it is less than the gain with olanzapine or clozapine111; on the other hand, among the conventional antipsychotics, fluphenazine is the one that appears to have less impact on weight.111 Among the atypical antipsychotics, ziprasidone and aripiprazole are the ones that appear to have less of a tendency to cause weight gain both in adult patients with schizophrenia111,112 or bipolar disorder113 and in children and adolescents with psychotic disorders or other disorders.114
Switching because of metabolic disordersAlthough a clinical trial is underway to evaluate the effect of switching to certain antipsychotics in patients with metabolic disorders, we do not yet have data available from randomised clinical trials that have evaluated this aspect of switching antipsychotics as a primary objective.
In the trial we were commenting on above—switching to aripiprazole in overweight patients treated with olanzapine—total cholesterol was reduced more in patients who received aripiprazole than in patients who continued taking olanzapine, and HDL cholesterol was increased in the patients taking aripiprazole but reduced in patients who continued taking olanzapine.108 On the other hand, in the study also previously mentioned on switching to quetiapine in overweight or obese patients, there were no significant differences between patients who switched to quetiapine and patients who remained on olanzapine in terms of changes in fasting blood sugar, glycosylated haemoglobin, or blood lipids.109 In Phase 2T of CATIE, also discussed above, patients who switched to olanzapine had a marked increase in total cholesterol and triglycerides while, in patients treated with ziprasidone and, to a lesser degree, those treated with risperidone, reductions were seen in these biochemical parameters.74 Again, these results with ziprasidone and risperidone are consistent with existing knowledge of the impact antipsychotics have on the lipid profile.115–117 However, according to a recent meta-analysis on patients with schizophrenia, risperidone appears to be associated with a greater increase in cholesterol than aripiprazole or ziprasidone.76
Regarding the presence of diabetes, the consensus of various American associations, including those for diabetes and psychiatry, is that ziprasidone and aripiprazole are the drugs associated with a lower risk of this metabolic complication.118 Although there is some conflicting information, the results from Phase 126 and Phase 274 of CATIE and from the latest observational studies appear to confirm this good profile for these 2 drugs. On the other hand, the results of a meta-analysis indicate that the conventional antipsychotics are associated with a lower risk of diabetes than the atypicals119; in this regard, in a certain study, a high-potency antipsychotic like haloperidol was the one associated with a lower risk of diabetes. Compared to risperidone and olanzapine, amisulpride has less of a tendency to induce weight gain—one of the mechanisms for the appearance of diabetes—and to increase glucose levels.120,121
Although specific clinical trials are lacking, these data suggest that, when there is a need to switch antipsychotics in a patient with metabolic disorders, the most appropriate options are amisulpride, aripiprazole, ziprasidone, and haloperidol.
Switching because of hyperprolactinemia or related problemsIn a randomised clinical trial on patients with schizophrenia and hyperprolactinemia under treatment with risperidone or conventional antipsychotics, switching to olanzapine was accompanied by a return to normal prolactin levels 4 months later in 90% of patients, compared to none of the patients who continued taking the previous antipsychotic.122 Olanzapine is believed to have very little tendency to cause hyperprolactinemia,123 but short-term clinical trials on adults showed that prolactin increased from normal to elevated levels in 30% of patients treated with olanzapine, compared to 10% of those receiving placebo101; in adolescents, these figures were increased to 47% and 7% for olanzapine and placebo, respectively. According to a meta-analysis, also, olanzapine increased prolactin somewhat more than aripiprazole, clozapine, and quetiapine.75 These data suggest that olanzapine should be considered a secondary option for switching antipsychotics in patients with hyperprolactinemia.
Quetiapine has not been associated with significant or sustained increases in prolactin levels within its dosage range.123 In clinical trials on adults, the incidence of increased prolactin up to clinically significant levels was 3.6% with quetiapine and 2.6% with placebo; in children and adolescents, these figures were 13.4% and 4%, respectively, in males, and 8.7% and 0%, respectively, in females.103 In a secondary analysis of a randomised clinical trial in which patients who had not responded to fluphenazine were randomised to receive quetiapine (600mg/day) or haloperidol (20mg/day), patients who switched to quetiapine had a significantly greater reduction in prolactin levels than patients who switched to haloperidol (−729.5mU/L vs −23.5mU/L, P<0.001).124 According to a meta-analysis of quetiapine compared to other atypical antipsychotics, this drug induced less of a prolactin increase than risperidone or ziprasidone.104
Aripiprazole has a neutral or even diminishing effect on prolactin levels over its entire dosage range,123 which is why the possible appearance of hyperprolactinemia is not among the warnings and precautions on its technical data sheet. Although there have been no randomised clinical trials conducted that evaluate switching to aripiprazole in patients with hyperprolactinemia, in a post hoc analysis125 of one of the trials on strategies for switching to aripiprazole,55 a significant reduction in prolactin levels was observed with switching to aripiprazole in patients coming off olanzapine, whose baseline prolactin levels were within normal limits, as well as in patients coming off risperidone, whose baseline prolactin levels were above normal limits.126 There are fewer side effects related to hyperprolactinemia or increased prolactin with aripiprazole than with olanzapine or risperidone, according to the results of a meta-analysis.127
Switching because of sexual dysfunctionIn the only randomised clinical trial conducted to date, patients with sexual dysfunction associated with risperidone therapy were switched to quetiapine; 6 weeks later, there were no significant differences between the patients who switched and those who continued taking risperidone in terms of sexual function, as evaluated by the Arizona Sexual Experience (ASEX) scale.128
These discouraging results aside, quetiapine and olanzapine are the antipsychotics that have been most robust and consistent—that is, in randomised clinical trials—in showing a lower incidence of sexual dysfunction.129–131 Serretti and Chiesa, conducting a meta-analysis of the impact of antipsychotics on sexual function, combined the results of observational studies (comparative or non-comparative) with those of randomised clinical trials.132 It is important to remember that there are major limitations inherent in combining the results of such a heterogeneous series of studies; nonetheless, these authors found that quetiapine, ziprasidone, and aripiprazole were the drugs associated with a lower frequency of sexual dysfunction, both overall and in most dimensions.132 Therefore, quetiapine, olanzapine, ziprasidone, and aripiprazole may be considered the best options for switching antipsychotics in patients with sexual dysfunction.
Switching because of other side effectsAlthough persistent sedation is an extremely common problem, there have been no randomised clinical trials conducted on switching antipsychotics because of this problem. If switching is the option chosen in these cases, an antipsychotic with a less sedating profile may be considered, such as amisulpride, aripiprazole, risperidone, or ziprasidone.
If switching antipsychotics is necessary because of QT prolongation, aripiprazole may be a reasonable option because it does not appear to be associated with this side effect.69
Switching because of therapeutic non-complianceIn patients with therapeutic non-compliance, the need may arise to switch to a long-acting antipsychotic. Among the atypical antipsychotics, as of December 2010, long-acting risperidone is available in Spain, and long-acting olanzapine will soon be available.
Specific recommendations have been made for switching to long-acting risperidone (risperidone-LA), which are summarized below60,61,133:
- -
Most patients receiving depot conventionals may be switched to risperidone-LA straight away.
- -
If patients have come off a non-depot antipsychotic, including the standard risperidone formulation, this drug should be continued for 3 weeks after initiating risperidone-LA therapy to ensure that the main release phase of risperidone from the injection site has begun. As an alternative, if the oral antipsychotic was not risperidone, other authors suggest first switching to oral risperidone and then to risperidone-LA.134
- -
If the previous antipsychotic was sedating (chlorpromazine, thioridazine, quetiapine, for example), consideration may be given to adding a hypnotic during the initial phases of the treatment and only for a short period of time.
- -
If patient is receiving anticholinergic medication, this medication should be continued until the first dose of risperidone-LA subsequent to interruption of the previous or supplementary antipsychotic. If it was a depot antipsychotic, however, continue the anticholinergic medication for 3–4 months to reduce the risk of dystonic reactions.
- -
In patients who have not taken oral risperidone previously, check for hypersensitivity with a single test dose of oral risperidone prior to initiating risperidone-LA therapy.
- -
The initial dose of risperidone-LA is 25mg/2 weeks, and the maximum dose is 50mg/2 weeks.
- -
The dose should be adjusted no more often than once every 4 weeks. A more rapid titration does not achieve a more rapid response.
Injectable, slow-release olanzapine (olanzapine-LA) has been approved by European health department officials, and its arrival in Spain appears to be imminent. According to its technical data sheet, the following recommendations for its use should be kept in mind135:
- -
Patients should remain under the supervision of properly qualified staff at a healthcare centre for at least 3h after each injection so that signs and symptoms of an olanzapine overdose may be detected.
- -
Before olanzapine-LA therapy is initiated, patients should first be treated with oral olanzapine to determine its tolerability and their response.
- -
The results of a post hoc analysis of a clinical trial with olanzapine-LA suggest that, when switching to this antipsychotic, there is no need to supplement with oral olanzapine.136
- -
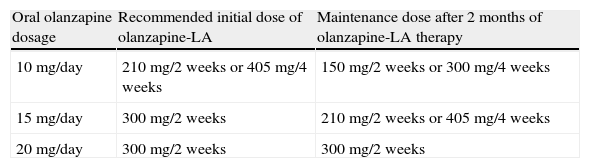
The recommended initial and maintenance doses equivalent to those for oral olanzapine are given in Table 6.
Table 6.Recommended dosage scheme between oral olanzapine and olanzapine-LA.
Oral olanzapine dosage Recommended initial dose of olanzapine-LA Maintenance dose after 2 months of olanzapine-LA therapy 10mg/day 210mg/2 weeks or 405mg/4 weeks 150mg/2 weeks or 300mg/4 weeks 15mg/day 300mg/2 weeks 210mg/2 weeks or 405mg/4 weeks 20mg/day 300mg/2 weeks 300mg/2 weeks Ref. [136].
- -
Olanzapine-LA should not be used in patients who are elderly or have renal insufficiency unless an effective and well-tolerated dosing regimen for oral olanzapine has been established. For these patients, a lower initial dose (150mg every 4 weeks) should be considered.
- -
A dosage reduction should be considered when more than 1 factor is present that could trigger a slowing of the metabolism (female sex, geriatric age, no tobacco habit). Increasing the dosage, if indicated, should be done with caution in these patients.
- -
Because the pamoate salt of olanzapine dissolves slowly to facilitate its steady slow release, which is not complete until approximately 6–8 months after the last injection, a doctor's supervision is required when switching to another antipsychotic drug considered medically appropriate—especially during the first 2 months after interrupting the olanzapine-LA therapy.
There are very few studies on switching antipsychotics in bipolar disorder, so, as a complement to the information reviewed above on pharmacokinetics, pharmacodynamics, and studies on switching antipsychotics in schizophrenia, the following special considerations are offered:
- •
Bipolar patients often receive an antipsychotic in conjunction with mood stabilizers and even with antidepressants. When switching antipsychotics, the potential pharmacokinetic and pharmacodynamic interactions with these drugs must be taken into account.
- •
Bipolar patients are more sensitive than schizophrenic patients to some of the antipsychotics’ side effects (extrapyramidal effects, for example).
- •
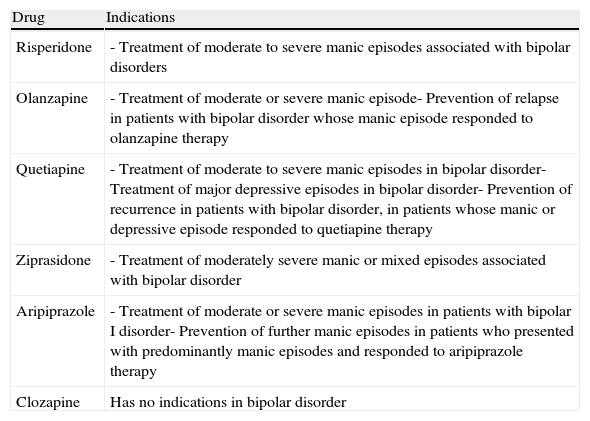
In Spain, the various atypical antipsychotics differ from each other in terms of their indications in bipolar disorder (Table 7).
Table 7.Indications in Spain for the different atypical antipsychotics in bipolar disorder (as of December 2010).
Drug Indications Risperidone - Treatment of moderate to severe manic episodes associated with bipolar disorders Olanzapine - Treatment of moderate or severe manic episode- Prevention of relapse in patients with bipolar disorder whose manic episode responded to olanzapine therapy Quetiapine - Treatment of moderate to severe manic episodes in bipolar disorder- Treatment of major depressive episodes in bipolar disorder- Prevention of recurrence in patients with bipolar disorder, in patients whose manic or depressive episode responded to quetiapine therapy Ziprasidone - Treatment of moderately severe manic or mixed episodes associated with bipolar disorder Aripiprazole - Treatment of moderate or severe manic episodes in patients with bipolar I disorder- Prevention of further manic episodes in patients who presented with predominantly manic episodes and responded to aripiprazole therapy Clozapine Has no indications in bipolar disorder - •
The antipsychotics have a different efficacy and tolerability profile, depending on the disease phase. When switching antipsychotics, the choice of drug should be influenced by the index episode and the drug's profile.
- -
Mania: anti-manic effectiveness
- -
Depression: antidepressant efficacy
- -
Maintenance: long-term efficacy profile according to predominant polarity and safety profile
- -
Based on the authors’ opinion and the consensus of a wide group of psychiatrists with extensive clinical experience, the 10 recommendations below represent a summary of the main conclusions that may be drawn from all the foregoing information. It is important to point out again that these recommendations for specific clinical situations do not necessarily mean that this group of experts believes switching antipsychotics to be the best or only therapeutic option. It is also important to bear in mind that the order in which the different antipsychotics are listed in these recommendations is simply alphabetical and does not in any way imply a preference for one antipsychotic over others. Lastly, the fact that an antipsychotic is named as an option for switching in a particular situation does not mean that this antipsychotic can be used in any given patient; as stated previously, pharmacokinetic and pharmacodynamic characteristics as well as the clinical features of the case may mean that its use is ill-advised.
- (1)
Prior to switching antipsychotics, the patient's clinical situation should be carefully evaluated to determine (1) the indications for switching, (2) the prospects for other therapeutic alternatives, including optimisation of the previous treatment (compliance, dose adjustment, potentiation, and evaluation of the treatment time, among others), and (3) the potential risks, depending on the patient's physical health status and possible pharmacological interactions.
- (2)
The patient should be properly informed of the potential benefits and risks of switching; it is recommended that the family and other professionals involved in the patient's care be informed, as applicable, including the family doctor. The patient must be followed more closely during the switch.
- (3)
There is no evidence that one switching strategy is better than any other, so the switch should be tailored to the patient's clinical situation, the possible implications for his/her quality of life, the characteristics of the new antipsychotic, and the risks that stem from continuing the side effects of the previous antipsychotic. It is generally recommended that an abrupt switch be avoided and that the antipsychotic be switched slowly (4–8 weeks). This group of experts believes that, in most cases, the most advantageous switching strategy is overlapping the new antipsychotic until therapeutic dosages are reached and then gradually and completing discontinuing the previous antipsychotic.
- (4)
The need to continue the patient's concomitant medication (anticholinergics, antidepressants, anti-epileptics, for example) should be evaluated; in general, however, these treatments should not be interrupted until the switch to the new antipsychotic has been successfully completed.
- (5)
If antipsychotics are being switched because of a lack of efficacy, there is no sufficient evidence for recommending a first-line antipsychotic, except for clozapine. In patients who have responded and are stabilized on clozapine therapy, antipsychotics should be switched only if there is a very solid clinical indication.
- (6)
The limited evidence available and clinical experience suggest that, when faced with certain efficacy issues, switching to certain antipsychotics is useful:
- (a)
Positive symptoms: haloperidol, olanzapine, risperidone
- (b)
Negative symptoms: some analyses support the direct effect of different atypical antipsychotics. However, the evidence is not consistent enough to enable a recommendation to be made
- (c)
Depressive symptoms: amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, ziprasidone
- (a)
- (7)
The limited evidence available and clinical experience suggest that, in the face of certain tolerability issues, switching to certain antipsychotics is helpful:
- (a)
Extrapyramidal side effects, excluding tardive dyskinesia: quetiapine
- (b)
Tardive dyskinesia: clozapine, quetiapine
- (c)
Weight gain: amisulpride, aripiprazole, perphenazine, ziprasidone
- (d)
Lipid disorders: amisulpride, aripiprazole, ziprasidone
- (e)
Hyperprolactinemia: aripiprazole, quetiapine
- (f)
Sexual dysfunction: aripiprazole, olanzapine, quetiapine, ziprasidone
- (g)
Hyperglycaemia/diabetes: amisulpride, aripiprazole, haloperidol, ziprasidone
- (h)
Persistent sedation: amisulpride, aripiprazole, risperidone, or ziprasidone
- (i)
QT prolongation: aripiprazole
- (a)
- (8)
In patients with therapeutic non-compliance, in addition to psychosocial measures, switching to a long-acting antipsychotic is recommended.
- (9)
The guidelines given for switching antipsychotics in adults are generally valid for children/adolescents. However, there are certain peculiarities that should be kept in mind:
- (a)
As of November 2010, the antipsychotics approved in Spain and their indications for use in children and adolescents are:
- •
Aripiprazole – patients with schizophrenia starting at 15 years of age
- •
Clozapine – resistant schizophrenia starting at 16 years of age
- •
Risperidone – behaviour disorder in mental retardation and autism spectrum disorder starting at 5 years of age
- •
Ziprasidone – Mania starting at 10 years of age
- •
- (b)
The patient as well as the legal guardians—who must give consent—should be properly informed of the potential risks and benefits of the switch.
- (c)
There is even less evidence than in adults to ensure that it is useful to switch antipsychotics for efficacy reasons.
- (d)
Regarding switching because of side effects, remember that children are especially vulnerable.
- (a)
- (10)
In patients with bipolar disorder, it is important to remember that they are particularly susceptible to certain side effects and to be aware of the potential for interactions with stabilizer and antidepressant drugs as well as the antipsychotic's differential profile with respect to mania and depression.
Dr. Bernardo has received research funds and/or has participated as consultant and/or speaker for activities organized by the following companies: Adamed, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Mylan, Pfizer, Roche, and Rovi.
Dr. Vieta has received research funds and/or has participated as consultant and/or speaker for activities organized by the following companies: Almirall, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Forest Research Institute, Geodon Richter, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Johnson & Johnson, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Qualigen, Sanofi-Aventis, Servier, Shering-Plough, Solvay, Takeda, United Biosource Corporation, and Wyeth.
Dr. Saiz-Ruiz has participated as consultant and/or speaker for activities organized by Lilly, GlaxoSmithKline, Lundbeck, Janssen, Servier, and Pfizer, and has received research funds from Lilly, Astra-Zeneca, Bristol-Myers, and Wyeth.
Dr. Rico-Villademoros has rendered consultancy services to the following companies: Adamed, Almirall, AstraZeneca, Bristol-Myers Squibb, Pfizer, Roche, Rovi, and Servier.
Dr. Álamo has participated as consultant and/or speaker for activities related to the field of antipsychotics organized by Janssen-Cilag, Bristol Myers Squib, Otsuka, and Pfizer.
Dr. Bobes has received research funds and/or has participated as consultant and/or speaker for activities organized by the following companies: Adamed, Almirall, AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Glaxo-Smith-Kline, Janssen-Cilag, Lundbeck, Merck, Novartis, Organon, Otsuka, Pfizer, Pierre-Fabre, Sanofi-Aventis, Servier, Shering-Plough, and Wyeth.
The Sociedad Española de Psiquiatría (SEP), the Sociedad Española de Psiquiatría Biológica (SEPB), and the authors of this article would like to thank Pfizer Laboratories for supporting this publication through an unconditional grant. No Pfizer employees participated in any of the meetings or teleconferences in which these recommendations were drawn up, nor have any Pfizer employees reviewed this article or made any comments on or suggestions for its content.
Luis Agüera (Madrid)
Celso Arango (Madrid)
Jose Luis Ayuso Mateos (Madrid)
Enrique Baca García (Madrid)
Inmaculada Baeza Pertegaz (Barcelona)
Antonio Benabarre (Barcelona)
Antonio Bulbena (Barcelona)
Manuel Camacho (Sevilla)
Fernando Cañas (Madrid)
Josefina Castro (Barcelona)
Benedicto Crespo (Santander)
Iñaki Eguíluz (Bilbao)
Manuel Franco (Zamora)
Paz García-Portilla (Oviedo)
Mauro García Toro (Palma de Mallorca)
Juan Gibert (Cádiz)
José Manuel Goikolea (Barcelona)
Ana González Pinto (Vitoria)
Manuel Gurpegui (Granada)
Miguel Gutiérrez (Vitoria)
José María Haro (Barcelona)
Miguel Ángel Jiménez Arriero (Madrid)
Fermín Mayoral (Málaga)
José Manuel Montes (Madrid)
José Manuel Olivares (Vigo)
Víctor Pérez-Solá (Barcelona)
Pedro Pozo (Murcia)
Miguel Roca (Palma de Mallorca)
Luis Rojo (Valencia)
Julio Sanjuán (Valencia)
Francisco Vidal (A Coruña)
Manuel Martín (Pamplona)
Julio Vallejo (Barcelona)
Carmen Díaz Sastre (Madrid)
Mikel Urretavizcaya (Barcelona)
Please cite this article as: Bernardo, M, et al. Recomendaciones para el cambio de antipsicóticos. Posicionamiento de la Sociedad Española de Psiquiatría y Sociedad Española de Psiquiatría Biológica. Rev Psiquiatr Salud Ment (Barc.). 2011;4:150–68. doi:10.1016/j.rpsm.2011.07.003.
Members of the RECAP Group are listed at the end of the article.