Vaccination for SARS-CoV-2 made it possible to reduce severe cases that require invasive mechanical ventilation (IMV) and care in the ICU. However, its impact on severe disease is not clear. The objective was to assess whether adults with severe SARS-CoV-2 pneumonia who required mechanical ventilation had a different clinical behaviour in terms of mortality, depending on their vaccination status.
MethodologyRetrospective cohort study, in adults with severe pneumonia due to SARS-CoV-2 requiring IMV and ICU. Clinical outcomes were evaluated according to vaccination status, controlling for comorbidities.
ResultsTwo hundred patients (24% vaccinated, age 61±13 years, men 68%) were analysed. The vaccinated had lower CRP at admission, distension pressure and PEEP requirement for IMV. Mortality (43.8 vs 36.8%, P: .335), hospital stay, ICU stay, and time on IMV were similar between groups. Acute kidney injury and haemodialysis requirement (0 vs 9.2%, P: .03) were more frequent in the unvaccinated. There was no association between mortality and receiving at least one dose of vaccination (RR 1.21; CI 95% 0.829–1.774, P: .335).
ConclusionsVaccination did not impact mortality. However, our data suggest that it may reduce the inflammatory state produced by the disease and the occurrence of acute kidney injury and the requirement for haemodialysis. Future studies will be required to assess the impact of the type of vaccine and/or the number of doses received.
La vacunación para SARS-CoV-2 permitió disminuir casos graves que requieren de ventilación mecánica invasiva (VMI) y atención en UCI. Sin embargo, no es claro su impacto en la enfermedad grave. El objetivo fue evaluar si los adultos con neumonía grave por SARS-CoV-2 que requirieron ventilación mecánica tuvieron un comportamiento clínico diferente en términos de mortalidad, según su estado de vacunación.
MetodologíaEstudio de cohorte retrospectiva, en adultos con neumonía severa por SARS-CoV-2 con requerimiento de VMI y UCI. Se evaluaron desenlaces clínicos según el estado de vacunación, controlando por comorbilidades.
ResultadosSe analizaron 200 pacientes (24% vacunados, edad 61 ± 13 años, hombres 68%). Los vacunados tenían menor PCR al ingreso, presión de distensión y requerimiento de PEEP para VMI. La mortalidad (43.8 vs 36.8%, p:0.335), estancia hospitalaria, estancia en UCI, y tiempo en VMI fueron similares entre los grupos. La lesión renal aguda y el requerimiento de hemodiálisis (0 vs 9.2%, p:0.03), fueron más frecuentes en los no vacunados. No hubo asociación entre mortalidad y recibir al menos una dosis de vacunación (RR 1.21; IC 95% 0.829–1.774, p: 0.334).
ConclusionesLa vacunación no impactó la mortalidad. Sin embargo, nuestros datos sugieren que puede reducir el estado de inflamación producido por la enfermedad y la aparición de lesión renal aguda y el requerimiento de hemodiálisis. Se requerirán estudios futuros para evaluar el impacto del tipo de vacuna y/o el número de dosis recibidas.
COVID-19, an infection caused by SARS-CoV-2, was declared a pandemic by the World Health Organisation on 11 March 2020.1,2 More than 600 million cases had been reported worldwide, with more than 6 million deaths by December 2022. Similarly, in Colombia, more than 5 million cases had been reported with more than 127 000 deaths.3
The implementation of vaccination has brought the rise in COVID-19 cases under control and has significantly reduced the number of severe cases requiring invasive mechanical ventilation (IMV) and intensive care unit (ICU) care. The reported effectiveness of the vaccines ranges up to 89% in preventing hospitalisation and up to 90% in preventing ICU admission with 2 doses of messenger RNA vaccination.4 With the implementation of the vaccination plan from January to September 2021, it was estimated that 445 193 new cases, 79 152 hospitalisations, 9839 ICU admissions, and 22 067 deaths were prevented in Italy.5
Some data show that there is additionally a potential impact on the course of severe forms of the disease; with less severity compared to non-vaccinated patients, less time requiring IMV, and fewer days of ICU stay.6
Despite the above, the impact of vaccination on mortality in patients who have already progressed to severe disease and required IMV remains unclear. The aim of this study was to evaluate in adults with severe SARS-CoV-2 pneumonia who required IMV whether being vaccinated compared to not being vaccinated had an impact on mortality, based on a cohort of patients managed in a referral hospital in Colombia.
MethodologyA retrospective cohort study was conducted, evaluating patients diagnosed with SARS-CoV-2 infection who required IMV and who were admitted to the ICU of the Hospital Universitario San Ignacio in Bogotá (Colombia), from February 2021 to June 2022. Inclusion criteria were: patients over 18 years of age, with SARS-CoV-2 infection confirmed by RT-PCR, antigen, or FilmArray, who had a clinical course of severe pneumonia, acute respiratory distress syndrome (ARDS), and who had received respiratory support with IMV and ICU admission. Exclusion criteria were patients referred from or to other institutions, co-infection with other viruses such as influenza A and/or B, respiratory syncytial virus or adenovirus, suspected or confirmed bacterial co-infection prior to IMV, and second episode of SARS-CoV-2 infection. The study was approved by the institutional ethics committee (approval code 180-2022).
The sample size calculation was performed according to the concept of “event of interest per variable” proposed by Freeman,8 seeking to include 10 mortality events for each of the 7 variables that were considered a potential confounder and for vaccination status. Assuming an expected mortality of 40%, the calculated sample size was 200 patients.
Data were obtained from an ICU database, where age, sex, weight, height, time of symptoms, comorbidities mentioned by the patient, admission laboratories to define severity, and SOFA on admission to the ICU were systematically recorded. Additionally, aspects related to mechanical ventilation such as time on IMV, characteristics of pulmonary mechanics (distensibility, distension pressure, plateau pressure, PEEP, and tidal volume), whether pronation was required and number of cycles, length of hospital and ICU stay, and in-hospital mortality. Finally, information was collected regarding vaccination status, number of doses, vaccine received, and time of vaccination on admission. To ensure data quality, the rate of missing data was monitored and information was verified when extreme data were found.
Severe pneumonia was defined according to the Colombian consensus for the management of SARS-CoV-2 infection (pneumonia with respiratory rate >30 rpm, respiratory distress or SaO2 <90% at ambient)1 and ARDS according to the Berlin criteria, without taking into account the PEEP criterion, since the diagnosis was considered by the treating physician prior to initiation of VMI.7 Obesity was considered when BMI was >30 kg/m.2
Categorical variables were described using absolute and relative frequency data. For continuous variables, mean and standard deviation were used if they met normal distribution criteria and median and interquartile range for those that did not. The Kolmogorov–Smirnov test was used to assess the assumption of normality. Comparison of mortality between the exposure groups (vaccinated vs unvaccinated) was performed using a t-test, Mann–Whitney U test or chi-square according to the characteristics of the variables. Considering the high frequency of mortality in the cohort, the risk ratio (RR) for in-hospital mortality was calculated, initially in a crude analysis and subsequently in an adjusted analysis controlling for possible confounding factors. This was done using a generalised linear model with a log link and binomial distribution.8 Potential confounding variables included were those that have been independently associated with mortality in previous studies (age, sex, hypertension, type 2 diabetes mellitus, obesity, chronic kidney disease, active cancer, and COPD) and biologically plausible interactions with vaccination (age and chronic kidney disease). The selection of the variables finally included in the adjusted model was made using the stepwisebackward method. A value of P<.05 was considered significant.The statistical package Stata® version 16 was used for the analysis.
ResultsA total of 200 patients were analysed, of whom 24% (n=48) had been vaccinated. The baseline clinical characteristics of these patients are presented in Table 1. The mean age was 61±13 years, with male predominance (68%). The median time of symptoms at admission was 7 days. The main comorbidities were hypertension and type 2 diabetes mellitus, as well as a history of smoking. The median SOFA score at ICU admission was 5 points. More than 70% had a lymphocyte count ≤1000 cells/μl and more than 40% had a D-dimer ≥1000 ng/ml. 73% required pronation and 81% neuromuscular relaxation.
Baseline clinical characteristics of the patients according to vaccination status.
| Characteristic | All (n=200) | Vaccinated (n=48) | Not vaccinated (n=152) | P value |
|---|---|---|---|---|
| Age (years), mean (SD) | 61 (13) | 64 (14) | 60 (12) | .073 |
| Male sex—n (%) | 137 (68.5) | 35 (72.9) | 102 (67.1) | .450 |
| Comorbidities—n (%) | ||||
| High blood pressure | 83 (41.5) | 18 (37.5) | 65 (42.8) | .519 |
| Tobacco habit | 42 (21.0) | 10 (20.8) | 32 (21.1) | .974 |
| Diabetes mellitus type 2 | 42 (21.0) | 10 (20.8) | 32 (21.1) | .974 |
| Obesity | 63 (33.5) | 12 (26.7) | 51 (35.7) | .265 |
| Hypothyroidism | 27 (13.5) | 8 (16.7) | 19 (12.5) | .461 |
| Chronic kidney disease | 17 (8.5) | 6 (12.5) | 11 (7.2) | .214 |
| Cancer active | 15 (7.5) | 3 (6.3) | 12 (7.9) | .706 |
| Chronic obstructive pulmonary disease | 14 (7.0) | 3 (6.3) | 11 (7.2) | .815 |
| Heart disease | 13 (6.5) | 2 (4.2) | 11 (7.2) | .452 |
| Length of symptoms on admission (days), median (IQR) | 7 (4–9) | 7 (4–10) | 7 (3–9) | .702 |
| Lab tests on admission | ||||
| Lymphocytes≤1000—cells/μl, n (%) | 145 (72.5) | 38 (79.2) | 107 (70.4) | .089 |
| D-dimer≥1000—ng/ml, n (%) | 87 (43.5) | 24 (50.0) | 63 (41.4) | .251 |
| Creatinine—mg/dl, median (IQR) | 0.95 (0.84–1.32) | 0.98 (0.84–1.31) | 0.94 (0.83–1.32) | .351 |
| LDH—U/L, median (IQR)) | 443 (316–587) | 405 (309–596) | 447 (316–576) | .467 |
| RCP—mg/dl, median (IQR) | 21.5 (14.5–27.5) | 18 (2–24) | 24 (17–33) | .050 |
| SOFA on ICU admission—n (%) | 5 (3–7) | 5 (3–7) | 5 (3–6) | .514 |
| Ventilatory parameters at start of IMV | ||||
| Static compliance [ml/cmH2O], median (IQR) | 38 (32–44) | 40 (37–45) | 37 (31–44) | .210 |
| Plateau pressure [cmH2O], median (IQR) | 22 (20–24) | 21 (19–23) | 23 (20–25) | .062 |
| Distension pressure [cmH2O], median (IQR) | 10 (9–12) | 10 (7–11) | 11 (9–12) | .000 |
| PEEP [cmH2O], median (IQR) | 10 (9–12) | 10 (10–12) | 12 (10–12) | .029 |
| Tidal volume [ml/kg], median (IQR)) | 7 (6.5–7) | 7 (6.5–7) | 7 (7–7) | .785 |
| Pronation—n (%) | 146 (73.0) | 32 (66.7) | 114 (75.0) | .257 |
| No. of pronation cycles, median (IQR) | 6 (4–8) | 6 (4–8) | 6 (4–7) | .358 |
| Neuromuscular relaxation—n (%) | 162 (81.0) | 28 (58.3) | 134 (88.2) | .011 |
ICU: intensive care unit; IMV: invasive mechanical mentilation; IQR: interquartile range; LDH: lactate dehydrogenase PEEP: positive end-expiratory pressure; RCP: reactive C protein; SD: standard deviation; SOFA: sequential organ failure assessment score.
When comparing the vaccinated vs unvaccinated group, RCP was lower in the vaccinated (median 18 vs 24 mg/dl, P: .05). Similarity was observed for ventilatory parameters at the start of IMV, where distensibility, plateau pressure, and distending pressure were better for the vaccinated, although only significantly different for the latter parameter (Table 1). Of the total vaccinated patients, 11 (22.9%) had received only 1 dose, 29 (60.4%) had received 2 doses, and 8 (16.6%) had received 2 doses and a booster. The vaccines received were mainly Sinovac, followed by Pfizer and finally Astrazeneca (Table 2) (see Table 3.)
Vaccination status.
| Characteristic | |
|---|---|
| Vaccination received—first dose (n=48), n (%) | |
| Sinovac | 28 (58.3) |
| Pfizer | 11 (22.9) |
| Astrazeneca | 9 (18.7) |
| Vaccination received—second dose (n=29), n (%) | |
| Sinovac | 19 (65.5) |
| Pfizer | 5 (17.2) |
| Astrazeneca | 5 (17.2) |
| Vaccination received—booster (n=8), n (%) | |
| Sinovac | 5 (62.5) |
| Pfizer | 3 (37.5) |
| Time until beginning of symptoms—first dose (days), median (IQR) | 84 (13–238) |
| Time of first dose—second dose (days), median (IQR) | 71 (32–112) |
| Time of second dose—booster (days), median (IQR) | 155 (75–198) |
| IQR (interquartile range) | |
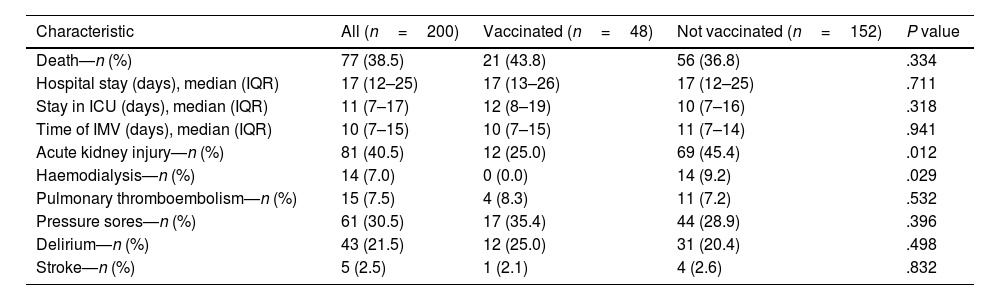
Clinical outcomes according to vaccination status.
| Characteristic | All (n=200) | Vaccinated (n=48) | Not vaccinated (n=152) | P value |
|---|---|---|---|---|
| Death—n (%) | 77 (38.5) | 21 (43.8) | 56 (36.8) | .334 |
| Hospital stay (days), median (IQR) | 17 (12–25) | 17 (13–26) | 17 (12–25) | .711 |
| Stay in ICU (days), median (IQR) | 11 (7–17) | 12 (8–19) | 10 (7–16) | .318 |
| Time of IMV (days), median (IQR) | 10 (7–15) | 10 (7–15) | 11 (7–14) | .941 |
| Acute kidney injury—n (%) | 81 (40.5) | 12 (25.0) | 69 (45.4) | .012 |
| Haemodialysis—n (%) | 14 (7.0) | 0 (0.0) | 14 (9.2) | .029 |
| Pulmonary thromboembolism—n (%) | 15 (7.5) | 4 (8.3) | 11 (7.2) | .532 |
| Pressure sores—n (%) | 61 (30.5) | 17 (35.4) | 44 (28.9) | .396 |
| Delirium—n (%) | 43 (21.5) | 12 (25.0) | 31 (20.4) | .498 |
| Stroke—n (%) | 5 (2.5) | 1 (2.1) | 4 (2.6) | .832 |
ICU: intensive care unit; IMV: invasive mechanical ventilation; IQR: interquartile range.
In crude analysis, no association was found between mortality and having received at least 1 dose of vaccination (relative risk 1.21 CI 95% 0.829–1.774, P: .334) unlike age ≥65 years (RR 2.42 CI 95% 1.71–3.44, P<.001) and history of chronic kidney disease (RR 2.37 CI 95%; 1.76–3.20, P<.001). In the adjusted analysis, again no significant association was found with vaccination status (RR 0.99 95% CI 0.68–1.45, P: .975), after controlling for age, sex, comorbidities, and interactions (Table 4).
Mortality-associated factors in patients with SARS-Cov2 pneumonia who required mechanical ventilation.a
| Variable | Crude analysis | Adjusted analysis | ||||
|---|---|---|---|---|---|---|
| Crude RR | 95% CI | P value | Adjusted RR | 95% CI | P value | |
| Vaccination, at least one dose | 1.21 | 0.82–1.77 | .334 | 0.99 | 0.68–1.45 | .975 |
| Age≥65 years | 2.42 | 1.71–3.44 | <.001 | 2.15 | 1.47–3.15 | <.001 |
| Male sex | 1.23 | 0.82–1.84 | .291 | |||
| High blood pressure | 1.31 | 0.93–1.86 | .119 | |||
| Diabetes mellitus type 2 | 1.14 | 0.76–1.70 | .533 | |||
| Obesity | 0.82 | 0.54–1.24 | .352 | |||
| Chronic kidney disease | 2.37 | 1.76–3.20 | <.001 | 1.79 | 1.31–2.45 | <.001 |
| Cancer active | 1.42 | 0.85–2.36 | .226 | |||
| Chronic obstructive pulmonary disease | 1.53 | 0.93–2.50 | .142 | |||
| Vaccination–age interaction | 1.49 | 0.96–2.31 | .114 | |||
| Interaction-chronic kidney disease | 1.76 | 0.97–3.19 | .153 | |||
In this study, we found that Colombian adults with severe SARS-CoV-2 pneumonia, who required IMV and ICU admission, did not have a significant difference in mortality rate, ICU stay, or IMV time according to vaccination status, after controlling for multiple comorbidities.
Our findings are similar to those reported by Graselli et al.9 who demonstrated how vaccination for SARS-CoV-2 decreases the likelihood of ICU admission, with RR 0.15 (95% CI 0.13–0.17), but once the patient requires admission to the unit, hospital stay (median 25 days IQR 17–36), ICU stay (median 13 days IRQ 7–24) and ICU mortality (25.9% [n=107] vs 32.4% [n=45]; P: .14) were no different between vaccinated and unvaccinated patients. In contrast, mortality during total hospital stay was higher among the vaccinated (29.9% [n=112/375] vs 40.2% [n=55/137], P: .03).
In contrast, in the study by Bruni et al.10 ICU stay was 11.4 (±7.1) days, which was lower in vaccinated patients, as was ICU mortality (38.5% vs 24.3%; P: .014). This study was conducted in Italy, where patients had been admitted to ICU, but with different oxygenation devices (not only IMV); vaccinated patients were older, more immunosuppressed, and/or had a malignancy. Differences in age, underlying comorbidities, and availability of management resources could explain the differences.
In our population, those vaccinated tended to be older, but had a similar frequency of comorbidities. This may be explained by the way in which the vaccination process for SARS-CoV-2 in Colombia was designed in stages, with the first stage including people >80 years of age and health workers, and the second stage including people between 60 and 79 years of age.11 This process was also similar in other countries,12 as evidenced in studies comparing the clinical behaviour of those vaccinated, who correspond to an older population with more risk factors for mortality.8,10
The studies with which the different SARS-CoV-2 vaccines have been developed have demonstrated their effectiveness in preventing severe disease.13 However, those who acquire the disease may also require ICU and IMV (albeit to a lesser extent).14 Our data suggest that vaccinated patients have less inflammation, reflected in less acute kidney injury and/or need for haemodialysis, lower CPR levels, as well as better respiratory mechanics at the start of IMV. This may be because once infection is acquired, thanks to vaccination, the body has the ability to react quickly by initiating immune activity and decreasing immune damage and disease severity.15
Of all the vaccines studied, the greatest effectiveness has been found in mRNA vaccines followed by inactivated virus vaccines,14 apparently due to their ability to generate a response mediated both by antibodies and by activation of cytotoxic T lymphocytes, which allows for a more rapid immune response.16 In our study, only 22.9% of those who received the first dose, 17.2% of the second dose, and 37.5% of those who received a booster had mRNA vaccination. Our sample size did not allow us to accurately assess whether vaccine type can lead to significant differences in clinical outcomes in this population. Further prospective, multicentre studies will be required to evaluate this hypothesis.
The limitations of this study are due to the retrospective nature of the study, which could have limited the assessment of comorbidities, as these were self-reported by the patient; however, if there is a misclassification bias, this would not be differential, as it would affect the entire population in a similar way, so the direction of the effect would not be deviated, but it could probably be underestimated. Similarly, some variables associated with mortality, such as heart failure or liver cirrhosis, could not be assessed in our study, since they were not assessed for all patients, making it possible that undocumented selection biases could have existed. Likewise, our size did not allow us to accurately assess the potential impact of the type of vaccine used, the combination of vaccines, booster doses, and timing of vaccine administration in relation to the time of disease acquisition. All of these factors could impact the conclusions if, for example, there were differences in mortality according to the type of vaccine used. In addition, our data may have limited external validity in populations other than Colombia, where different vaccination schedules were used. Prospective, multicentre studies will be needed to evaluate the effect of different vaccination schedules in multiple populations.
ConclusionsVaccination did not significantly impact the mortality rate in Colombian patients with severe COVID-19 requiring IMV and ICU. However, our data show that vaccination may reduce the inflammatory state produced by the disease and the occurrence of outcomes such as acute kidney injury and the need for haemodialysis. Studies with larger sample sizes will be required to assess the impact of type of vaccine and/or number of doses received in different populations.
Ethical responsibilitiesThe procedures followed have been carried out in accordance with the ethical standards of the institutional ethics committee and in accordance with the World Medical Association and the Declaration of Helsinki.
AuthorshipYina Benítez, Luis Trianab, Oscar Muñoz y Viviana López contributed to the conceptualisation of the research. Yina Benítez, Laura Niño and Santiago Bottia collected the data. Yina Benítez, Oscar Muñoz, and Viviana López carried out the data analysis and wrote the draft and final manuscript. All authors contributed to, read and approved the submitted version of the manuscript.
FundingNone.
Please cite this article as: Benítez Patiño YD, Triana LC, Muñoz Velandia OM, López Ramírez VY, Niño Guerra LM, Bottia Córdoba S. Impacto de la vacunación en la mortalidad de adultos colombianos con síndrome de dificultad respiratoria aguda por SARS-CoV-2 que requirieron ventilación mecánica invasiva. Vacunas. 2023. https://doi.org/10.1016/j.vacun.2023.07.004