Breast cancer is a prevalent and fatal cancer worldwide. Neoadjuvant chemotherapy is a treatment option used to reduce tumor size in patients with locally advanced breast cancer. The neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR), and platelet–lymphocyte ratio (PLR) are inflammatory markers that have been studied as prognostic factors in breast cancer. This cross-sectional study aimed to investigate the role of NLR, LMR, and PLR in the clinical response to neoadjuvant chemotherapy in women with locally advanced breast cancer.
Materials and methodsWe used a cross-sectional research design for this study with the aim of observing the relation between NLR, LMR, and PLR and the clinical response of neoadjuvant chemotherapy. The study was conducted in Cipto Mangunkusumo General Hospital. We analyzed the medical records of 84 patients treated with neoadjuvant chemotherapy between 2016 and 2021.
ResultsOur study found that majority of the subjects receives CAF combination of NAC with inoperable breast cancer. Most of the subjects have luminal B type of breast cancer and no clinical response to chemotherapy regimen. Surgery has significant association with clinical response in patients receiving NAC (P = <.001). There are no significant correlation between NLR, LMR, and PLR with clinical response to neoadjuvant chemotherapy. However, we did observe a significant association between NLR and 1-year mortality rate.
ConclusionIn conclusion, significant association between NLR and 1-year morality rate suggest that NLR may serve as a useful prognostic factor in breast cancer patients receiving neoadjuvant chemotherapy.
El cáncer de mama es un cáncer prevalente y mortal a nivel mundial. La quimioterapia neoadyuvante es una opción de tratamiento utilizada para reducir el tamaño del tumor en pacientes con cáncer de mama localmente avanzado. La proporción de neutrófilos-linfocitos (NLR), la proporción de linfocitos-monocitos (LMR) y la proporción de plaquetas-linfocitos (PLR) son marcadores inflamatorios que se han estudiado como factores pronósticos en el cáncer de mama. Este estudio transversal tuvo como objetivo investigar el papel de NLR, LMR y PLR en la respuesta clínica a la quimioterapia neoadyuvante en mujeres con cáncer de mama localmente avanzado.
Materiales y métodosUtilizamos un diseño de investigación transversal para este estudio para observar la relación entre NLR, LMR y PLR y la respuesta clínica de la quimioterapia neoadyuvante. El estudio se llevó a cabo en el Hospital General Cipto Mangunkusumo. Analizamos las historias clínicas de 84 pacientes tratados con quimioterapia neoadyuvante entre 2016–2021.
ResultadosNuestro estudio encontró que la mayoría de los sujetos reciben una combinación CAF de NAC con cáncer de mama inoperable. La mayoría de los sujetos tienen cáncer de mama luminal tipo B y no tienen respuesta clínica al régimen de quimioterapia. La cirugía tiene una asociación significativa con la respuesta clínica en pacientes que reciben NAC (p = <0,001). No existe una correlación significativa entre NLR, LMR y PLR con la respuesta clínica a la quimioterapia neoadyuvante. Sin embargo, observamos una asociación significativa entre el NLR y la tasa de mortalidad a 1 año.
ConclusiónEn conclusión, la asociación significativa entre la NLR y la tasa de moralidad a 1 año sugiere que la NLR puede servir como un factor pronóstico útil en pacientes con cáncer de mama que reciben quimioterapia neoadyuvante.
Breast cancer is a highly prevalent type of cancer with significant morbidity and mortality rates. According to the Global Cancer Observatory (GLOBOCAN) data by the World Health Organization (WHO) in 2022, breast cancer is the second most common cancer worldwide, with an incidence of 2 million people annually and a mortality rate of more than 620 000 people globally.1 The increase in the incidence of breast cancer is mainly attributed to the rise in local-stage disease, which has grown from 75 per 100 000 in 2004 to 86 per 100 000 in 2019. The improvement in the diagnosis and management of breast cancer over the last 2 decades has resulted in a significant increase in survival rates, mainly due to various screening programs.2 However, limited resources in developed countries have led to limited treatment choices for locally advanced breast cancer (LABC), with neoadjuvant chemotherapy becoming the cornerstone of treatment.3,4 The response of breast cancer to chemotherapy plays a crucial role in the prognosis and survival of patients.5 Immunological and inflammatory processes also play an essential role in tumor development and prognosis, affecting every stage of tumor formation, including initiation, promotion, invasion, and metastasis.6 Inflammatory processes characterized by an increase in inflammatory mediators such as C-reactive protein (CRP) or an increased number of leukocytes indicating an inflammatory response have prognostic value in breast cancer.7 Several inflammation indicators that can be used in cancer are neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR), and platelet electrolyte–lymphocyte ratio (PLR). Neutrophil–lymphocyte ratio (NLR), lymphocyte–monocyte ratio (LMR), and platelet–lymphocyte ratio (PLR) are inflammation indicators that can be used in cancer and have predictive value for assessing response to neoadjuvant chemotherapy.8,9 However, systematic review studies show inconclusive results on the effect of NLR, PLR, and LMR on response to neoadjuvant chemotherapy.8 Therefore, this study aims to analyze NLR, PLR, and LMR on the clinical response of locally advanced breast cancer.
Materials and methodsThe study was conducted in Cipto Mangunkusumo General Hospital from October 2021 to January 2022 using a cross-sectional study design with consecutive sampling to analyze the association between immunological indicators (NLR, LMR, and PLR) and clinical response in patients with locally advanced breast cancer receiving neoadjuvant chemotherapy. Inclusion criteria for this study are locally advanced breast cancer patients (Stage IIIA and IIIB) who received neoadjuvant chemotherapy for the first time followed by mastectomy. Exclusion criteria are patients with a history of chemotherapy or radiation therapy, patients with acute infection, chronic infection, or autoimmune disease, and incomplete patient data. Data containing clinicopathological characteristics, laboratory examination results, and treatment given to the patients were obtained. The NLR, LMR, and PLR values were obtained during first admission before chemotherapy was given. The clinical response was assessed using Miller-Payne system. Statistical Package for Social Science (SPSS) was used to analyze the data. Bivariate analysis was done using unpaired t-test or Mann–Whitney test for numerical data and Chi-Square or Fisher Exact test for categorical data. The Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo General Hospital ethics committee approved this study proven by ethics number KET-1035/UN2.F1/ETIK/PPM.00.02/2021.
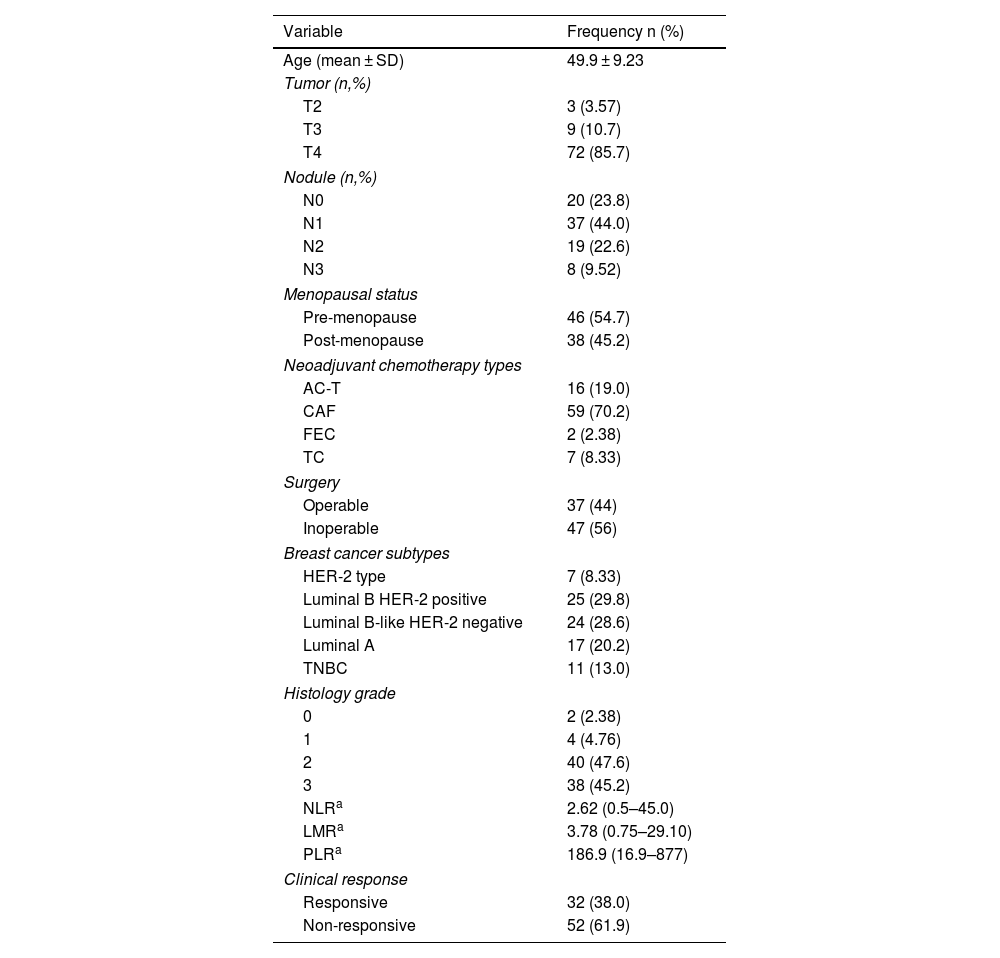
ResultsIn this study, there were 84 research subjects including patients with locally advanced breast cancer who received NAC therapy. Data analysis shows that the average age of the subjects is 50 years and that most of the subjects have a clinical stage of T4, and N1. The majority of the study subjects are pre-menopausal women with luminal B breast cancer and positive HER2 receptor expression on immunohistochemistry examination. They received the cyclophosphamide, doxorubicin hydrochloride, and fluorouracil (CAF) combination type of chemotherapy, of which most of the patients did not undergo surgery. Histopathology examination reveals that the majority of the tumors have a histologic grade of 2 or 3, and that the clinical response was non-responsive in most cases (Table 1).
Characteristics of the study subjects.
| Variable | Frequency n (%) |
|---|---|
| Age (mean ± SD) | 49.9 ± 9.23 |
| Tumor (n,%) | |
| T2 | 3 (3.57) |
| T3 | 9 (10.7) |
| T4 | 72 (85.7) |
| Nodule (n,%) | |
| N0 | 20 (23.8) |
| N1 | 37 (44.0) |
| N2 | 19 (22.6) |
| N3 | 8 (9.52) |
| Menopausal status | |
| Pre-menopause | 46 (54.7) |
| Post-menopause | 38 (45.2) |
| Neoadjuvant chemotherapy types | |
| AC-T | 16 (19.0) |
| CAF | 59 (70.2) |
| FEC | 2 (2.38) |
| TC | 7 (8.33) |
| Surgery | |
| Operable | 37 (44) |
| Inoperable | 47 (56) |
| Breast cancer subtypes | |
| HER-2 type | 7 (8.33) |
| Luminal B HER-2 positive | 25 (29.8) |
| Luminal B-like HER-2 negative | 24 (28.6) |
| Luminal A | 17 (20.2) |
| TNBC | 11 (13.0) |
| Histology grade | |
| 0 | 2 (2.38) |
| 1 | 4 (4.76) |
| 2 | 40 (47.6) |
| 3 | 38 (45.2) |
| NLRa | 2.62 (0.5–45.0) |
| LMRa | 3.78 (0.75–29.10) |
| PLRa | 186.9 (16.9–877) |
| Clinical response | |
| Responsive | 32 (38.0) |
| Non-responsive | 52 (61.9) |
The results of the comparative analysis between the characteristics of the subjects and the clinical response of the primary tumor showed that T stage, N stage, menopause status, type of NAC, breast cancer subtypes, and histology grade do not have significant association with clinical response. The analysis shows that surgery types have a significant association with clinical response (P = <.001, OR 19.05, 95% CI: 4.8–74).
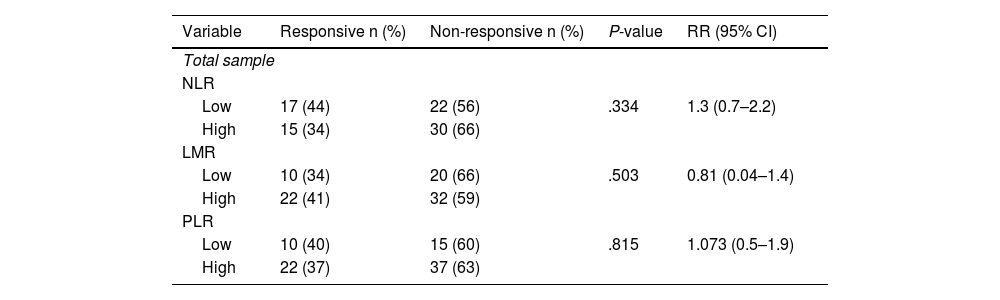
In subjects with good clinical response, modified radical mastectomy was done while there is no surgery done in non-responsive subjects. Bivariate analysis shows that NLR, LMR, and PLR are not significantly associated with clinical response, with P-value of .334, .504, and .815 respectively (Table 2).
Comparison between NLR, LMR, and PLR to clinical response post-neoadjuvant chemotherapy.
| Variable | Responsive n (%) | Non-responsive n (%) | P-value | RR (95% CI) |
|---|---|---|---|---|
| Total sample | ||||
| NLR | ||||
| Low | 17 (44) | 22 (56) | .334 | 1.3 (0.7–2.2) |
| High | 15 (34) | 30 (66) | ||
| LMR | ||||
| Low | 10 (34) | 20 (66) | .503 | 0.81 (0.04–1.4) |
| High | 22 (41) | 32 (59) | ||
| PLR | ||||
| Low | 10 (40) | 15 (60) | .815 | 1.073 (0.5–1.9) |
| High | 22 (37) | 37 (63) | ||
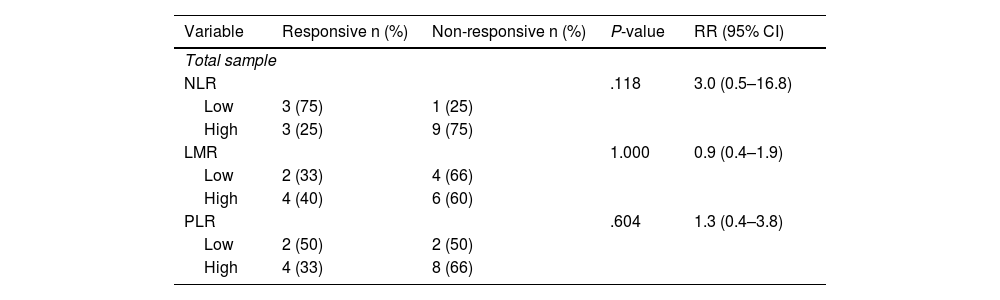
Stratified analysis conducted on different types of NAC shows a higher prevalence of non-responsive outcomes in the group with high NLR. It is found that there is an increase in the incidence of responsive clinical response in the low NLR group by 3 times compared to the high NLR in patients receiving the AC-T regimen (Table 3 and Table 4).
Association between immunological indicators (NLR, LMR, and PLR) and clinical response in post-neoadjuvant chemotherapy patients with AC-T regimen.
| Variable | Responsive n (%) | Non-responsive n (%) | P-value | RR (95% CI) |
|---|---|---|---|---|
| Total sample | ||||
| NLR | .118 | 3.0 (0.5–16.8) | ||
| Low | 3 (75) | 1 (25) | ||
| High | 3 (25) | 9 (75) | ||
| LMR | 1.000 | 0.9 (0.4–1.9) | ||
| Low | 2 (33) | 4 (66) | ||
| High | 4 (40) | 6 (60) | ||
| PLR | .604 | 1.3 (0.4–3.8) | ||
| Low | 2 (50) | 2 (50) | ||
| High | 4 (33) | 8 (66) | ||
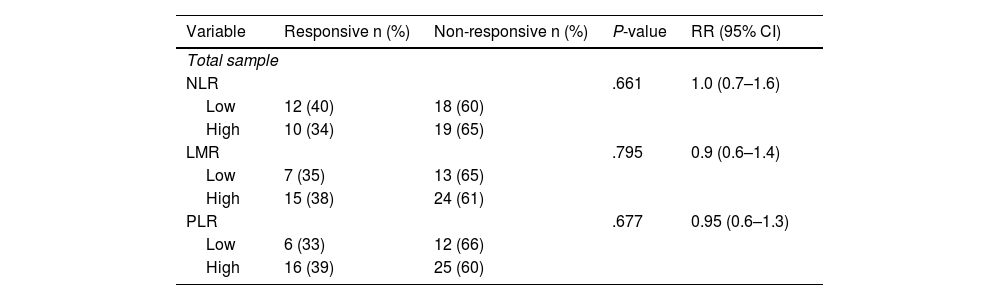
Association between immunological indicators (NLR, LMR, and PLR) and clinical response in post-neoadjuvant chemotherapy patients with CAF regimen.
| Variable | Responsive n (%) | Non-responsive n (%) | P-value | RR (95% CI) |
|---|---|---|---|---|
| Total sample | ||||
| NLR | .661 | 1.0 (0.7–1.6) | ||
| Low | 12 (40) | 18 (60) | ||
| High | 10 (34) | 19 (65) | ||
| LMR | .795 | 0.9 (0.6–1.4) | ||
| Low | 7 (35) | 13 (65) | ||
| High | 15 (38) | 24 (61) | ||
| PLR | .677 | 0.95 (0.6–1.3) | ||
| Low | 6 (33) | 12 (66) | ||
| High | 16 (39) | 25 (60) | ||
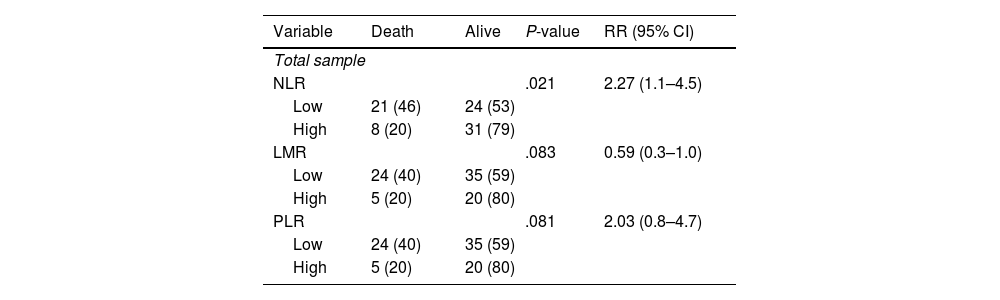
There is a significant association between NLR and 1-year mortality as shown in Table 5 (P =.021). The RR value obtained in the NLR group was the highest, with 2.27 times higher risk of death.
Association between immunological indicators (NLR, LMR, and PLR) and 1-year mortality.
| Variable | Death | Alive | P-value | RR (95% CI) |
|---|---|---|---|---|
| Total sample | ||||
| NLR | .021 | 2.27 (1.1–4.5) | ||
| Low | 21 (46) | 24 (53) | ||
| High | 8 (20) | 31 (79) | ||
| LMR | .083 | 0.59 (0.3–1.0) | ||
| Low | 24 (40) | 35 (59) | ||
| High | 5 (20) | 20 (80) | ||
| PLR | .081 | 2.03 (0.8–4.7) | ||
| Low | 24 (40) | 35 (59) | ||
| High | 5 (20) | 20 (80) | ||
The results of the study indicate that although there is no statistically significant association between NLR and clinical response, there is an increase in clinical response in subjects with low NLR values. The finding is consistent with Suppan et al.,10 who reported a lack of significant association between pre-chemotherapy NLR values and chemotherapy response in early-stage breast cancer patients. The conflicting findings in previous studies may be due to the fact that NLR examination alone may not be sufficient to be a predictive or prognostic marker, as it is influenced by various factors such as liver and kidney dysfunction and normal thyroid function. The study conducted by Eryilmaz et al.11 also reported an insignificant association between NLR values and clinical response to chemotherapy. However, one of the limitations of their study was the non-standardized chemotherapy regimen. In contrast, Eren et al. found a significant association between low NLR values and complete response in locally advanced breast cancer patients. They reported that low NLR values are independent predictive factors for complete response (OR 3.438, 95% CI 2.66–5.419, P-value <.001).12 Kim et al. also found a trend of lower NLR values in subjects with a complete response to neoadjuvant chemotherapy, although this was not statistically significant (OR 4.13, 95% CI 1.49–11.43, P-value = 0.006).9
In summary, although the P-value for the association between NLR and clinical response was not statistically significant in this study, the increase in clinical response in subjects with low NLR values should be taken into consideration. Future studies with larger sample sizes and standardized chemotherapy regimens may provide more definitive conclusions about the predictive value of NLR in breast cancer chemotherapy response.
Based on the statistical analysis, there was no significant relationship between LMR and clinical response in patients with locally advanced breast cancer. This finding is consistent with the findings of Eren et al. who reported that the LMR value was not significantly related to clinical response.12 Similarly, the study conducted by Goto et al. showed that the LMR value did not have a significant association with the response to neoadjuvant chemotherapy using the FEC regimen.13 In contrast, Ma et al. reported that LMR with a cut-off value of 6.2 was significantly associated with neoadjuvant chemotherapy response. However, it should be noted that Ma et al. conducted a study of breast cancer patients with clinical stages I–IV and there was a statistically significant difference in the proportion of clinical stages between groups with LMR≤6.2 and LMR>6.2 (P = .019).14 These findings may not be able to be generalized to subjects with locally advanced breast cancer, which is the main focus of this study. In general, studies examining the relationship between LMR and chemotherapy response reported that higher LMR values were associated with complete response to neoadjuvant chemotherapy as well as better survival rate in breast cancer patients.13,14 Lymphocytes play a significant role in the host's immune response against various types of tumors and are capable of infiltrating the tumor microenvironment and expressing various factors that influence tumor growth and damage tumor cell proliferation and metastasis.14
Based on statistical analysis, there was no significant relationship between PLR and clinical response of locally advanced breast cancer patients. This finding is consistent with the findings of Eren et al.,12 Ma et al.,14 and Graziano et al.15 who reported that PLR value was not significantly related to clinical response. On the other hand, these findings contradict the study conducted by Cuello-López et al. who reported that low PLR with a cut-off value of <150 was significantly associated with complete response to neoadjuvant chemotherapy for all subtypes of breast cancer.16 In terms of molecular subtypes, the characteristics of the subjects in the study of Cuello-López et al. are also similar to the characteristics of the subjects in this study, with a predominance of luminal cancer. However, approximately 40% of the subjects in their study had T1 and T2 tumors, so the findings from their study may not be extrapolated to a population of locally advanced breast cancer patients such as our study subjects. A high PLR value is associated with an increase in number of platelets and a relative decrease in the number of lymphocytes, possibly caused by the non-specific inflammatory response of cancer cells so that high PLR value is thought to be associated with a worse prognosis in cancer patients.14 Previous studies have reported this finding. A higher pre-operative PLR value is a predictor of poor outcomes, both in breast cancer and other cancers.12,14,15,17 A study analyzing non-metastasis breast cancer patients found that higher PLR values were associated with overall survival and lower cancer-specific survival.15 However, in contrast to survival outcomes, existing studies show inconsistent results regarding the relationship between PLR and neoadjuvant chemotherapy response in patients with breast cancer.12,14,15 Ma et al. reported a significant relationship between PLR and survival rate of breast cancer patients but found no significant association between PLR and chemotherapy response, and between PLR and post-therapy disease-free survival.14 The inconsistent findings may be related to differences in the target population and the inadequate sample size in this study.
In this study, a significant association was found between NLR and 1-year mortality in patients with locally advanced breast cancer who received NAC. Previous studies have also shown an association between NLR and breast cancer mortality. A meta-analysis study conducted by Ethier et al. showed an increase in hazard ratio of 2.56 times to total survival in breast cancer patients with NLR value higher than 2.5.18 This finding is in line with our study in which we found that an increased risk of death with RR value of 3 in the group with NLR value higher than 2.5. This finding can be caused by the relationship between high NLR values and inflammation.18 In addition, neutrophils have also been shown to inhibit the immune system and promote tumor growth by inhibiting lymphocyte activity and the response of T cells.19 The relationship between PLR and LMR in this study shows insignificant findings. However, the calculated RR value shows that there is a 2-fold increase in the incidence of death at 1 year in a group with higher PLR value. The analysis also showed a 1.3-fold increase in the incidence of death at 1 year in a group with low LMR. Previous research conducted by Zhu et al. demonstrated an increase in total survival of the high PLR group.20 Another study showed a worse symptom-free survival rate in a group with lower LMR, which is consistent with the results obtained in this study.13 There are no statistically significant correlation between LMR and 1-year mortality (P = .083), and PLR and 1-year mortality (P = .081). However, these results are highly suggestive of correlation between the two. Therefore, subsequent study can be conducted to prove the correlation between the variables. To sum up, the results obtained in this study are quite consistent with previous studies.
LimitationThis study has several limitations that need to be addressed. Limited availability of subjects' laboratory data, clinical response, and histopathology results caused suboptimal number of research subjects and decreased research power. This limitation can also impact the statistical analysis done in this study. Multivariate analysis could also not be performed considering that the results of bivariate analysis did not meet the requirements to proceed.
ConclusionIn this study, the median NLR, PLR, and LMR values in locally advanced breast cancer patients receiving neoadjuvant chemotherapy are 2.62, 186.9, and 3.78, respectively. A clinical response of 38% was found in locally advanced breast cancer patients who received NAC and in the subsequent analysis of the group with low NLR value, it is found that the prevalence of patients who experienced clinical response increased to 44%. There was no significant association between high NLR, high PLR, and low LMR, and clinical response to NAC in locally advanced breast cancer patients. In patients with breast cancer undergoing neoadjuvant chemotherapy, there is a substantial correlation between NLR and the 1-year survival rate, indicating that NLR may be a valuable prognostic indicator.
FundingNo funding.
Ethical considerationsThe Faculty of Medicine, Universitas Indonesia, Cipto Mangunkusumo General Hospital ethics committee approved this study proven by ethics number KET-1035/UN2.F1/ETIK/PPM.00.02/2021. Informed consent was obtained from each study subjects.
Patients consentThe authors declare that they obtained the patient's consent for publication of the article.
Author contributionsSSP and AG contribute to the study conception and design. Data collection was done by SSP and AG. NK validated the pathology examination and TAP validated the statistical analysis. Analysis and interpretation of results were done by AG, NK, TAP, and SJH. SSP, AG, and SJH drafted the manuscript, while NK and TAP revised the manuscript. All authors reviewed the results and approved the final version of the manuscript.
We would like to express our gratitude to the individuals and organizations who have made substantive contributions to this research. Without their support, this study would not have been possible.
We would like to thank Surgical Oncology Division, Department of Surgery, Cipto Mangunkusumo General Hospital and Oncology Division, Dharmais Cancer Hospital for their assistance with providing the basic data that were needed to start this research and for their support throughout this research project. Their contribution was invaluable in completing this research. We apologize if we have inadvertently omitted anyone who contributed to this work.