Apathy is a negative symptom of schizophrenia and is associated with poor real world functioning. Therefore, it is important to have validated psychometric instruments to assess this symptom. This is the first study to validate the Spanish adaptation of the self-rated version of the Apathy Assessment Scale (AES-S) in patients with schizophrenia.
Materials and methodsNaturalistic, cross-sectional, validation study in 104 patients with schizophrenia evaluated using the following scales: Clinical Global Impression-Severity (CGI-S), Personal and Social Performance (PSP), Clinical Assessment Interview for Negative Symptoms (CAINS), Self-report of Negative Symptoms (SNS), Motivation and Pleasure Scale-Self-Report (MAP-SR), Calgary Depression Scale for Schizophrenia (CDSS), and Apathy Evaluation Scale-self-rated version (AES-S).
ResultsReliability: Internal consistency (Cronbach's alpha) was 0.908. Convergent validity: The Pearson correlation coefficient between AES-S and CAINS-MAP total scores was −0.483 (p<0.001). For SNS, total and avolition subscale scores were −0.803 and −0.639 (p<0.001), respectively. With the MAP-SR, the correlation coefficient was −0.727 (p<0.001). Divergent validity: The Pearson correlation coefficient between AES-S and PSP total scores was 0.504 (p<0.001). Furthermore, with the CDSS, the correlation coefficient was −0.431 (p<0.001). Discriminant validity: The AES-S discriminated between different levels of illness severity according to CGI-S scores. Factor analysis: A three-component solution explained 57.32% of the variance. Pearson correlations between coefficients were 1–2=0.265, 1–3=0.464, and 2–3=0.060.
ConclusionThe Spanish AES-S is a reliable and valid instrument for assessing apathy in Spanish patients with schizophrenia. It seems to be appropriate for use in everyday clinical practice as a means of monitoring apathy in these patients.
La apatía es un síntoma negativo de la esquizofrenia, y está asociada a un mal funcionamiento del mundo real. Por tanto, es importante disponer de instrumentos psicométricos validados para valorar este síntoma. Este es el primer estudio que valida la adaptación al español de la versión auto-evaluada de la escala de evaluación de la apatía (AES-S) en pacientes esquizofrénicos.
Material y métodosEstudio naturalista, transversal y de validación realizado en 104 pacientes esquizofrénicos evaluados utilizando las escalas siguientes: Clinical Global Impression-Severity (CGI-S), Personal and Social Performance (PSP), Clinical Assessment Interview for Negative Symptoms (CAINS), Self-report of Negative Symptoms (SNS), Motivation and Pleasure Scale-Self-Report (MAP-SR), Calgary Depression Scale for Schizophrenia (CDSS) y Apathy Evaluation Scale-self-rated version (AES-S).
ResultadosFiabilidad: La consistencia interna (alfa de Cronbach) fue de 0,908. Validez convergente: El coeficiente de correlación de Pearson entre las puntuaciones totales de AES-S y CAINS-MAP fue de −0,483 (p<0,001). Para SNS, las puntaciones totales y de la subescala de abulia fueron de −0,803 y −0,639 (p<0,001), respectivamente. En cuanto a la escala MAP-SR, el coeficiente de correlación fue de −0,727 (p<0,001). Validez divergente: El coeficiente de correlación de Pearson entre las puntuaciones totales de AES-S y PSP fue de 0,504 (p<0,001). Además, en la escala CDSS, el coeficiente de correlación fue de −0,431 (p<0,001). Validez discriminante: La escala AES-S discriminó entre los diferentes niveles de gravedad de la enfermedad, conforme a las puntuaciones CGI-S. Análisis factorial: Una solución de tres componentes explicó el 57,32% de la varianza. Las correlaciones de Pearson entre los coeficientes fueron de 1-2=0,265, 1-3=0,464 y 2-3=0,060.
ConclusiónLa versión española de AES-S es un instrumento fiable y válido para valorar la apatía en los pacientes esquizofrénicos españoles, que parece adecuado para utilizar en la práctica clínica diaria, como medio de cribado de la apatía en dichos pacientes.
Schizophrenia is a severe, complex, multidimensional disorder characterized by negative, positive, affective, and cognitive symptoms. Negative symptoms are heterogeneous. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5)1 groups it into five subdomains: apathy/avolition, anhedonia, asociality, alogia, and affective flattening. However, there is a consensus that it can be grouped into two domains: avolition/apathy and diminished emotional expression.2,3
These symptoms affect around 60% of patients with schizophrenia.4 This seems to be associated with different biomarkers (IL-2, IL1β, and LPO),5,6 but no treatments have yet emerged as reliably and robustly effective.7 Furthermore, these negative symptoms (including apathy) are not specific to schizophrenia and can be confused with an antipsychotic adverse event; thus, they may be difficult to evaluate. In particular, apathy has been described as one of the most determining symptoms of the residual and chronic stages of schizophrenia.8,9 However, as previously mentioned, this symptom is not specific to schizophrenia; apathy can occur independently10 or in combination with symptoms of depression or dementia.11 Due to the similarity of symptoms, it can be very complex to differentiate apathy as a symptom of schizophrenia from a symptom of depression. Although both apathy and anhedonia indicate lack/decrease of interest, the latter presents a state of decreased experienced pleasure in activities whilst apathy is characterized by a lack of primary motivation and affective dullness.12
Depending on the repercussions on patients’ lives, apathy was strongly associated with higher levels of psychopathology and poorer functioning and quality of life in patients 10 years after the first psychotic episode.13 Thus, it is very important to assess the level of apathy to prevent repercussions on patients’ lives. However, there are few validated instruments that specifically assess apathy, the Apathy Evaluation Scale (AES)14 is one of them. The AES was developed by Marin et al.14 to characterize apathy in adult patients, regardless of their nature; the main symptom versus a symptom belonging to a major syndrome such as dementia, stroke or major depression. The authors simultaneously developed versions to be used by three different sources: patients (AES-S), clinicians (AES-C) and informants or proxies (AES-I). The AES-S showed good reliability (internal consistency 0.86; test-retest reliability 0.76) as well as a fairly good convergent validity with the scores of the clinicians (AES-C, r=0.72) but not of the informants (AES-I, r=0.43).14 The scarce literature on the AES-S factorial structure is controversial. On the one hand, Marin et al.14 found a common structure for the three versions consisting of 3 factors; a general apathy factor that explained 32–53% of the variance (information is not provided for each of the versions separately), curiosity or novelty seeking accounting for 5–10%, and, the third factor, structuring daily activities, that accounted for 7–8% of the variance. On the other, Clarke et al.15 in patients with dementia identified two factorsfor the AES-S version, apathy accounting for 36.4% of the variance, and “other” that explained a further 6.9%. The factorial structure of the AES-C was also analyzed in patients with Parkinson disease,16 Alzheimer disease,17 and with a first episode of psychosis (FEP).18,19 However, to the best of our knowledge, no study has validated this scale in either the Spanish population or patients with established schizophrenia.
Therefore, the aim of this study was to validate the self-reported version of the AES (AES-S) in European Spanish and assess its psychometrics properties (including floor and ceiling effects, reliability, and construct and discriminant validity) in patients with schizophrenia. We decided to validate the self-report version, since self-assessment is a time-efficient method that provides the patient's point of view on the experience of their negative symptoms, and facilitates shared decision-making in daily clinical practice.
Materials and methodsStudy designThis is a secondary analysis of a cross-sectional, naturalistic study carried out in three outpatient centres in Spain with the aim of validating two self-assessment instruments for the negative symptoms of schizophrenia (the Self-Evaluation of Negative Symptoms-SNS- and the Motivation and Pleasure Self-Report-MAP-SR-). It was approved by the Clinical Research Ethics Committee of one of the centres, Hospital Universitario Central de Asturias, Oviedo, Spain (ref. no. 140/150) and was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All subjects gave their written informed consent prior to enrolment.
SubjectsA total of 104 patients who had completed the AES-S in the SNS and MAP-SR validation study were included in this secondary analysis. Inclusion criteria were (1) schizophrenia diagnosis according to ICD-1020 criteria; (2) patients with stable schizophrenia (stability was defined as those patients who were clinically stable and had not required any change in their current pharmacological treatment during the past 3 months); (3) older than 17 years of age; (4) receiving outpatient treatment at one of the three centres; and (5) written informed consent to participate in the study. Exclusion criteria were designed to be minimal, and only those with intellectual developmental disorder or acquired brain injury or who refused to participate in the study were excluded.
Psychometric measuresParticipants were assessed by trained psychologists. The assessment included an ad hoc questionnaire for collecting demographic and clinical information. The Spanish versions of the following instruments were also used. We used the Clinical Global Impression-Schizophrenia scale (CGI-SCH)21 to assess severity of illness. The level of functioning was assessed using the Personal and Social Performance scale (PSP).22 Negative symptoms were assessed using the Clinical Assessment Interview for Negative Symptoms (CAINS),23 the Self-report of Negative Symptoms (SNS),24 and the Motivation and Pleasure Scale-Self-Report (MAP-SR).25 In addition, depressive symptoms were assessed with the Calgary Depression Scale for Schizophrenia (CDSS).26 Information was collected from the patients themselves and, when possible, from the main caregiver.
Finally, we employed the Spanish adaptation of the Apathy Evaluation Scale-self-rated version (AES-S).14 The AES-S is a self-reported 18-item scale that assesses apathy in the past 4 weeks. Each item is rated on a 4-point Likert-type scale from 1 (not at all true) to 4 (very true). Additionally, there are three inverse items (6, 10, and 11) that have to be recoded. The total AES-S score range is 18–72 with lower scores indicating greater apathy.
In the review of Weiser and Garibaldi27 the AES has been validated in individuals with Alzheimer's disease, Parkinson's disease, other types of dementia, stroke and subarachnoid haemorrhage, first episode of psychosis (FEP), major depressive disorder, and the general population.
Two Spanish clinical psychologists who are fluent in the English language (LGA, TBB) first translated the original instrument into Spanish. Then, a Spanish psychiatrist (EFE) fluent in English back-translated the Spanish version, and finally the original authors approved it.
Statistical analysisThe statistical analysis was done using SPSS 17.0. The two-tailed level of significance used was 0.05.
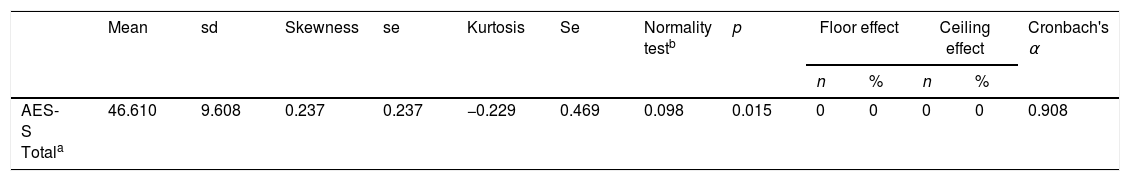
Skewness and kurtosis were calculated to measure the shape of the distributions (values of skewness and kurtosis±1 were considered good). The coefficient of variation (standard deviation/mean) and ceiling and floor effects were also determined (number of patients with scores greater than 95% and less than 5%, respectively).
The internal consistency of the AES-S was calculated using the Cronbach's alpha coefficient at the item level.
To calculate divergent validity, we used the Pearson correlation coefficient between the total AES-S score and total scores on the PSP and CDSS using the hypothesis that a moderate coefficient would be found, as they are related but different constructs. Convergent validity was calculated using the Pearson correlation coefficient between the total AES-S score and total scores on the CAINS, SNS, and MAP-SR and the SNS avolition subscale score using the hypothesis that higher coefficients would be found with self-rated measures (SNS and MAP-SR).
For analysing the discriminant validity, patients were classified into three groups based on their CGI-S negative subscale scores: mildly ill (CGI-S=2–3), moderately (CGI-S=4), and severely ill (CGI-S=5–7). An ANOVA test (Duncan post hoc) was used to identify statistically significant differences in the AES-S scores according to severity groups. An exploratory factor analysis (EFA) using the principal component analysis (PCA) method with oblimin rotation was used to explore the structure of the 18 AES-S items.
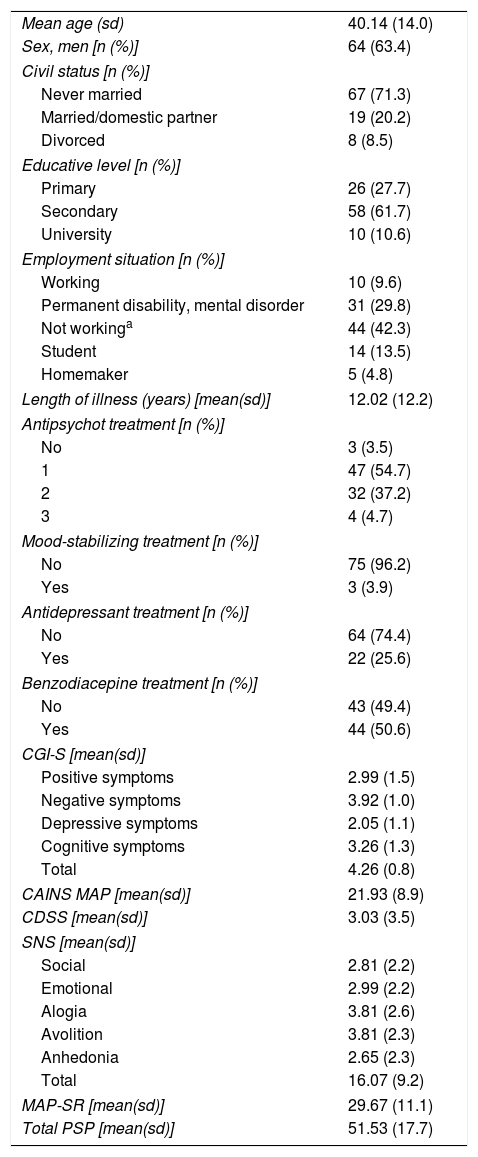
ResultsA total of 104 patients with schizophrenia were included. The mean age was 40.11 (sd=14.08), 63.4% were men, and the mean number of years of disease progression was 12.02 years (sd=12.23). Most of the subjects were on antipsychotic monotherapy (54.7%), the 25.6% received antidepressants, and 50.6% had prescribed at least one benzodiazepine. Table 1 shows patient demographic and clinical characteristics.
Clinical and demographic characteristics of the patients included in the study.
| Mean age (sd) | 40.14 (14.0) |
| Sex, men [n (%)] | 64 (63.4) |
| Civil status [n (%)] | |
| Never married | 67 (71.3) |
| Married/domestic partner | 19 (20.2) |
| Divorced | 8 (8.5) |
| Educative level [n (%)] | |
| Primary | 26 (27.7) |
| Secondary | 58 (61.7) |
| University | 10 (10.6) |
| Employment situation [n (%)] | |
| Working | 10 (9.6) |
| Permanent disability, mental disorder | 31 (29.8) |
| Not workinga | 44 (42.3) |
| Student | 14 (13.5) |
| Homemaker | 5 (4.8) |
| Length of illness (years) [mean(sd)] | 12.02 (12.2) |
| Antipsychot treatment [n (%)] | |
| No | 3 (3.5) |
| 1 | 47 (54.7) |
| 2 | 32 (37.2) |
| 3 | 4 (4.7) |
| Mood-stabilizing treatment [n (%)] | |
| No | 75 (96.2) |
| Yes | 3 (3.9) |
| Antidepressant treatment [n (%)] | |
| No | 64 (74.4) |
| Yes | 22 (25.6) |
| Benzodiacepine treatment [n (%)] | |
| No | 43 (49.4) |
| Yes | 44 (50.6) |
| CGI-S [mean(sd)] | |
| Positive symptoms | 2.99 (1.5) |
| Negative symptoms | 3.92 (1.0) |
| Depressive symptoms | 2.05 (1.1) |
| Cognitive symptoms | 3.26 (1.3) |
| Total | 4.26 (0.8) |
| CAINS MAP [mean(sd)] | 21.93 (8.9) |
| CDSS [mean(sd)] | 3.03 (3.5) |
| SNS [mean(sd)] | |
| Social | 2.81 (2.2) |
| Emotional | 2.99 (2.2) |
| Alogia | 3.81 (2.6) |
| Avolition | 3.81 (2.3) |
| Anhedonia | 2.65 (2.3) |
| Total | 16.07 (9.2) |
| MAP-SR [mean(sd)] | 29.67 (11.1) |
| Total PSP [mean(sd)] | 51.53 (17.7) |
sd: standard deviation; CGI-S: Clinical Global Impression Severity scale; CAINS: The Clinical Assessment Interview for Negative Symptoms; PSP: Personal and Social Performance; CDSS: Calgary Depression Scale for Schizophrenia; MAP-SR: Motivation and Pleasure Scale-Self-Report; SNS: Self-report of Negative Symptoms.
The distribution characteristics of the total AES-S score are shown in Table 2. Total AES-S scores exhibited symmetrical and mesokurtic distributions. The AES-S did not show ceiling or floor effects.
ReliabilityThe AES-S scale had good internal consistency for patients with schizophrenia(Cronbach's alpha of 0.908), and with the exception of items 6 and 11, all the Corrected Item-Total Correlation Values were >0.3 (they ranged between 0.411 for item 10 and 0.775 for item 18) (Table 2).
Convergent validityThe Pearson correlation coefficient between the AES-S total score and the total score of the CAINS-MAP was −0.483 (p<0.001). The correlation coefficients were greater with the self-reported measures. Thus, Pearson correlation coefficients were −0.803 (p<0.001) and −0.639 (p<0.001) with the SNS total and avolition subscale scores and −0.727 (p<0.001) with the MAP-SR. When controlling for scores on the CDSS, all the coefficients slightly decreased, ranging from −0.414 (with the CAINS-MAP) to −0.748 (with the total SNS).
Divergent validityThe Pearson correlation coefficient between the AES-S total score and the total score of the PSP was 0.504 (p<0.001). Again, when controlling for scores on the CDSS, the correlation coefficient decreased to 0.426 (p<0.001). The correlation between AES-S and CDSS was −0.431 (p<0.001).
Discriminant validityThe AES-S was able to discriminate among the different levels of illness severity according to CGI-S negative symptom scale scores. AES-S scores decrease as the severity of negative symptoms increases: mildly ill: 55.62 (sd=8.21), moderate ill: 43.38 (sd=6.46), and severely ill: 38.25 (sd=6.01)(F=42.644, p<0.001). The Duncan post hoc analysis demonstrated that each group was significantly different from the other two groups.
Exploratory factor analysis and principal component analysisThe 18 items of the AES-S were subjected to principal components analysis (PCA). The Kaiser–Meyer–Olkin value was 0.87, exceeding the recommended value of 0.60, and Bartlett's Test of Sphericity (Chi2=9333.07, p<0.001) reached statistical significance, supporting the factorability of the correlation matrix.
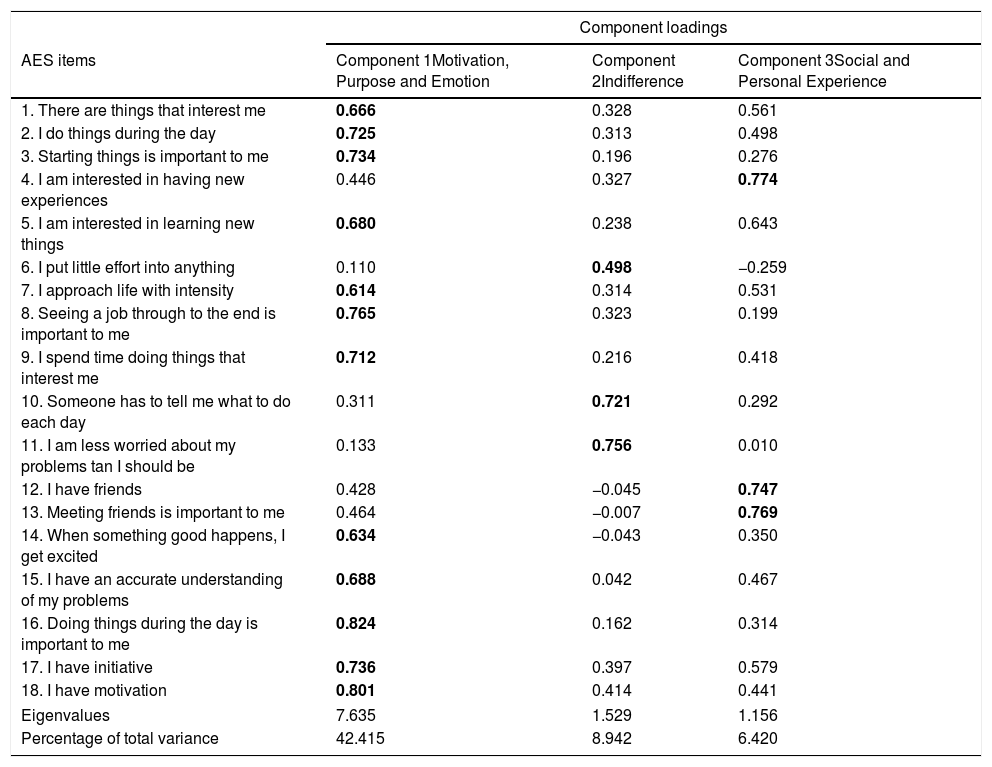
Principal component analysis revealed the presence of four components with eigenvalues exceeding 1, explaining 42.41%, 8.49%, 6.42%, and 6.27% of the variance, respectively. An inspection of the scree plot revealed a clear break after the third component; therefore, it was decided to retain three components for further investigation.
The three-component solution explained a total of 57.32% of the variance, with component 1 contributing 42.41%, component 2 contributing 8.49%, and component 3 contributing 6.42% (Table 3). To aid in the interpretation of these three components, an oblimin rotation was performed. The interpretation of the three components was consistent with previous research, with motivation-, purpose-, and emotion-related items loading strongly on component 1, indifference items loading strongly on component 2, and social and personal experience items loading strongly on component 3. There was a weak correlation among the three factors (they ranged between 0.060 and 0.464). Therefore, the results of this analysis support an underlying three-factor structure.
AES rotated three-component structure and component loadings.
| Component loadings | |||
|---|---|---|---|
| AES items | Component 1Motivation, Purpose and Emotion | Component 2Indifference | Component 3Social and Personal Experience |
| 1. There are things that interest me | 0.666 | 0.328 | 0.561 |
| 2. I do things during the day | 0.725 | 0.313 | 0.498 |
| 3. Starting things is important to me | 0.734 | 0.196 | 0.276 |
| 4. I am interested in having new experiences | 0.446 | 0.327 | 0.774 |
| 5. I am interested in learning new things | 0.680 | 0.238 | 0.643 |
| 6. I put little effort into anything | 0.110 | 0.498 | −0.259 |
| 7. I approach life with intensity | 0.614 | 0.314 | 0.531 |
| 8. Seeing a job through to the end is important to me | 0.765 | 0.323 | 0.199 |
| 9. I spend time doing things that interest me | 0.712 | 0.216 | 0.418 |
| 10. Someone has to tell me what to do each day | 0.311 | 0.721 | 0.292 |
| 11. I am less worried about my problems tan I should be | 0.133 | 0.756 | 0.010 |
| 12. I have friends | 0.428 | −0.045 | 0.747 |
| 13. Meeting friends is important to me | 0.464 | −0.007 | 0.769 |
| 14. When something good happens, I get excited | 0.634 | −0.043 | 0.350 |
| 15. I have an accurate understanding of my problems | 0.688 | 0.042 | 0.467 |
| 16. Doing things during the day is important to me | 0.824 | 0.162 | 0.314 |
| 17. I have initiative | 0.736 | 0.397 | 0.579 |
| 18. I have motivation | 0.801 | 0.414 | 0.441 |
| Eigenvalues | 7.635 | 1.529 | 1.156 |
| Percentage of total variance | 42.415 | 8.942 | 6.420 |
The numbers represent the load of each item in each of the three factors, and the items belonging to each factor are highlighted in bold.
This is the first study to investigate an apathy self-report scale in Spanish patients with stable schizophrenia. The aim was to adapt and validate the AES-S instrument into European Spanish and assess its psychometrics properties in Spanish patients with stable schizophrenia. Our results confirm that the Spanish version of the AES-S has appropriate psychometric properties and may therefore be used by Spanish clinicians when evaluating patients with schizophrenia in order to obtain the patients’ perspective on their level of apathy.
The internal consistency of the overall scale was adequate and similar to previous studies,19 although items 6 and 11 could be removed since they showed corrected item-total correlation values <0.3. With respect to convergent validity, we found a highly significant correlation between AES-S and the other self-reported scales used in this study, SNS and MAP-SR, indicating strong convergent validity. However, a moderate correlation was found with the CAINS-MAP, which is a clinician-administered scale. The awareness of apathy may vary more among lay people in general, including patients, than among research clinicians who are trained to provide high inter-rater agreement.19 However, apathy is an internal experience and therefore more accessible and suitable for self-reporting than observation-based expressive deficits.28 Thus, we think that obtaining information from patients themselves is of great value and should be considered complementary to the clinician's point of view.
Concerning divergent validity, moderate correlation coefficients were obtained with total PSP and CDSS scores, demonstrating that these instruments measure related but not identical constructs. The moderate correlation found among scores for apathy and functioning and depression is in keeping with the results of Faerden et al.19 This may be related to the negative influence of apathy on real world functioning, as the negative symptom is more highly associated with poor functional outcome.29
As hypothesized, AES scores discriminate between different levels of negative symptom severity according to CGI-S negative symptom scores. Our results show that the AES-S total score decreases by more than five points for each level increase in the CGI-S.
We obtained a three-component solution as in the original validation study.14 Following the definition of apathy proposed by other authors,30,31 the interpretation of these factors is as follows: the first component, Motivation, Purpose, and Emotion, is associated with a reduction in initiation and persistence in motivation and goal-directed activities. The second factor, Indifference, can be associated with affective dullness, which characterizes apathy. Finally, the two previous factors would result in increased associability, which reflects our third factor, Personal and Social Experiences. Since other studies have identified two or three factors with similar components to ours, including interest, cognitive behaviour, social indifference, insight, and social contacts,14–18 in their validation study of the original AES, Marin et al.14 conclude that this scale was predominantly a single-factor structure.
One of the limitations of this study is the generalizability of our results, since all patients were outpatients from the same region of Spain (Asturias), and there was a lack of patients with extremely severe negative symptoms, such as institutionalized or acutely hospitalized individuals. Another limitation is the cross-sectional design of the study that does not allow us to obtain information about the ability of the AES-S to detect changes in apathy over time. The main strength of this study consists of the non-restrictive inclusion and exclusion criteria, so our patients are very similar to those seen in routine outpatient clinical practice.
In conclusion, the Spanish version of the AES-S is an instrument that is reliable and valid for measuring apathy in patients with stable schizophrenia. As a self-reported instrument, it seems to be appropriate for use in routine clinical practice as a means of identifying apathy in this population. Furthermore, it is feasible to use since it is not time-consuming, and the information obtained should be considered complementary to the clinician's point of view.
Funding sourceThis work has been financially supported by BICIBERSAM, the Government of the Principality of AsturiasPCTI-2018-2022 IDI/2018/235, and Fondos Feder.
Conflict of interestThe authors declare that there is no conflict of interest. The funding sources had no participation in the development of this study.