The high detection rate of the sentinel lymph node (SLN) in breast cancer is a quality criterion of the procedure. The aim of our work is to identify the failure factors (FF) of the SLN biopsy in tumors larger than 4 cm.
Material and methodsThis is a prospective series of patients with breast tumors larger than 4 cm without clinical axillary involvement. The colorimetric method was used in 52% of cases and the dual method in 48% of cases.
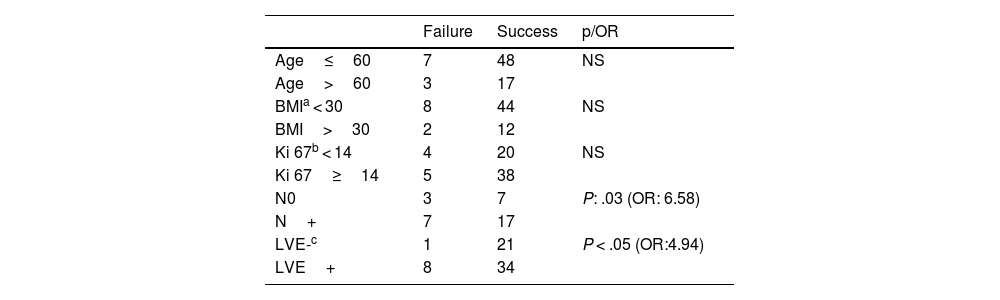
ResultsThe failure rate (FR) is 13%. The FF with a statistically significant difference are axillary invasion (P: .03; OR: 6.58) and the presence of lympho-vascular emboli (LVE) (P < .05; OR: 4.95). It should be noted that lympho-vascular emboli only concern those found in the peri-tumoral area. On the other hand, age over 60 years, Body mass index (BMI) over 30 and Ki 67 over 14 are not determining factors.
DiscussionSener finds a more important FR when more than 10 lymph nodes of the axillary dissection are reached compared to the axillary hollows free from any neoplastic infiltration (P: .002; OR: 9.19). The FR for Gimbergues was statistically correlated with lymph node status (0% for pN0 tumors, versus 55% for pN1–N2 tumors). For Brenot-Rossi, the existence of LVE is a significant failure factor (P = .004).
For other factors (obesity, advanced age), the data in the literature are contradictory.
ConclusionThe infiltration of the axillary fossa and the existence of LVE constitute the main FF of SLN biopsy in patients with tumors larger than 4 cm without clinical axillary invasion.
La elevada tasa de detección del ganglio linfático centinela (GLC) en el cáncer de mama es un criterio de calidad del procedimiento. El objetivo de nuestro trabajo es identificar los factores de fracaso (FF) de la biopsia del GLC.
Material y métodosSe trata de una serie prospectiva de pacientes con tumores de mama mayores de 4 cm sin afectación clínica axilar. Se utilizó el método colorimétrico en el 52% de los casos y el método dual en el 48% casos.
ResultadosLa tasa de fracaso (FR) es del 13%. Los FR con una diferencia estadísticamente significativa son la invasión axilar (p: 0,03; OR: 6,58) y la presencia de émbolos linfo-vasculares (ELV) (p < 0.05; OR: 4.95). Cabe señalar que los émbolos linfovasculares sólo afectan a los que se encuentran en la zona peritumoral.
DiscusiónSener encuentra un FR más importante cuando más de 10 ganglios linfáticos de la disección axilar se alcanzan en comparación con los axilares huecos libres de cualquier infiltración neoplásica (p: 0,002; OR: 9,19). El FR de Gimbergues se correlacionó estadísticamente con el estado de los ganglios linfáticos (0% para tumores pN0 frente al 55% para los tumores pN1-N2). Para Brenot-Rossi, la existencia de EVL es un factor de fracaso significativo (p = 0,004). Para otros factores (obesidad, edad avanzada), los datos en la literatura son contradictorios.
ConclusionesLa infiltración de la axila y la existencia de ELV constituyen los principales FF de la biopsia SLN en pacientes con tumores mayores de 4 cm sin invasión axilar clínica.
The sentinel lymph node (SLN) is the first lymph relay reached in a tumoral progression pattern. The SLN biopsy is tending to become the gold-standard in the exploration of the axillary fossa of patients with breast neoplasms of less than 5 cm, without clinical and radiological axillary invasion.
A high detection rate is a guarantee of the quality of the SLN technique. The failure rate (FR) of this procedure ranges from 2% to 8%.
Very few studies have examined the feasibility of the SLN technique in large breast tumors.1,2 Our question was whether large breast tumors present the same factors for failure as smaller tumors when the sentinel node procedure is applied? The aim of our work is to identify the factors causing failure (FF) of the SLN technique, in patients with breast tumors larger than 4 cm without clinical axillary invasion.
Materials and methodsThis is a prospective series of 75 SLN detections in patients with breast neoplasms larger than 4 cm without clinical axillary invasion (cT2N0M–cT3N0M0). The rate of cT2N0M0 patients represents 25% of the cases, and the rate of cT3N0M0 patients represents 75% of the series.
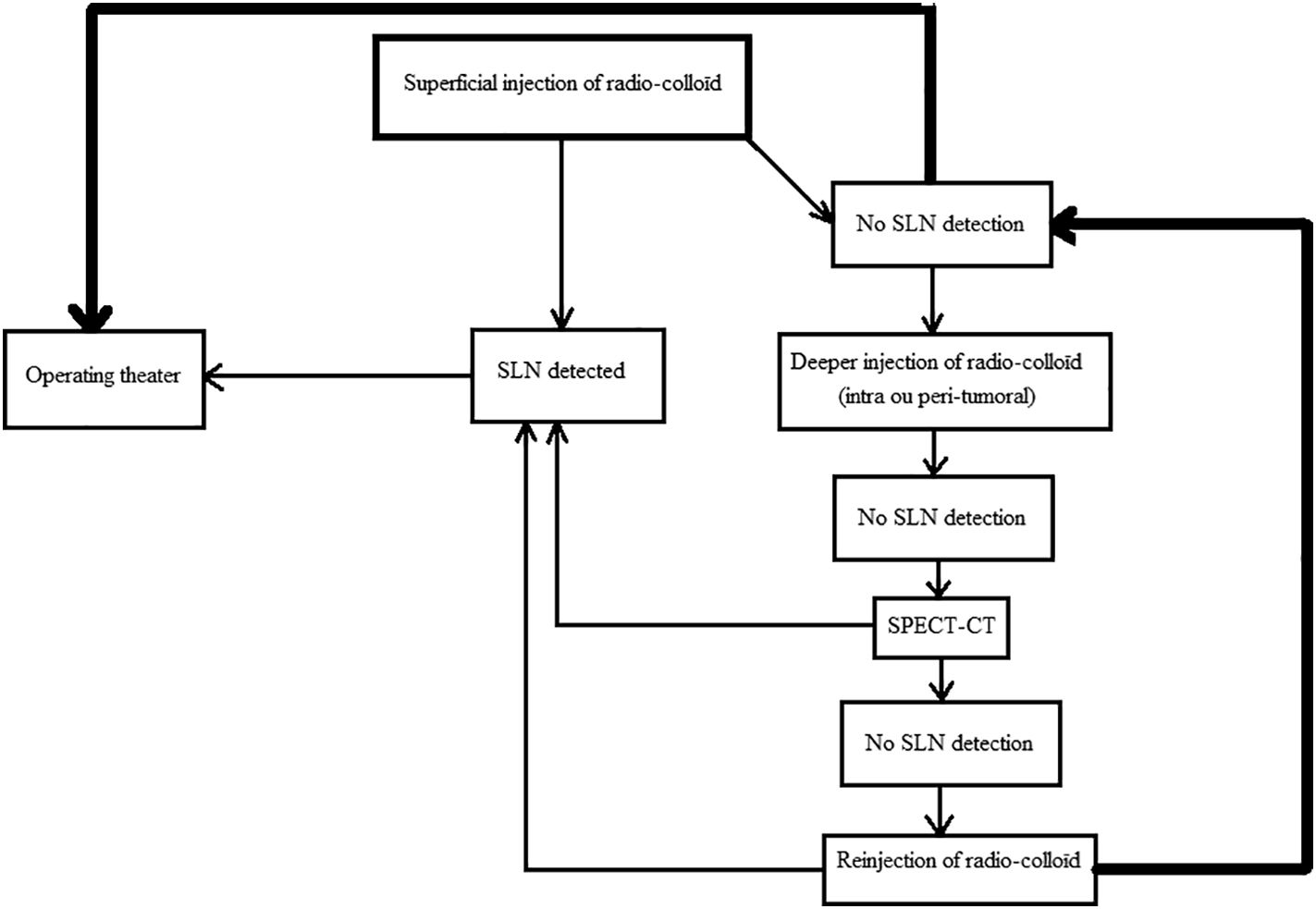
The product used during lympho-scintigraphic time is Nanocoll * (GE Healthcare*, Italy). The product is injected in a peri-areolar fashion at the 4 cardinal points. The total dose of radioactivity used is 1.2 milliCurie. A 20 min breast massage follows, and then planar lympho-scintigraphic images of the face and profile are taken in searching of SLN. If no SLN is detected, a SPECT–CT (Single Photon Emission Computed Tomography) is performed to search for a deep or atypical localization of the SLN.
Intraoperatively, the dye used is patent blue (Laboratoire Guerbet*, Aulnay-sous-bois, France). The injection is made peri-areolar and a 10 min breast massage is performed to promote migration of the dye to the lymph nodes of the axillary hollow.
An elective incision at the hottest point of the previously marked axillary hollow is made. Any hot lymph node detected using the gamma-acoustic probe (Eurorad* Strasbourg, France) and or stained is taken.
The failure of the isotopic technique is declared when the lympho-scintigraphic image is white (Fig. 1B) and/or no radioactivity is detected intraoperatively.
The failure of the colorimetric technique is represented by the intraoperative non-detection of lymph nodes stained with patent blue.
For the dual detection method, failure of one technique is considered procedural failure.
Axillary lymph node dissection is performed systematically. Histological analysis of SLN and axillary lymph nodes dissection is performed using hematoxylin and eosin staining. If no infiltration is detected after standard histological analysis of GS, an immunohistochemical study is carried out to avoid missing micrometastases or isolated cell clusters.
The colorimetric method alone was used in 39 cases (52%) and the dual method (isotopic and colorimetric) in 36 cases (48%).
All of our patients with SLN technique failure underwent radical surgery (mastectomy). Axillary dissection was performed systematically in our series, whatever the result of the histological analysis of the SLN. This attitude is justified by the fact that the procedure for detecting SLN is not fully validated for large breast tumors. On the other hand, by calculating the intrinsic values, this approach will enable us to extend the indications of the SLN technique to larger breast tumors if our results are convincing.
We carried out the final analysis of the data using SPSS Version 22.0.
We obtained informed consent from all participants in our study.
ResultsThe average age of our patients who presented with a failed SLN procedure was 55 years (range 46–75). The mean BMI of these patients is 30.2 (range 25.87–41.2). The overall failure rate is estimated at 13%: 8 failures in the double method and 2 failures in the colorimetric method.
Regarding the immuno-histochemical profile of these 10 patients according to the Saint-Gallen classification is A luminal in 4 cases, B luminal in 5 cases, and the triple negative type in 1 case.
Among these 10 patients, the axillary hollow was invaded in 5 cases (with massive invasion N3 in 2 patients) and free from any neoplastic infiltration in the remaining 5 cases (Table 1).
Factors influencing the success or failure of the sentinel node procedure for breast tumors larger than 4 cm without clinical axillary invasion.
Using the Chi-square test after Yates correction, there is no statistically significant difference between the technique failure rate in patients under 60 and over 60 years old. Likewise, no statistically significant difference was noted between the detection rate in cases of BMI less than 30 or in obese patients with BMI greater than 30. The proliferation index (Ki 67) does not constitute a significant failure factor between the two subgroups of patients: the first grouping together patients with a Ki67 less than 14%, and the second subgroup of patients with a Ki 67 greater than or equal to 14%.
On the other hand, we noted a statistically significant difference (P = .03) between the failure rate in patients with an infiltrated axillary hollow (N+), compared to patients with an uninfiltrated axillary hollow (N0). The odds ratio of FR in axillary N+ hollows to N0 hollows was estimated to be 6.58 (CI 95%) [1.51–28.61].
The presence of LVE is correlated with more SLN technique failure than its absence (P < .05), with an odds ratio of 4.94 (CI 95%) [i-1.32071] (Table 1).
The other FF that could be identified are: a defective batch of the radio-colloïd in one case, lymphatic stasis in one case, and a history of breast abscess in another patient. In 2 cases, no FF could be found.
DiscussionIn our study population, 2 determining factors are at the risk of failure of the SLN technique: in one hand, the invasion of the axillary hollow, and in the other hand, the presence of LVE in the histological analysis of the breast cancer.
The other FF do not seem to be so decisive.
Sener et al. observed more failure of the SLN biopsy technique in patients over 70 years compared to younger patients (OR 3.14 and P = .018). The FR in patients with more than 10 infiltrated lymph nodes is 40.9% of cases, on the other hand, it is only of the order of 5.3% in the case of non-infiltrated axillary nodes (OR: 9.19 and P: .002),1 which matches our results.
In the Gimbergues series where the SLN was taken after systemic chemotherapy, the FR was statistically correlated with the lymph node status (0% for pN0 tumors, against 55% for pN1–N2 tumors). The introduction of chemotherapy, despite its potentially therapeutic effect on infiltrated lymph nodes, does not seem to reduce the FR of the SLN technique in infiltrated hollows.3
For Straalman et al., FF are represented in multivariate analysis by high age and BMI, central location of the tumor and lack of operator experience.4 Conversely, Derossis et al. had high identification rates of 96.6% in patients with BMIs over 30 using the dual detection method after analyzing its 2495 procedure series.5 Failure associated with advanced age is not found in all studies: Ogasawara et al. and Johnson et al. do not see advanced age as a factor for failure.6,7 Benzidane and Bendib from the Pierre and Marie Curie Center (PMCC) in Algiers, found in their series of 374 patients all tumor stages combined (T0, T1, T2, T3, N0, N1) a FR of the SLN technique of 14.7%. The FF they were able to identify are as follows:
- •
When lumpectomy or mastectomy are performed before the injection of blue dye.
- •
Excessive breast volume greater than 1000 g.
- •
Multifocal tumors.8
Schrenk et al. looked for SLN in 30 patients who had undergone prior axillary surgery. FF are represented by a white lympho-scintigraphic image preoperatively (P < .001) and an anterior axillary dissection having removed more than 10 lymph nodes (P < .02).9
For Goyal et al., the FF he revealed are represented by: obesity (P < .001), tumor localization outside the UOQ (P = .008) and the non-visualization of SLN on planar images during lympho-scintigraphy (P < .001).10
Brenot-Rossi et al. (N = 332 patients) found in their series of invasive breast tumors (T0, T1, or T2 of less than 3 cm all N0) in multivariate analysis that the only independent factor correlated with the non-detection of SLN in lymphoscintigraphy was the number of infiltrated SLN greater than 4. In univariate analysis, in addition to this factor, the existence of LVE significantly constitutes a failure factor with a P = .004,11 which is consistent with our study.
Johnson et al. in 2009 found more detection failures in the preoperative lympho-scintigraphic image and non-intraoperative in patients with morbid obesity (BMI>40), and that lymph node infiltration is not correlated with detection failure.7 Other authors agree with Johnson et al., finding that elevated BMI affects preoperative detection on lympho-scintigraphy and not intraoperative detection.12,13 For the experts of the ASCO panel in 2017, there is no robust evidence according to the data in the literature to consider the advanced age of the patient as a limit, or even a factor of failure of the technique, compared to younger patients. Likewise, BMI should not be involved in the choice of procedure for approaching the axillary hollow in patients with breast neoplasms.14
The influence of the learning curve is not insignificant, and tends to reduce FR as teams gain expertise, as illustrated by Wu et al., seeing a reduction in FR from 11.1% the first year, to 9.1% the second year and to 1.4% in the third year.15 In the series by Johnson et al., the FR went from 18% during the first 100 procedures to 2% after the 200th procedure, then to 1% after the 300th procedure.7 As for intraoperative detection, the results improve after the 100th procedure for the same department.7 But individually, a surgeon can achieve a 90% success rate after the 23rd procedure, and a 95% success rate after 53 procedures.16,17
In the face of all this data, the failure of the SLN technique should not be inevitable. Preventive measures can be used to increase the detection rate, such as preoperative hydration to help opening the lymphatic channels in fatty breasts from post-menopausal women. Prolonged breast massage (at least 20 min for the radio-colloid which has a high molecular weight and at least 10 min for the blue dye lighter) to ensure sufficient migration of these products. The SLN should never be taken after surgery on the breast (lumpectomy or mastectomy), but always before. The mentoring of young surgeons by seniors is the key to the success of this technique, especially for the colorimetric method which is more difficult to acquire than the double detection.
If the SLN is not visualized on planar images, do not hesitate to inject a larger volume of radio-tracer (especially in the case of big breasts) or change the injection site altogether, and switch from a superficial injection to a deep injection (see algorithm in Fig. 2).18–20
This relatively high failure rate in our study (13%) is due to the fact that this series includes large tumors (75% of cT3N0M0), knowing that lymph node invasion, itself a source of failure, is correlated with tumor size.
Our study has certain limitations which are as follows: First, the use of 2 different methods of detecting SLN (colorimetric and combined method) which creates bias in the detection rate. Then the small sample size, which may have made certain factors such as obesity or old age less significant.
ConclusionFrom this limited series, it appears that the dominant failure factors in our Algerian study population are lymph node invasion of the axillary fossa and the presence of LVE. Therefore, careful axillary exploration preoperatively can be predictive of failure, if there are several infiltrated lymphadenopathies. Likewise, the presence of LVE on the breast microbiopsy specimen, should alert the surgeon to the possibility of failure of the SLN procedure and consider applying all preventive measures to increase detection. The other factors (age, obesity) are less significant, especially when applying the double detection method. Do not give up in case of a white image on the lympho-scintigraphy, and always try an intraoperative detection. We must work to respect the consensual and validated indications of the SLN biopsy in breast cancer, and always try to present patient files in multidisciplinary consultation meetings in senology.
The ethics committee and the scientific committee of our institution gave us their approval before starting this work.
FundingThe authors have not received any funds to finance this work.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent to participateInformed consent was obtained from all individual participants included in the study.
Author contributionsAll authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Kheïra MEDJAHER. The first draft of the manuscript was written by Saïd HADDADI and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.