The present review focuses on innate–adaptative immune stimulation by COVID-19 vaccines, especially by mRNA-iLNP vaccines. It describes iLNP and nucleoside-modified mRNA technologies, reverse transcription, inflammatory signals linked to reactogenicity, including vascular endothelial growth factor-mediated vascular cross-talk, induced by systemic and spike protein, which mimic COVID-persistent. Finally, the connection between the manifestation of severe forms of adverse reactions to vaccination and molecular mimicry, the production of particular autoantibodies and the role of certain vaccine adjuvants are discussed in detail.
ObjectivesTo identify articles that publish information on the adverse effects produced after the administration of COVID-19 vaccines in order to demonstrate their therapeutic potential in the treatment–prevention of disease; as well as to demonstrate the association of causality and temporal ocurrence.
MethodologySystematic review of the scientific literature published between July 2021 and July 2023, which analyses all reports of inflammatory signatures of serious adverse effects caused by COVID-19 vaccines.
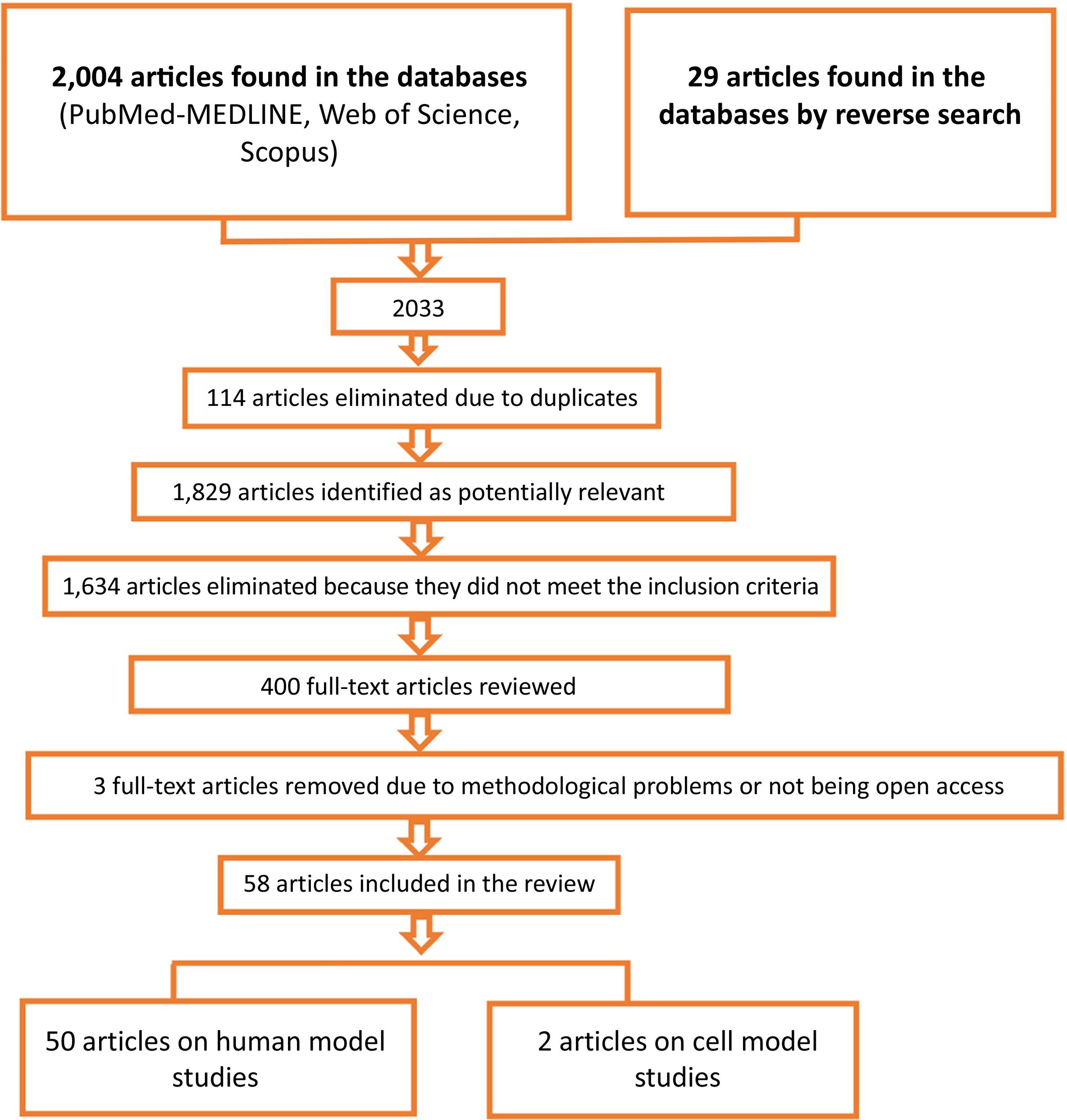
ResultsThe systematic review identified 2033 records which, after a screening process according to the inclusion criteria and the elimination of duplicated papers, work with methodological problems and work without open access, were reduced to 58 articles, of which 50 articles are human models and 2 are cellular models.
ConclusionThe results of this systematic review reveal the causal and temporal association of the various serious adverse events following administration of COVID-19 vaccines and the “peak effect” of COVID-19 vaccines is recognised.
La presente revisión se centra en la estimulación inmunitaria innata-adaptativa por las vacunas COVID-19, especialmente por las vacunas ARNm-iLNP. Se describen las tecnologías iLNP y ARNm modificado con nucleósidos, la transcripción inversa, las señales inflamatorias vinculadas a la reactogenicidad, incluye la diafonía vascular mediada por el factor de crecimiento endotelial vascular (VEGF), inducida por la proteína pico con efecto sistémico y, que imitan el COVID-persistente. Por último, se discuten en detalle la conexión entre la manifestación de las formas graves de las reacciones adversas a la vacunación y el mimetismo molecular, la producción de autoanticuerpos particulares y el papel de ciertos adyuvantes de las vacunas.
ObjetivosIdentificar los artículos que publican información sobre los efectos adversos producidos después de la administración de las vacunas COVID-19 para demostrar su potencial terapéutico en el tratamiento y/o prevención de la enfermedad; así como evidenciar la asociación de causalidad y ocurrencia temporal.
MetodologíaRevisión sistemática de la literatura científica publicada entre julio de 2021 y julio de 2023, que analiza todos los reportes sobre firmas inflamatorias de efectos adversos graves causados por las vacunas contra la COVID-19.
ResultadosLa revisión sistemática ha permitido identificar 2033 registros que, tras un proceso de cribado de acuerdo con los criterios de inclusión y la eliminación de trabajos duplicados, de trabajos con problemas metodológicos y de trabajos sin acceso libre se redujeron a 58 artículos, de ellos, 50 artículos son modelos humanos y 2 corresponden a modelos celulares.
ConclusiónLos resultados de esta revisión revelan la asociación causal y temporal de los distintos efectos adversos graves posteriores a la administración de las vacunas COVID-19 y se reconoce el «efecto de pico» de las vacunas COVID-19.
The leading vaccine technologies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) include recombinant glycoprotein, weakened/inactivated adenovirus, and lipid-nanoparticle (LNP)-encapsulated mRNA (Pfizer-BioNTech Comirnaty, Moderna Spikevax).1,2 Nucleoside-modified mRNA vaccines against coronavirus disease 2019 (COVID-19) are the first mRNA products to be approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These vaccines' main components are: nucleoside-modified mRNA, which has the capacity to encode antigenic protein, in this case the SARS-CoV-2 spike (S) protein, and lipid nanoparticles containing ionizable lipid (iLNP), which function as a delivery vehicle for intact mRNA to the cytoplasm of cells that will translate the encoded protein.3–5 These nucleoside-modified mRNA-iLNP vaccines are highly effective in inducing spike-specific adaptive immune responses in humans, especially neutralising antibodies to create protective immunity against SARS-CoV-2 infection, as well as promoting humoral immunity via T cells and preventing severe forms of COVID-19.1,3,6–8 However, we know very little about the dynamics and structure of the spike protein of vaccine platforms, how innate immune pathways regulate adaptive immunity, or which immune responses are protective and which are dispensable.
The present review focuses on innate/adaptive immune stimulation by COVID-19 vaccines, especially by mRNA-iLNP vaccines. In the first part, we define the concept of iLNP and nucleoside-modified mRNA technologies, and provide a detailed study on the dynamics and structure of the S protein of COVID-19 vaccines, reverse transcription, exposure and subsequent dissemination to the cell line and integration into the host genome. Then, the inflammatory signals linked to reactogenicity, including vascular endothelial growth factor (VEGF)-mediated cross-talk, induced by the spike protein with systemic effect and mimicking long COVID. Finally, we discuss in detail the connection between the manifestation of severe forms of adverse reactions to vaccination and molecular mimicry, the production of particular autoantibodies, and the role of certain vaccine adjuvants.
Nucleoside-modified mRNA and ionisable lipid nanoparticle technologiesSafe and effective vaccines must stimulate the innate immune system in such a way that they achieve a balance between immunogenicity and reactogenicity. They must deliver the signals necessary to maintain and prime adaptive immune responses. This refers to the ability of vaccines to act in a specific situation without causing excessive local and systemic inflammatory effects.3
A number of factors must be considered that stand in the way of using synthetic mRNA for biomedical (vaccine or therapeutic) applications, such as the highly inflammatory mechanical and biocompatible properties for recognising mRNA molecules by innate sensors in subcellular compartments and the inefficient cytosolic delivery of mRNA in vivo.3,9–11
First, it is important to highlight the need to select a manufacturing method adapted to innate immune recognition of mRNA. Karikó et al. (2005) identified the expression of certain modified ribonucleosides working as immune sensors discriminating self-RNA from foreign RNA versus unmodified ribonucleosides. In particular, the replacement of uridine with natural uridine derivatives (pseudouridine [ψ] and its derivatives) is used to attenuate inflammation and facilitate translation. The aforementioned mechanical properties are essential for mRNA to mitigate or escape detection by most immune sensors and mimic their microstructural features. This technological advance was essential to create the approved COVID-19 mRNA vaccines, uridines are replaced with N1-methylpseudouridine (m1ψ).3,4,12,13
Finally, a key requirement in the manufacture of COVID-19 vaccines is that it is biocompatible. It must be chosen based on mRNA delivery efficiency, biodegradability, and tolerability to withstand cell culture manipulation and the biological activities of the host. The approved COVID-19 mRNA-iLNP vaccines use ionisable lipids (ALC-0315 in the Pfizer-BioNTech vaccine and SM-102 in the Moderna vaccine). iLNPs comprise a polyethylene glycol (PEG)-conjugated lipid that confers platform stability, cholesterol, a cationic “ionisable” amino lipid, and 1,2-diestearoyl-sn-glycero-3-phosphocholine.3,14,15
The iLNP technology not only enables the delivery of mRNA into innate immune cells after vaccination, but also plays a special role with strong adjuvant activity in this type of vaccine platform. The main characteristic of nucleoside-modified mRNA is its ability to not produce inflammation, mRNA-iLNP vaccines are not immunosilent, as verified by the strong innate immune activation and local and systemic adverse event reports post-COVID-19 vaccination in humans.1,3,12,16–53 This would change our understanding of the current mRNA vaccine paradigm, of how it activates the innate immune system, and would raise the prospect for refining the design for more effective and safer mRNA vaccines and treatments in the near future. The advantage of mRNA-iLNP platforms is that they do not require the addition of adjuvants to induce robust protective immune responses against various pathogens.1,3
It is necessary to understand how nucleoside-modified mRNA components versus iLNP excipients function in the overall immune response elicited by the new generation of COVID-19 mRNA vaccines. As we shall discuss below, the main driver of adjuvanticity and reactogenicity of the mRNA-iLNP vaccines is the iLNP carrier. It activates a variety of specific signals including pro-inflammatory cytokines and chemokines. For example, granulocyte-macrophage colony-stimulating factor (GM-CSF); tumour necrosis factor (TNF); interferon-gamma (IFN-γ); interleukins (IL): IL-1β, IL-6; CC chemokines, e.g., CC motif chemokine ligand 2, 3, and 4 (CCL2, CCL3, CCL4); CXC motif chemokine ligand: 2 and 10 (CXCL2, CXCL10).3,15,16,54–57
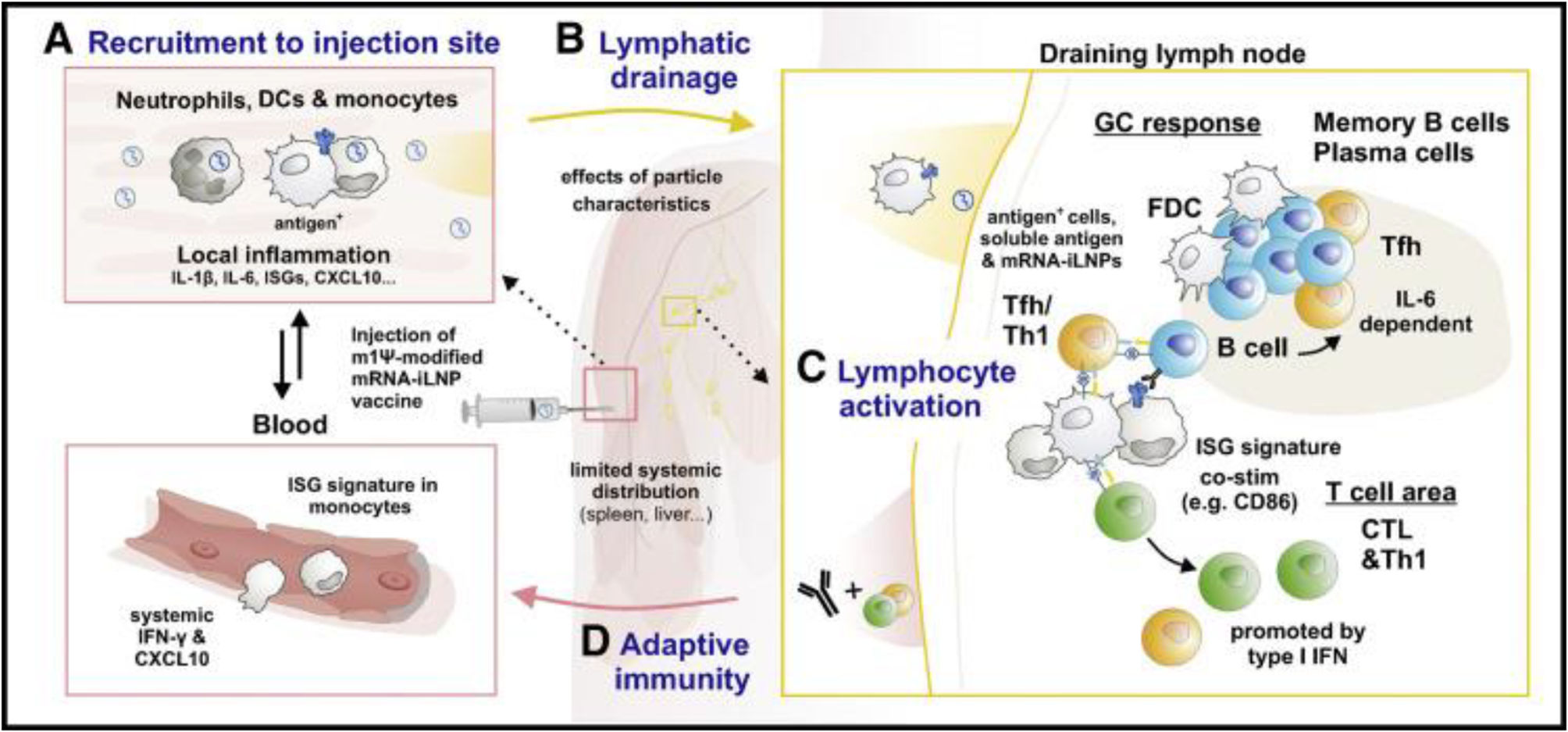
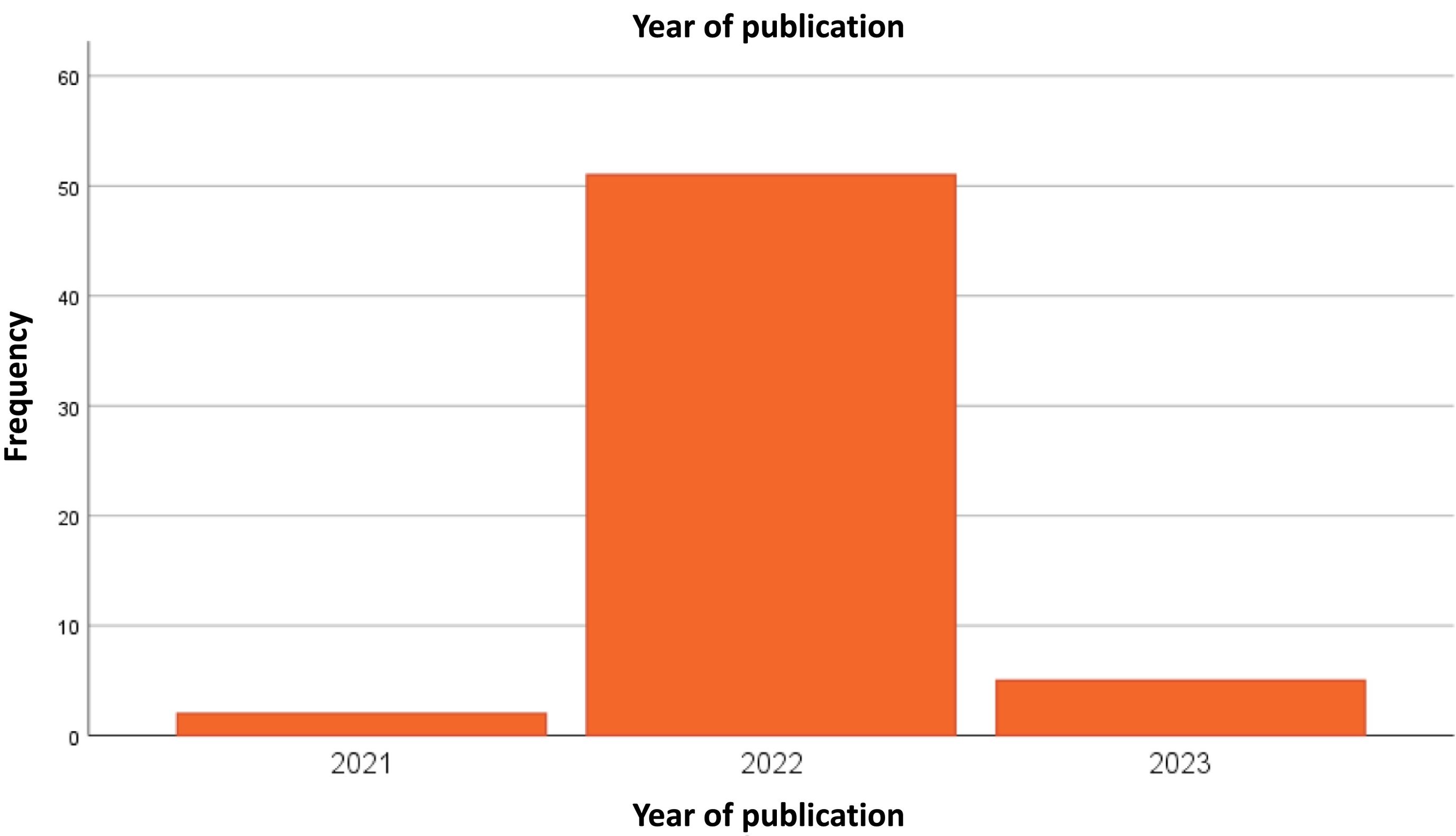
Pharmacokinetics of nucleoside-modified mRNA-iLNP vaccines. Biodistribution and dynamics of innate immune cells after administration of mRNA-iLNP vaccinesmRNA-iLNP vaccines travel in the body according to the route of administration and iLNP formulation. Intramuscular administration of COVID-19 mRNA vaccines and other similarly designed vaccines results in the uptake and production of the encoded antigen at the site of inoculation and subsequent drainage into the lymph nodes (LNs). In addition, limited mRNA and/or lipid spread was detected in other non-draining tissues such as lungs, liver, spleen, and LNs.3,14,15,54 Verbeke et al. (2022) identify a similar biodistribution in adjuvant protein subunit vaccines.3,58 Other studies showing lower seropositivity of the SARS-CoV-2 spike protein in plasma from humans and mice receiving Pfizer's BNT162b2 vaccine show how the spike protein, or its mRNA template (mRNA-iLNP or cell-associated mRNA) can spread systemically after intramuscular inoculation.3,15,58–60Fig. 1 summarises the mechanisms of innate immune cells after administration of mRNA-iLNP vaccines (biodistribution and dynamics).
Dynamics and biodistribution of innate immune cells following mRNA-iLNP vaccination. (A) Intramuscular administration of nucleoside-modified mRNA-iLNP vaccines leads to local inflammation, recruitment of neutrophils, dendritic cell (DC) subsets, and monocytes through the production of chemokines and other inflammatory mediators involved in immune cell extravasation. (B) Antigen expression. mRNA-iLNPs spread to lymph nodes and drain. Biodistribution, cellular uptake, and protein uptake (opsonisation), limited by surface characteristics and size of innate immune cells. (C) Monocytes/macrophages and DCs are involved in antigen expression and T-cell priming. (D) Induction of affinity maturation. Follicular helper T cells (Tfh) drive B cells in germinal centre (GC) reactions in the presence of follicular DCs. Dysfunction in inflammatory signalling, e.g., iLNP-induced IL-6 in stimulating B cell Tfh and GC responses, IFN type 1 induces CTL (cytotoxic T lymphocyte) responses. Verbeke et al (2022).2,3,15,17,57
Proteins produced after mRNA-iLNP inoculation appear rapidly. In pre-clinical studies, they peak 4–24h after administering the vaccine and decline progressively, ranging from several days to weeks or months. This process will depend on the encoded protein, the mRNA dose, the route of mRNA-iLNP administration, and the type of iLNP.3,13–15,25,35,43,59,61,62 These features not only offer high and sustained antigen availability, but also favour more robust antibody responses, making them support for adherent cells, and improve survival of cell infiltration and differentiation, favouring protein production from mRNA vaccination compared to other vaccine platforms. For example, both spike-encoding mRNA and spike protein are detectable 60days after the second dose of BNT162b2 and mRNA-1273 in vaccinated humans, located in axillary germinal centres (GCs).1,3,16,25,27,31,38,40,59,63,64
There is little literature on the dynamics of protein/iLNP interactions for lymphatic and cellular dissemination, although it is acknowledged that the opsonisation of mRNA-iLNP induces detection and uptake by innate immune cell receptors. Nucleoside-modified mRNA-iLNP vaccines elicit T follicular helper cell (Tfh) responses and the creation of GCs, essential for the generation of particular autoantibodies, with high affinity and persistence.3,65 Verbeke et al. (2022) report a persistence of more than 6months after the second dose of 30μg mRNA in draining LNs in humans, the resulting GC B and Tfh cells leading to the generation of affinity mature B cells and long-lived bone marrow plasma cells. The versatility of COVID-19 mRNA vaccines in humans, COVID-19 mRNA vaccines induce antigen-specific circulating Tfh cells, as well as CD4+ T cells with T helper 1 (Th1) polarisation and IFN-γ-producing CD8+ T cells which remain detectable up to 6months post-vaccination.1,3,15,66–71 Another point to consider is the strong immunogenicity associated with the surface decoration of nanoparticles with PEG that modifies the topological structure to prevent aggregation of nanomaterials and facilitate distribution in the lymphatic system, slowing opsonization processes, and their phagocytosis.3,15,57 Accordingly, PEG lipids desorb from the iLNPs, to support binding between endogenous proteins and lipids in the extracellular space, forming a biomolecular “corona” around the iLNP, including various lipoproteins, immunoglobulins, and complement fragments abundant in blood.3,72–74 It is also possible that neutrophils could compete with other immune cells for the uptake of MRNA-iLNP vaccines by efficiently internalising at the injection site, but they show weak reporter protein encoding. In contrast, monocyte, and dendritic cell (DC) subsets take up and translate mRNA-iLNP better.3,15,57,75
In these studies, neutrophils are dispensable for B-cell Tfh and GC responses compared to frequencies of monocytes and/or myeloid DC subsets and macrophages that are increased in draining LNs and express a greater number of co-stimulatory markers such as CD80 and CD86 compared with cells in the contralateral non-draining LNs.3,60,76 The mechanisms of iLNP-mRNA uptake by the innate immune system and their physicochemical parameters such as surface composition, morphology, and size of the iLNPs are not yet well understood, which may condition the pharmacokinetics of these vaccines.
Immunological identification of nucleoside-modified and unmodified mRNAThere is little public information on the exact methods of RNA preparation (transcription, protection, and purification) used by Pfizer-BioNTech, Moderna, and other RNA vaccines, which makes research in this field difficult. Three categories are investigated of innate immune sensing of synthetic mRNA, which are: (1) uridine-dependent recognition of various RNA species,68,77 (2) recognition of double-stranded RNA (dsRNA),78–80 and (3) recognition of the 5′ end of mRNA if not properly capped.3,81–83 Identification of uridine-containing RNAis associated with increased expression of pro-inflammatory cytokines, particularly type 1 interferon (IFN-1), which further promotes the expression of RNA sensors, causing inhibition of antigen expression from the mRNA via protein kinase R (PKR) and 2′,5′-oligoadenylate synthetase (OAS).3,4 The modification of nucleosides is required for clinical success and the widespread deployment of these vaccines today. However, it is not clear how nucleoside modification per se induces reactions in GCs compared to unmodified RNA. Verbeke et al. (2022) propose several hypotheses that are not mutually exclusive: (1) The modified mRNA protein is presented with higher and longer parameters over time than unmodified mRNA. This would drive GC reactions, which are promoted by prolonged antigen availability, giving the platform better kinetics,3,64 (2) the main difference between modified and unmodified mRNA is not (only) the overall protein expression, but also differential expression in key antigen-presenting cell types (monocytes, macrophages, and DC). These cell types may be especially sensitive to the translation-inhibiting effects of unmodified mRNA due to higher expression of RNA sensors, for example, TLR7/8 genes, hindering a key pathway of T-cell priming: direct presentation of translated antigens to CD4+ and CD8+ T cells,3,13,54,75,84 (3) the cytokine dysfunction generated by modified mRNA-iLNP would produce increased reactogenicity, conditioning GC responses compared to responses induced by unmodified mRNA-iLNP.3
Unmodified mRNA can also elicit protein expression and neutralising antibody reactions, thus promoting strong antigen expression and immunogenicity.3,11 This theory verifies the decrease in inflammation caused by uridine following mRNA administration. However, the likelihood of yielding an immunosilent mRNA is low; not all uridines can be removed from the mRNA sequence. The alternative could be partial removal of uridine,1,3,6,17 this would explain the differences in immunogenicity and reactogenicity between COVID-19 vaccines.
In addition, dsRNA (unwanted RNA) is involved in “deleterious” innate immune recognition. In vitro transcription (IVT) by T7 RNA polymerase yields the desired RNA, but also a set of unwanted RNA species, including short abortive transcripts. It also produces antisense RNAs transcribed from the promoter-less end of the DNA template.3,85 This could drive the formation of dsRNA, inducing a potent inflammatory response and translational blockade via the recognition of various intracellular receptors: PKR, OAS, melanoma differentiation-associated protein 5 (MDA-5), endosomal Toll-like receptor 3 (TLR3), retinoic acid-inducible cytosolic receptor gene I (RIG-1), laboratory of genetics and physiology 2 (LGP-2), mitochondrial antiviral signalling protein (MAVS), DEAH-box helicase 33 (Homo sapiens, human [DHX33]), etc., initiate an interferon response to single-stranded RNA.3,12,13,15,78,86 DsRNA sensors play an essential role in translational blockade, these are the IFN-inducible cytosolic sensors OAS and PKR. Secondary and tertiary double-stranded structures also form based on the sequence of the mRNA.3 It can be argued that the preparation of the mRNA vaccine involves destroying any therapeutic mRNA. These deleterious innate immune responses can be reduced chemically by 2 methods; first, as discussed above, the inclusion of modified nucleosides such as ψ, m1ψ, and 5-methylcytidine to reduce the activation of PKR and OAS sensors, and second, the removal of dsRNA from IVT mRNA by another chemical treatment known as purification.3,12,13 Finally, the 5′ co-transcriptional capping strategy impacts the translatability and immune activation of IVT mRNA.3,83
The use of modified uridines is now recognised as a key aspect of the effectiveness of COVID-19 mRNA vaccines used by Pfizer-BioNTech and Moderna. Unfortunately, both the inflammatory capacity of the mRNA component and its translation are altered by other factors.
Activity of vaccine adjuvants. Inflammatory signature similar to long COVID. Innate immune sensing of ionizable lipid nanoparticles (iLNP)iLNPs function as delivery agents, both empty iLNPs and mRNA-iLNP complexes act as adjuvants. The ionisable lipid component is necessary for the adjuvant effect, alone they do not induce robust antibody responses. Importantly, mRNA-iLNP and iLNP-adjuvanted protein vaccines induce similar, much stronger, humoral, and cellular immune responses in Tfh and GC B cells.3,13,15,16,65 An understanding is beginning to emerge of the potent adjuvant activity of iLNPs and the inflammatory milieu that they stimulate. After immunisation, in the draining LNs, both nucleoside-modified mRNA-iLNPs and empty iLNPs or iLNPs comprised of non-coding RNA stimulate the production of several CC- and CXC-motif chemokines (CXCL1, CXCL2, CXCL5, CXCL10, CCL3, CCL4) and cytokines IL-1β, IL-6, leukaemia inhibitory factor (LIF) and GM-CSF.3,16,57 The rapid, efficient, and potent production of these inflammatory signals explains the induction of Tfh cells and GC B cells, as well as the infiltration of immune cells in the injected tissues.3,56
Available data on innate immune activation in humans following mRNA-iLNP administration are limited, however, the studies published to date are consistent with previous reports in animals. They indicate a similar inflammatory signature in the serum of mice after vaccination with BNT162b2: CXCL10, CCL4, IL-6, interferon alpha and gamma (IFN-α, IFN-γ).54 Li et al. (2022) highlight how vaccine-stimulated IFN-I and IFN-stimulated gene (ISG) signatures in various cell types including monocytes, macrophages, DCs, natural killer (NK) cells, peak at day 1 and return to baseline levels by day 7, whereas NK cells and T cells exhibit a continuous increase in expression of cell-cycle- and transcription-associated genes (analysed by single-cell transcriptional profiling in draining LNs). Six hours after administration of a second vaccine dose, serum IFN-γ is 8.6-fold higher, coming largely from CD4+ and CD8+, compared with 6h after the first dose, when most of the IFN-γ is derived from NK cells.3,54 It is not clear which component of the mRNA-iLNP vaccine can induce type 1 IFNs. They state that IFN-γ signalling activates antigen-presenting cells, but, in this condition, plasmacytoid DCs produce IFN-α only when exposed to mRNA-iLNP and not empty LNPs, supporting the theory that the mRNA component may be responsible for inducing type I IFN.75
A higher concentration of IFN-γ is generally observed, i.e., a general immune dysfunction following vaccination, consistent with reports of adverse effects following approved mRNA COVID-19 vaccinations, mainly for systemic reactions, and it is theorised that enhanced T cell and myeloid cell activation results in cross-talk between lymphocytes and myeloid cells that increases their responsiveness to subsequent vaccine encounters and/or COVID-19 infection.2,3,31,54,74,86–91 It is suggested that mRNA-iLNP vaccines have persistent enhancing effects on trained immunity (myeloid cells).3,60,91,92 There may be important species-dependent differences in inflammatory responses that need to be considered when testing vaccines in animal models, including reactogenicity, fever, and induction of other inflammatory cytokines stimulated by mRNA-iLNP. Human peripheral blood mononuclear cells (PBMCs) treated in vitro with m1ψ-modified mRNA-iLNPs formulated with SM-102 lipid release IL-β, IL-6, TNF, CCL5, vascular endothelial growth factor (VEGF-A), GM-CSF, and other molecules have been detected, and research is needed in this area.3,15,55 Plant-Hately et al. (2022) note the stimulation of basophils in the generation and release of histamine within the vascular system.50,57
The innate immune signalling pathways implicated in the iLNP adjuvant effect are: (1) oxidised phospholipids or metabolised and/or modified lipid products, e.g., oxidative impurities of ionisable lipid93; (2) individual cholesterol and lipids, e.g., ionisable lipids16,56,57; (3) the entire nanoparticle or other multimolecular structures; (4) endogenous molecules (apolipoprotein (ApoE) or complement proteins) that bind to iLNPs after inoculation with the mRNA vaccine are involved in both sensing mechanisms and receptor-mediated uptake by innate immune cells; (5) finally, the presence of a cellular receptor, such as a TLR, that specifically detects iLNPs.3,13,15 Certain vaccine components such as LNPs and cationic liposomes are sensed by nucleotide binding domain, TLR2, TLR4, the stimulator of interferon genes (STING), and protein 3 (including the leucine-rich repeat pyrin domain-containing protein 3 [NLRP3]).3,57,94–96 In contrast, Li et al. (2022) and Pichmair et al. (2009) suggest that certain nucleic acid and microbial lipid sensors, including inflammasome mediators, are not required for a strong immune response to this vaccine.54,97 It is therefore possible that SARS-CoV-2 vaccines composed of viral vectors (AstraZeneca's Vaxzevria and Johnson & Johnson's Janssen) and protein subunits (Novavax's Nuvaxovid) deliver more attenuated inflammatory signals or use other mechanisms that contribute to innate immune activation, not just the mRNA encapsulated in lipid nanoparticles of Pfizer's Comirnaty BioNTech, Moderna's Spikevax, and Curevac. For example, Li et al. (2022) analyse the immunogenicity of the BNT162b2 vaccine (CD8+ T-cell and antibody response), it is not attenuated in the absence of TLR2, TLR4, TLR5, TLR7, protein 3 including leucine-enriched pyrin repeat domain (NLRP3), apoptosis-associated Speck-like protein also containing caspase (CARD/ASC), cyclic GMP-AMP synthetase (cGAS), or the stimulator of interferon genes (STING). They suggest the possibility that mRNA (modified with m1ψ and without dsRNA) or its degradation products may be sensed by the above sensors sending signals, supporting the theory of its function as an adjuvant.3,12,16,17,22,26–28,30,31,33,34,36,37,39,41–44,46–54,57,98
Some of the mechanisms are detailed below:
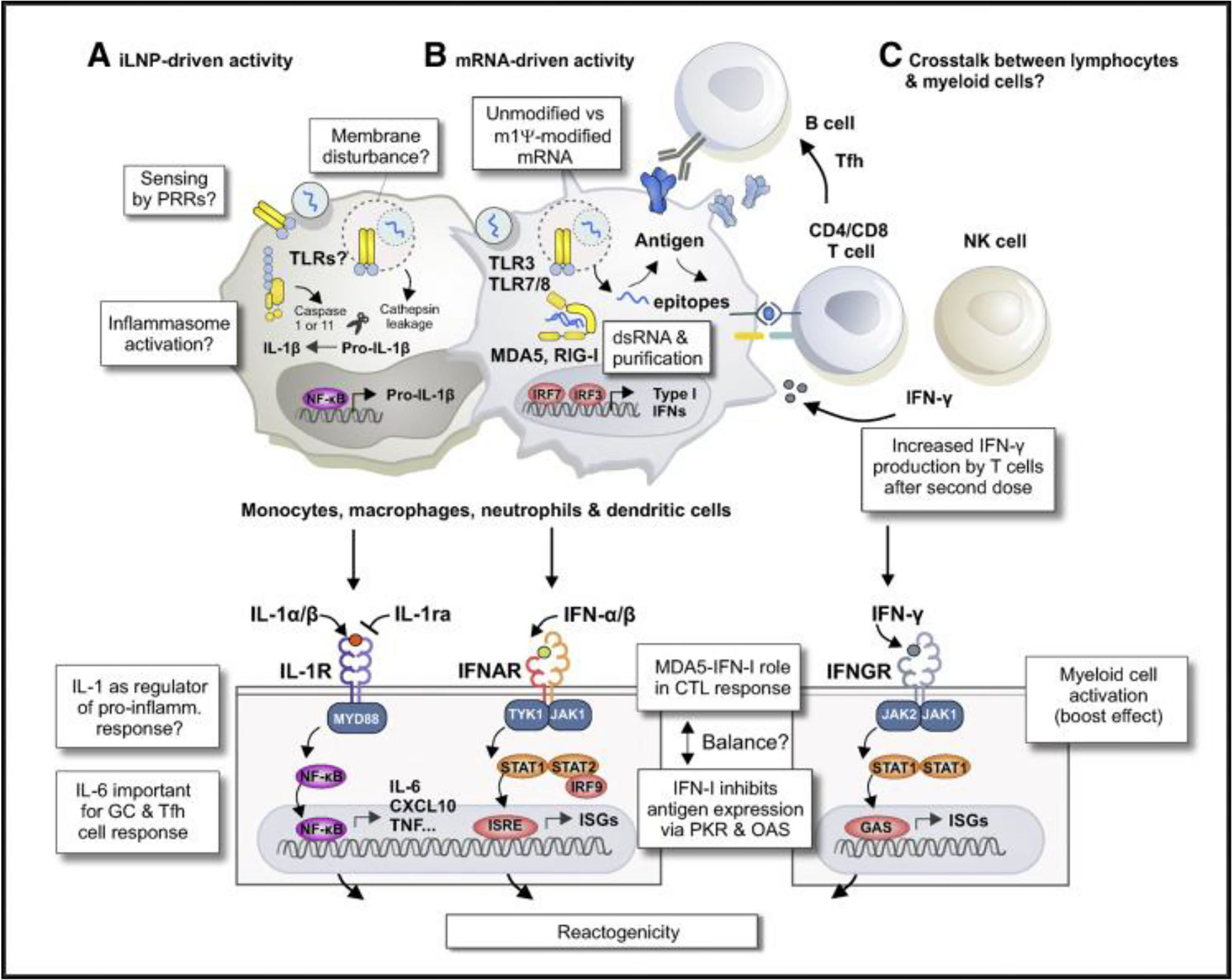
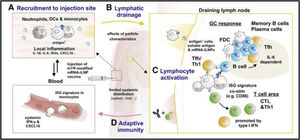
As mentioned above, a particular sensor is capable of directly and/or indirectly sensing iLNPs or their degradation products. For example, Ndeupen et al. (2021) describe how a polycytidine (non-coding) mRNA-iLNP triggers expression of a necroptosis-associated gene set, which could cause the release of inflammatory damage-associated molecular patterns (DAMPs) from dying cells.3,56 Recently, Plant-Hately et al. (2022) tested a major pro-inflammatory histamine-like immunoregulatory mediator, IL-1β. It is generally stimulated through the exposure of immune cells to various microbial-associated molecular patterns and DAMPs through inflammasomes such as NLRP3. It has been shown that liposomes containing Moderna's ionisable cationic lipid SM-102 can induce the release of IL-1β from peripheral blood cells, suggesting intracellular pattern recognition through a receptor such as NLRP3.57 Also, sensing this mRNA vaccine associated with secondary and tertiary mRNA structures or other elements involved in priming, such as incomplete dsRNA removal, is possible.3 Another innate immune signalling pathway proposed by Holm et al. (2012) involves sensing membrane disturbances caused by fusion of iLNPs with the plasma or endosomal membrane (morphological changes, cationic membrane patches, etc.).3,99 Inflammasomes include a set of pattern recognition receptors and adaptor proteins that respond to a variety of danger signals. For example, the cytokine IL-β1 has a potent iLNP adjuvant effect, is commonly detected in PBMCs, animals, and humans exposed to empty iLNPs or mRNA-iLNPs.3,54 It has been shown that iLNPs are designed to be fusogenic and that they are able to penetrate the endolysosomal membrane into the cytosol, a common feature of viral infections.3 Verbeke et al. (2019) state that RNA sensing in bridging innate and adaptive immune responses to viral infections, and also impede the therapeutic role of mRNA vaccines, hindering clinical success by suppressing the synthesis of the encoded antigen and causing adverse reactions.3,10,13Fig. 2 summarises the innate immune sensing mechanisms of synthetic mRNA and its lipid transport.
Innate immune mechanisms involved in the immunogenicity and reactogenicity of mRNA-iLNP vaccines. (A) Uptake of empty iLNPs by innate immune cells and other cell types induces local and systemic inflammation, characterised by the release of pro-inflammatory cytokines such as IL-1β and IL-6. (B) Preparation of synthetic mRNA. The incorporation of modified uridines and purification process of IVT mRNA lowers the recognition of IVT mRNA by TLR3, TLR7, TLR8, and other RNA sensors. These modifications are important to minimise the negative effects of type I IFN-stimulated RNA sensors on protein expression of antigen-encoding mRNA and prevent cytotoxicity. Signalling pathway associated with melanoma differentiation-associated gene 5-interferon type α (MDA5-IFN-α) in the induction of CTL to BNT162b2 in a mouse animal model indicates residual type I IFN activity in the current generation of mRNA vaccines. (C) After administration of the second dose of vaccine, strong boost in T cell responses associated with increased IFN-γ production. Enhanced activation of T cells and myeloid cells after booster vaccine reflects cross-talk between lymphocytes and myeloid cells. Verbeke et al. (2022).2,3,15,17,57
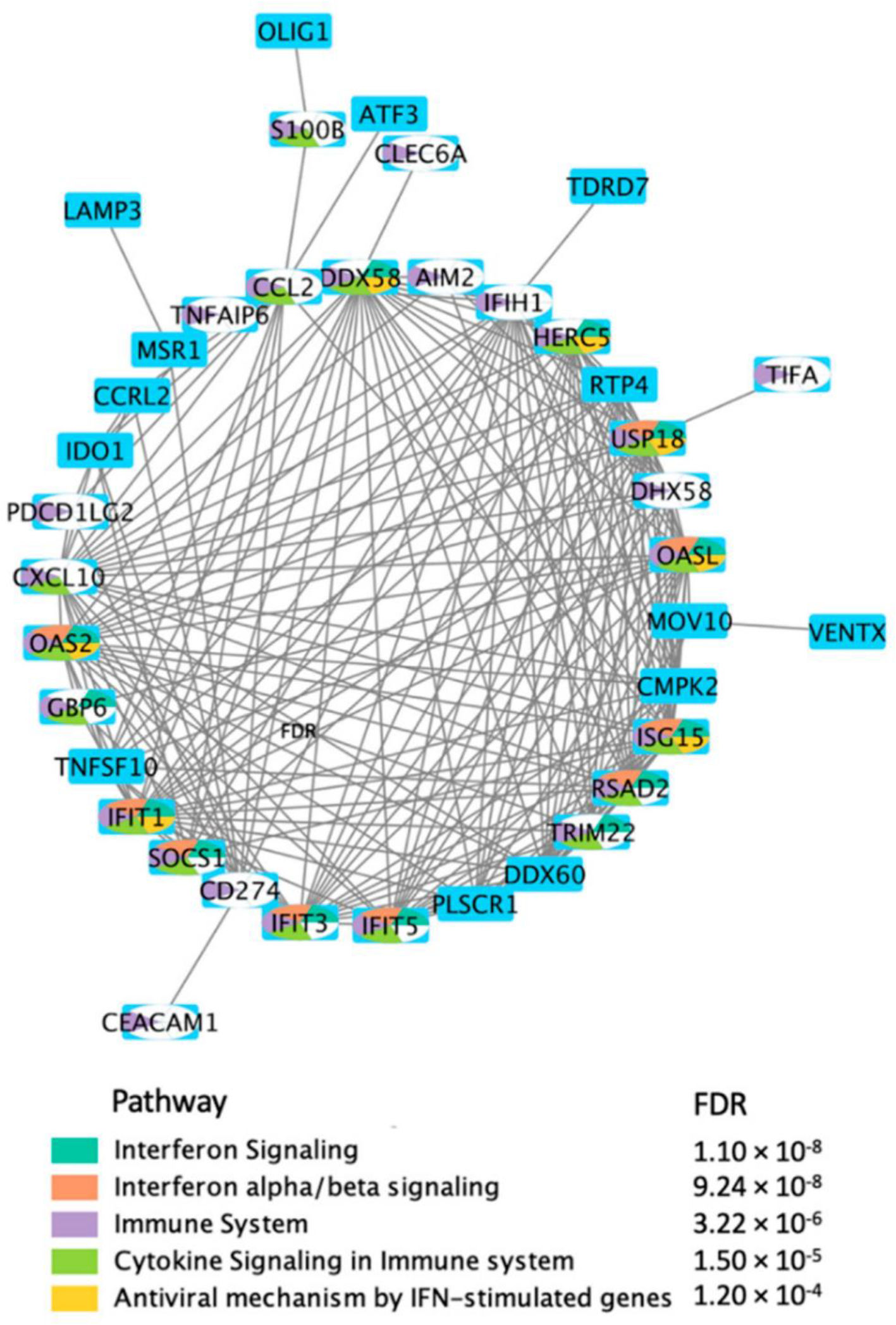
Direct protein–protein interaction network (PPI) using the DEGs, in response to BNT162b2 vaccine on day 22. Hajio et al. (2022).156
Szebeni et al. (2022) indicate additional adjuvants as favouring immune sensing and antigen presentation through complement activation (CARPA), antigen-depot effects, and/or the possibility that iLNPs could deliver mRNA to specific cell types or subcellular compartments. Lipids and lipid-based nanoparticles induce chemokines, whereas therapeutic nucleic acids (e.g., mRNA) induce interferon responses.15
Understanding the molecular mechanisms by which the inflammatory signature induced by COVID-19 vaccines, in particular synthetic mRNA and its lipid carrier iLNP, is sensed will help in the design of the next vaccines.
Severe adverse effects after vaccination. Molecular mimicry theory-reverse transcriptionSome of the reported adverse effects of COVID-19 vaccines, which may overlap with manifestations reported during post COVID-19 or long COVID-19, include: new-onset neuroimmunological disorders (myasthenia gravis [MG], Guillain-Barre syndrome [GBS] or atypical “pseudo-Guillain-Barré” variant, seizures), varicella zoster virus reactivation, cranial neuropathies (vestibular neuritis, Bell's palsy, abducens nerve palsy, olfactory dysfunction, sensorineural hearing loss), neuromyelitis optica, transverse myelitis (TM), neuromyelitis optica spectrum disorder (NMOSD), longitudinal extensive transverse myelitis (LETM), acute encephalopathy, acute disseminated encephalomyelitis (ADEM), small vessel vasculitis, narcolepsy, neuroleptic malignant syndrome (NMS), chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME); flares or risk of conversion to multiple sclerosis, myositis, dysautonomia (POTS), temporary headaches (intermittent or persistent); cognitive impairment (subjects report loss or impairment of executive, planning, memory and lexical-semantic functions); haematological autoimmune diseases (secondary immune thrombocytopaenia (ITP), immune thrombotic thrombocytopaenic purpura (iTTP), autoimmune haemolytic anaemia (AIHA), Evans syndrome, aplastic anaemia, antiphospholipid syndrome (APS), catastrophic APS (CAPS), and vaccine-induced thrombotic thrombocytopaenia (VITT); vasculitis (cutaneous, IgA vasculitis, ANCA-associated vasculitis, large vessel vasculitis); cerebrovascular complications (cerebral venous sinus thrombosis, ischaemic stroke, haemorrhagic stroke); other rare clinical neurological conditions (Tolosa-Hunt syndrome, Parsonage-Turner syndrome, small fibre neuropathy), and functional neurological disorder (FND); glomerular disease (immunoglobulin A nephropathy [IgAN]); hearing disorders (tinnitus, progressive loss without remission, vertigo with/without spontaneous nystagmus, and dizziness); intestinal dysbiosis; cardiovascular diseases (myocarditis, pericarditis, perimyocarditis/myopericarditis, heart failure, acute coronary syndrome, arrhythmias, palpitations, haemodynamic instability, and autonomic dysregulation); visual disturbances (referred to as decreased visual acuity, floaters, flashes of light, photopsia, vision-obscuring curtains, visual field defects, greyish spots or blurred vision, proptosis, red eye, scalp pain, ophthalmoplegia, retrobulbar pain, temporal headache, uveitis, optic neuritis, etc.); myeloid/lymphoid neoplasms; menstrual cycle irregularities and specific pregnancy outcomes, including maternal and foetal data (miscarriages, pre-mature births, congenital conditions, maternal and neonatal mortality rates); systemic inflammatory response syndrome (SIRS); anaphylactic and anaphylactoid allergic reactions (type III and IV HSR, fatal fulminant necrotising eosinophilic myocarditis); skin disorders (macrophage activation syndrome, systemic erythema).1,12,16,23,24,26–39,41,42,44–53,100–106 These long-term effects will affect physical and mental health, interfering with work activity, therefore these symptoms must be identified immediately and treated as soon as possible to avoid progression of the damage and disability they cause.107,108 The pathogenesis of these diagnoses is mainly neurological, digestive, and cardiovascular, attributable to the dysfunctional mechanism triggered by the S protein used for immunisation or during infection, which could bind to the neurovascular receptor NRP-1 and act as an antagonist to VEGF binding. It also suggests the “spike effect”, known as “spike intoxication” in the context of saturation and subsequent impairment of angiotensin II-converting enzyme receptor (ACE2) function.12,16,19,21,23,25–29,34,39–43,46,50–53,101,103,107,109,110Table 1 summarises the current evidence, which supports the hypotheses on molecular mimicry, inflammatory signals attributed to post-vaccination reactogenicity, their systemic spread, and the possibility of a “cumulative effect”, i.e. the relationship between elevated antibody levels (anti-S IgG, anti-RBD IgG) post-vaccination and their temporal persistence, suggesting post-COVID-19 mimicry.
Cellular mechanisms and pathogen/viral, autoinflammatory, autoimmune, and paraneoplastic pathways associated with adverse effects in recipients of COVID-19 vaccines.
| Cellular mechanisms and hypotheses associated with serious adverse events following vaccination | Cellular mechanisms | Association with reported adverse effects of COVID-19 vaccines | References | ||
|---|---|---|---|---|---|
| INVERSE TRANSCRIPTION | Aldén et al. (2021) present the first study on the effect of the COVID-19 mRNA vaccine BNT162b2 on the human liver cell line Huh7 in vitro. They show rapid entry of BNT162b2 into cells (6h after exposure to BNT162b2) and subsequent intracellular reverse transcription of BNT162b2 mRNA into DNA, they indicate a possible mechanism for reverse transcription via endogenous reverse transcriptase with the long interspersed nuclear element 1 (LINE-1), and nucleus distribution of the LINE-1 protein being elevated by BNT162b2 and leading to robust expression of the BNT162b2 antigen9 | They suggest studying whether liver cells also present the vaccine-derived SARS-CoV-2 spike protein, which could make liver cells targets for pre-primed spike-reactive cytotoxic T cells consistent with reported cases of autoimmune hepatitis following BNT162b2 vaccination.At this stage of reverse transcription, the authors are concerned about BNT162b2-derived DNA integrating into the host genome and affecting the integrity of genomic DNA, which would lead to genotoxicity.9 In the toxicity report of BTT162b2, neither genotoxicity nor carcinogenicity studies have been provided,72 the authors point out that they do not know whether the DNA reverse transcribed from BT162b2 integrates into the cell genome.9 | Further studies are needed to verify the effect of BNT162b2 on genomic integrity, including whole genome sequencing of cells exposed to BNT162b2 and tissues from human subjects receiving BNT162b2 vaccine. They also indicate endogenous LINE-1 expression and/or elevated levels associated with viral infection, including SAR-CoV-29 infection.9 The exact regulation of LINE-1 activity in response to BNT162b2 should be further studied | The cell model used in this study is a carcinoma cell line with active DNA replication that differs from non-dividing somatic cells, showing significantly different expression of proteins and genes. However, cell proliferation is also active in several human tissues such as: bone marrow, basal layers of the epithelium and during embryogenesis. In addition, they report effective retrotransposition in non-dividing and terminally differentiated cells, such as human neurons.9 Pfizer's evaluation report also showed that BNT162b2 distributes in the spleen, adrenal glands, ovaries, and testes.27 Therefore, the effect of BNT162b2 on genomic integrity under such conditions needs to be investigated. No data on placental transfer of BNT162b2 are available in the EMA assessment report on Pfizer | 9,27,72 |
| HYPERSENSITIVITY RESPONSES TO COVID-19 VACCINES | Plant-Hately et al. (2022) in their study discussed above, related to the cellular mechanisms of basophils, showed that cell surface proteins (CD63, CD203c, and CD164) have upregulated expression during immune system activation in response to allergen recognition111 | It is useful to look at the level of expression of inflammatory biomarkers. In this case, the 3 selected basophil activation markers exhibit distinct expression patterns that may not be mutually exclusive, suggesting several activation pathways. These mechanisms occur in eosinophils during granulation.111 | – | – | 111 |
| Szebeni et al. (2022) describe another useful model in the evaluation of hypersensitivity responses to mRNA-based SARS-CoV-2 vaccines. They describe all components of mRNA-iLNP vaccines (mRNA, carrier, excipients and expressed antigen), which have diverse immunostimulatory effects on a broad spectrum of effector and target cells (antigen presenting cells [APCs], T and B lymphocytes, platelets, NK cells, and myocytes) and biochemical pathways (complement and coagulation), all of which are necessary to achieve vaccine efficacy. The same components also contribute to hypersensitivity reactions (HSRs) and other types of immune-mediated adverse effects (IMAEs) through their wide inter-individual variability in both quality (spectrum of inflammatory mediators) and quantity (complement split products, cytokine levels, tryptase and induced and/or pre-existing antibodies)15 | Anaphylaxis is an IMAE in the type-1 immediate-type hypersensitivity category (ITH) category. Classical ITH leads to the release of preformed and newly synthesised mediators such as histamine, tryptase, interleukins, prostaglandins, and leukotrienes, among others. Although cytokines are produced by activated immune cells during ITH and other types of HSR, some authors separate anaphylaxis from cytokine release syndrome, they also highlight the function of biomarkers to define the genotypes that underlie the different phenotypes of anaphylaxis. It is known that mediator binding to tissue and cellular receptors induces local and systemic symptoms that affect the skin, respiratory, gastrointestinal, and cardiovascular systems1,2,15,16,35-39,41,42,45-53,82,86,88,110,112-114 | Anaphylaxis can occur without immunoglobulin E (IgE) through newly described (IgG) G-coupled protein receptors, by direct activation of mast cells and/or basophils and by activation of the complement system (anaphylactoid reaction or CARPA pseudoallergy). The enzymatic cleavage of complement will create anaphylatoxins that act as cytokines and activate immune cells to stimulate secondary inflammation mediators. Anaphylatoxins can also activate mast cells, both IgG and allergen-specific immunoglobulin M (IgM) are involved in the activation of the complement system1,2,15,41,42,48,50,52,82 | Reported delayed reactions to COVID-19 vaccines suggest type III and IV HSR by their timing of onset and symptoms, most have been reported in the context of IMAE to SARS-CoV-2 vaccines, especially mRNA.1,2,15,41,42,48,50,52,82Mast cell activation through IgE and non-IgE mechanisms must be taken into account, this condition occurs in people with clonal and non-clonal mast-cell disorders, including asthma, mastocytosis, acute myeloid leukaemia, and myelodysplastic syndrome. They may have high levels of proteases (tryptases), histamine, heparin, and cytokines that are associated with strong mast cell activation. It could be considered that they could develop an increased predisposition to HSR to SARS-CoV-2 mRNA vaccines without symptoms of anaphylaxis, a pre-vaccination risk assessment is required2,15 | 1,2,15,16,35-39,41,42,45-53,82,86,88,110,112-114 | |
| In another study, Yoshida et al. (2023) confirm the potential risk of experiencing more adverse reactions after vaccination with BNT162b22,115 | The authors suggest that people with allergic disease, such as asthma, hay fever, allergic rhinitis, atopic dermatitis, food allergies and/or intolerances, who are potentially susceptible to COVID-19, are associated with the development of systemic adverse reactions and a worsening of their chronic disease after vaccination with BNT162b2, which is also a risk factor for anaphylactic shock and HSR115 | – | – | 2,115 | |
| Granados Villalpando et al. (2022) conclude that the BNT162b2 and ChAdOX1 nCOV-19 vaccines are strongly associated with the occurrence of allergic reactions, with OR of 1.6 (95% CI; 1.18–2.3) and 1.87 (95% CI; 1.35–2.6), respectively. They also associate a higher likelihood of developing adverse effects and/or allergic reactions associated with COVID-19 vaccines with female sex, older age, and prevalence of comorbidities after vaccination and COVID-19 infection after vaccination. They report a significantly high prevalence of asthma (7.8%) in this setting, already assessed in other studies75,116 | Another variable to consider is the genetic diversity of the human population that influences the great inter-individual variation in cytokine responses. One dose of the same immune adjuvant produces an optimal cytokine response in one subject, which may be too strong or too weak for another subject. In summary, innate and adaptive immune responses vary between individuals due to their cellular and biochemical components. In addition, the SARS-CoV-2 spike protein contains the sequence and structural motif of a superantigen, causing the hyperinflammatory syndrome that involves direct T-cell stimulation and excessive cytokine hyperproduction1,2,12,15,16,20,24,26,28,29,31,35-39,41,44,45,47-52 | These cytokines interact with complement systems which, in addition to producing inflammation and cross-talk between innate-adaptive immunity, activate the coagulation system and increase the permeability of the endothelial layer in a time- and dose-dependent manner, in turn promoting their distribution to the systemic circulation, resulting in elevated inflammation1,2,12,15,16,20,24,26,28,29,31,35–39,41,44,45,47–52 | In fact, the fall in RANTES (regulated upon activation, normal T cell expressed and secreted) is known to increase the risk of cardiovascular events in patients with atherosclerosis. The low plasma concentration of RANTES may reflect its increased deposition in the vascular endothelium of areas with atherosclerosis which in turn leads to an increase in its human CC chemokine receptor type 5 (CCR5) and worsens inflammatory organ damage. These data would explain the recruitment of IFN-gamma-secreting T cells to the vessel wall (confined to the adventitia and intima) and reinforce the suggestion that it may be a site of immune privilege, i.e., recruitment, retention, and infiltration of IP-10 and RANTES chemokines. The CCR5 receptor is a G-protein-coupled receptor (GPCR) that plays an important role in inflammation and is involved in cancer, HIV, and COVID-19117–119 | 1,2,12,15,16,20,24,26,28,29,31,35-39,41,44,45,47-52,75,116-119 | |
| PLATELET ACTIVATION | Both L5NP-mRNA and the spike protein activate platelets15,20,24,26–28,39,41,47–50,52 | This study specifies the involvement of platelets in inflammation, proposed as a biomarker of anaphylaxis, CARPA, and HSR through the release of biologically active molecules (ATP, thromboxane, and chemokines) and lipid inflammatory molecules such as platelet activating factor (PAF), which triggers the degranulation of perivascular mast cells and the release of thromboxane and serotonin, also causing inflammatory responses and tissue injury15,20,24,26–28,39,41,47–50,52 | – | – | 15,20,24,26-28,39,41,47-50,52 |
| OXIDATIVE STRESS | Another study attributes oxidative stress to nanoparticle-mediated toxicity of some drugs15 | Related to HSR, it inhibits the negative regulation of complement and induces complement-mediated toxicities. Individuals with common variable immunodeficiency (CVID) may develop HSR to SARS-CoV-2 mRNA vaccines. They also discuss the variation of human leukocyte antigens (HLA) associated with an increased risk of reactions mediated by T cells and likelihood of reactions to SARS-CoV-2 mRNA vaccines for the development of HSR1,15 | – | – | 1,15 |
| NEUROVASCULAR CROSS-TALK MEDIATED BY VASCULAR ENDOTHELIAL GROWTH FACTOR (VEGF) AND INDUCED BY THE SARS-COV-2 SPIKE PROTEIN | Neurological and cardiovascular adverse effects simulating prolonged COVID-19 have been reported in recipients of COVID-19.1,12,16,21,24,26-28,30,34,35,37-39,41,42,44-47,49-52,103 The clinical similarity is due to the shared pathogen of the SARS-CoV-2 spike (S) protein used for immunisation and produced by the virus. The study by Talotta (2022) describes the impaired vascular endothelial growth factor (VEGF)-mediated neurovascular cross-talk induced by SARS-CoV-2 spike protein associated with the side effects of COVID-19 vaccines and explaining prolonged COVID-19 symptoms107 | The S protein can bind to neuropilin 1 (NRP-1), which normally functions as a co-receptor for vascular endothelial growth factor A (VEGF-A). By antagonising the docking of VEGF-A to NRP-1, the S protein could disrupt physiological pathways involved in angiogenesis and nociception. The consequence would be an increase in unbound forms of VEGF that could bind to other receptors. People vaccinated and/or infected with SARS-CoV-2 exhibit elevated plasma levels of VEGF during convalescence and acute illness, which could be responsible for diffuse neurological and microvascular damage. Other studies are indicated suggesting that serum VEGF may also be a potential biomarker for COVID-19 vaccines, and prolonged COVID infection1,16,22,27,107,104 | More research is needed to better understand the clinical course, pathogenesis, and treatment of adverse effects following vaccination. Recent research suggests that the adverse effects of COVID-19 vaccines are the result of a profound endotheliopathy caused by both direct viral damage and hyperactivation of the immune response. In addition to favouring viral entry, the SARS-CoV-2 S protein interferes with the physiological function of bound receptors, e.g., NRP-1/VEGF-A-mediated pathways, resulting in dysfunctional complications and thus producing symptoms, even in the absence of organ damage16,27,28,30,35,39,41,44-47,49,51,53,104,107,120 | But the evidence in favour of this mechanism is scant given the lack of experimental data | 1,12,16,21,22,24,26-28,30,34,35,37-39,41,42,44-47,49-53,103,104,107,120 |
| CALPROTECTIN, NEUTROPHIL EXTRACELLULAR TRAPS (NET) AND SYNDECAN LEVELS AS PREDICTORS OF SEVERITY | The study by Hetland et al. (2022) recently demonstrated that levels of neutrophil extracellular traps (NETs) correlate with the severity of side effects of the ChAdOx1 vaccine. Calprotectin, NETs, and syndecans are key inflammatory markers with physiological effects involved in innate immune activation and vascular endothelial damage, respectively. Calprotectin is the main cytosolic protein in neutrophil granulocytes, used as a sensitive faecal marker in gastrointestinal diseases, and has antimicrobial and cytotoxic properties through zinc binding. It has been associated as a predictor of severe prognosis in COVID-1946 | NETs are webs of DNA surrounded by histones and granular proteins, which are expulsed from neutrophils to trap and destroy invading pathogens in the extracellular space. Elevated levels of NETs have been demonstrated in post-COVID-19 and NETosis has been associated with thrombosis formation in patients with vaccine-induced immune thrombotic thrombocytopaenia (VITT). Circulating NETs are removed from the blood by extracellular DNAases that digest free DNA. Syndecan-1 is a glycocalyx marker for damaged endothelium in the vasculature, elevated levels of syndecan-1 have been demonstrated in COVID-1946 | Vascular endothelial damage and dysfunction is suggested as a prognostic indicator for post-COVID-19, a disease in which there is a direct effect of immune response dysregulation on endothelial damage, which, as discussed in this study, is an essential pathological response to infection that in severe cases results in certain side effects of vaccination46 | Therefore, if elevated NET and calprotectin levels can be detected early after vaccination with ChAdOx1, these biomarkers may be useful predictors for a severe outcome of vaccine complications. They could be used after vaccination with the Janssen vaccine, which is also an adenoviral vector vaccine with the potential to trigger VITT46 | 46 |
| LOSS OF ACE2RECEPTOR ACTIVITY | Angelini et al. (2022) note that spike proteins produced after vaccination have the native-like mimicry of the SARS-CoV-2 spike protein's receptor binding functionality and prefusion structure. COVID-19 vaccines increase endogenous synthesis of SARS-CoV-2 spike proteins. Once synthesised, the free-floating spike proteins released by the destroyed cells previously targeted by the vaccines circulate in the blood and interact with ACE2 receptors (a zinc metalloproteinase) expressed by other cells, promoting ACE2 internalisation and degradation, mimicking the pathological features of SARS-CoV-2121 | This mechanism can increase the imbalance between angiotensin II (Ang II) overactivity and Ang1–7 deficiency through loss of ACE2 receptor activity, triggering inflammation, thrombosis, increased blood pressure and other adverse reactions, the mechanism known as the “spike effect” of COVID-19 vaccines. Also, the detrimental effects of deficiency of other angiotensins (prolyl oligopeptides[POP] and prolyl carboxypeptidases [PRCP]) on blood pressure, thrombosis, and inflammation have been well studied121,101 | The authors compare the relationships between different mechanisms of Ang II cleavage and accumulation, offering the possibility of closing the pathophysiological loop between the risk of progression to severe forms of COVID-19 and adverse reactions to SARS-CoV-2 vaccination101,121 | The hypothesis of loss of ACE2 receptor activity due to interaction between these receptors and free-floating spike proteins can be observed at all stages of cardiovascular disease progression. They add that increased catalytic activity of POP and PRCP is not common in the young population, but is more frequent in the elderly with comorbidities or previous cardiovascular events. Therefore, reported adverse reactions with Ang II accumulation associated with COVID-19 vaccination will be more likely in young, previously healthy subjects101,121 | 101,121 |
| NEUROINVASION AND NEUROIMMUNE CROSS-TALK AS INDUCERS OF NEUROLOGICAL SYMPTOMS PRODUCED BY SARS-CoV-2 VACCINATION | The above-mentioned study by Talotta (2022) details the physiopathological cross-talk between NRPs and VEGFs, reflecting the interconnection between vessels and nerves from both an anatomical and functional perspective, their role in tumour progression and invasiveness of several types of cancer has been extensively studied due to the mitogenic effects of VEGF in NRP-overexpression in malignant cells and in multiple sclerosis lesions43,107,122,123 | Impaired NRP-1 activity can cause dysfunctional neurological symptoms, expressed in a variety of cells such as endothelial cells, neurons, glial cells, and immune cells. NRP-1 is involved in cell proliferation, migration, survival, and invasion during embryogenesis and carcinogenesis. It is involved in immunological synapse formation, B-cell differentiation, and immunotolerance18,91,107,124,125 | VEGF-A is closely associated with systemic inflammation. Neuropilins 1 and 2 (NRP-1, NRP-2) act as co-receptors enhancing the binding of VEGF-A to VEGF receptor 2 (VEGF-R2) in blood vessels and the binding of VEGF factor C (VEGF-C) to VEGF receptor 3 (VEGF-R3) in lymphatic vessels, respectively.107,125-128 The physiological functions of NRP-2 is less well studied, this receptor can bind to other types of semaphorin and VEGF ligands and participate in the development of the lymphatic system during embryogenesis. NRP-2 controls functions such as phagocytosis, chemotaxis, and antigen presentation, as it is abundant in immune cells107,129,130 | The author describes a single route for the pathogenesis of the NRP-1/VEGF-A pathway, the NRP-1-dependent entry route for human T-cell lymphotropic virus type 1 (HTLV-1), a human retrovirus able to infect CD4+ and CD8+ T lymphocytes, monocytes/macrophages, and DCs. Infection causes lymphoproliferative, inflammatory disorders and/or neuromyelopathies, including neuroinflammatory alteration, and alter the blood–brain barrier, which may explain the development of the adverse effects cited above.1,16,33-39,41,45,51,114,122,123,131 In addition, like other viral infections, VEGF-A variations may reflect changes in circulating pro-inflammatory mediators, such as tumour necrosis factor alpha (TNF-α), IL-1β, IL-6, IL-8, monocyte chemotactic protein 1 (MCP-1, also known as chemokine ligand 2 [CCL2]), and IFN-γ-inducible protein 10 (IP-10). Searching for VEGF-A in plasma samples could accurately reflect the actual concentrations of this mediator in peripheral blood, it is known that the concentration of VEGF in serum samples could be affected by the number of platelets, platelets being a major source of VEGF-A3,107,112,127 | 1,3,16,18,33-39,41,43,45,51,91,107,112,114,122-131 |
| Chen et al. (2022) propose neuroinvasion and neuroimmune cross-talk as inducers of neurological symptoms produced by SARS-CoV-2 vaccination. On the one hand, the shared SARS-CoV-2 pathogen can infect the brain directly, via haematogenous propagation and retrograde axonal transport, except for the olfactory neuron pathway. On the other hand, it can induce indirect neurotoxicity through immune-mediated pathogenesis and gastrointestinal infection132 | They report 13 cases of classical GBS associated with vaccination (infection), with mild pulmonary involvement and negative SARS-CoV-2 tests in cerebrospinal fluid. They conclude that not all neurological symptoms require direct infection of the nervous system; indirect neurotoxicity secondary to immune-mediated pathogenesis may occur. They also report autoantibodies binding to endothelial and epithelial cells, which could induce some of the cell lyses107,132 | Similarly, in patients with post-COVID-19 infection, antibodies to the virus may attack antigens of endothelial cells in cerebral vessels. Gastrointestinal infection also threatens the nervous system by increasing the transport of aberrant proteins like alpha-synuclein (α-synuclein), which are associated with neurodegenerative diseases132 | They highlight the importance of aggravated brain damage caused by nerve injury leading to abnormal intestinal blood flow and intestinal dysmotility, which could further promote entry of metabolites and bacterial components into the blood and brain parenchyma132 | 107,132 | |
| OREXINERGIC SYSTEM. ASSOCIATION BETWEEN REDOX IMBALANCE, INFLAMMATION, AND ENERGY METABOLISM | Garrido Suárez et al. (2022) postulate the hypothesis of the orexinergic (Ox) system linked to inflammatory signals, specifically its involvement in reactogenic somnolence following peripheral activation of the innate immune system by COVID-19 vaccines. They call for their consideration by pharmacovigilance and, further understanding of possible additional long-term inflammatory mechanisms in future studies to preserve confidence in these vaccines. Further research identifying biomarkers linked to the reactogenicity of COVID-19 vaccines is needed to better understand their immunogenicity133 | They suggest a mechanistic link between reactogenic inflammatory parameters and hypothalamic circuits regulating the sleep–wake cycle. They propose that pro-inflammatory cytokines INF-γ, TNF-α, and IL-1β may propagate a peripheral inflammatory response after vaccination, activating a subset of lateral hypothalamic area neurotensin-expressing GABAergic neurons (LHA Nts), as well as inhibitory neurons from sleep-inducing areas providing inhibitory input to Ox neurons that that control wakefulness. The adenosinergic modulation of sleep–wake signals could also be involved.133,134 In this case, immunity and sleep are bidirectionally linked, but the cellular and molecular mechanisms underlying these interactions are limitless and not entirely understood | In some cases, somnolence, narcolepsy, or other sleep disorders (prolonged sleep deficiency) are reported as a moderate adverse effect of vaccination, most commonly reported in Pfizer-BioNTech COVID-19 vaccination. Prolonged sleep deficiency can lead to chronic low-grade systemic inflammation, associated with several diseases that share an inflammatory component (neurodegeneration, cancer, diabetes, and atherosclerosis)12 | This would indicate that the vaccine is effective because it may produce immunological memory, in response to pronounced immune activation. The sleep-modulating effect is triggered by pathogen components and decomposition products, including the pathogen-associated molecular patterns of PAMPs (endotoxins), peptides, lipids, and viral double-stranded RNA discussed above. These factors are recognised in macrophages or dendritic cells resident in various tissues, as well as downstream pathways like the nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB), controls transcription and inflammasomes, which induce cytokine and prostaglandin expression. Vaccine antigens as potential pathogens causing autoimmune reactions through the mechanisms of molecular mimicry and T-cell activation are recognised, further research is warranted1,2,12,13,16,24,26-29,31,35-39,41,44,47,48,50-53,105,135 | 1,2,12,13,16,24,26-29,31,35-39,41,44,47,48,50-53,105,133-135 |
| Other mediators involved in the neurobiological bases and regulation of sleep are hormones of the hypothalamus–pituitary–adrenal (HPA) axis, neuropeptides such as orexin, the hormone melatonin, and classical neurotransmitters (γ-aminobutyric acid [GABA], glutamate, serotonin, acetylcholine, histamine, noradrenaline, and dopamine). These molecules are imbalanced in the functional interaction between the immune system and the nervous system135,136 | In addition, dense projections from Ox neurons in spinal, diencephalic, and cortical regions are involved in regulating diverse physiological functions. Therefore, Ox neurons not only regulate eating, energy metabolism, and the sleep–wake cycle, but also closely regulate cardiovascular control, cognition and mood, nociception, reproduction, stress, reward and addiction18 | Recent studies support the elevated co-expression of the leptin receptor (LepRb) in some subpopulations of Ox neurons, which are activated by the adipose tissue-derived hormone leptin,137 and also specify the role of galanin (GAL) as a potential mediator of leptin to modulate nutrient reward by inhibiting orexin neurons via GAL receptor 1 (GalR1).138 They speculate that following vaccination with COVID-19 vaccines, pro-inflammatory cytokines may propagate an inflammatory response to the hypothalamus causing sleep disorders through 2 pathways: leptin, which has pro-inflammatory properties and up-regulates the secretion of multiple inflammatory cytokines and the subset of GABAergic sleep-inducing neurons. In both cases, the action of pro-inflammatory brain cytokines may be mediated by prostaglandins or nitric oxide produced in endothelial cells of cerebral blood vessels and perivascular macrophages133 | Another potential biomarker is myeloperoxidase (MPO), a pro-inflammatory enzyme that triggers oxidative stress and brain neuroinflammation during the acute and chronic phases of COVID-19. Oxidative stress from MPO occurs by promoting the production of reactive oxygen and nitrogen species, which in turn causes damage to the lungs (chronic hypoxia) causing some of the symptoms seen in the adverse effects of COVID-19 vaccines and in long COVID. In turn, the damage caused by this factor to the blood–brain barrier leads to neuroinflammation, by activation of microglia, also leading to a drop in cerebral perfusion which generates, among other typical symptoms, chronic fatigue and mental confusion86,139,140 | 18,86,133,135-140 | |
| POTENTIAL BIOMARKERS THAT INCREASE NEUROVASCULAR, CARDIOVASCULAR, HEPATIC (HEPATOCELLULAR DAMAGE), AND INTESTINAL MUCOSAL INFLAMMATION WITH REGIONAL IMMUNE SYSTEM AND NEURO/MYOENTERIC DYSFUNCTION | Other potential biomarkers have been studied and could be proposed to investigate adverse effects of COVID-19 vaccines. Growth differentiation factor 11 (GDF-11) is expressed in several organs and tissues, including skeletal muscle, intestine, pancreas, heart, nervous system, olfactory system, retina, and kidneys. It is also highly concentrated in platelets.135,141,142 Low plasma concentration of this bioprotective factor has been shown to be associated with ageing in mice losing their reported actions to promote skeletal muscle regeneration, muscle dysfunction.141,143 Findings from subsequent studies show that GDF-11 may cause skeletal muscle atrophy rather than regeneration13,141,144 | However, in humans, results related to circulating plasma levels are variable, showing a decrease, increase/trend to an increase or no change with ageing.141,135,136,145 Falling plasma levels of GDF-11 in the context of adverse effects of COVID-19 vaccines could contribute to myalgia and chronic fatigue/asthenia or symptoms referred to as functional component fibromyalgia in patients, some of which are reported by pharmacovigilance systems, such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Although it should first be ensured that myalgia symptoms (muscle pain without elevated creatine phosphokinase levels) are not caused by statin treatment (up to 10% of statin-treated patients have myalgia)141 | Therefore, the GDF-11 factor may be involved in the metabolism, regulation of inflammation and may be involved in thyroid pathophysiology.141 Recently, BMP11 target genes were found to be down-regulated by Reference microRNA (miRNA) and YAE1 and RSU1 by the SARS-CoV-2 Delta variant, a diagnostic predictor validated through the differentially expressed gene (DEG) dataset).58,146 Falling plasma levels of fibroblast growth factor 21 (FGF21) contribute to glucose intolerance, higher blood insulin levels, and the development of fatty liver. FGF21 promotes weight loss through increased fatty acid oxidation and decreases triglyceridaemia and blood glucose by improving insulin sensitivity. This peptide functions as a hormone with anti-inflammatory, anti-diabetic, and anti-obesity effects. It is produced in the liver, adipose tissue, skeletal muscle, and pancreas, although its endocrine action depends mainly on the liver. FGF21 is induced in situations of muscle stress, particularly in mitochondrial myopathies58 | A fall in both factors could increase neurovascular, cardiovascular, and hepatic vulnerability (hepatocellular damage), leading to intestinal mucosal damage and inflammation, and regional immune system and neuro/myenteric dysfunction. The disorders reported by pharmacovigilance systems suggest the persistence of these symptoms over time and are experienced as: gastrointestinal disorders (intestinal dysmotility, abdominal inflammation and pain, dyspepsia), musculoskeletal pain (myalgia), cognitive impairment (difficulty concentrating, loss of memory, executive functions, and lexical-semantic functions), headaches, sleep disorders, and chronic disabling fatigue.1,16,42,49,51,58,141,146 The association between redox imbalance, inflammation, and energy metabolism must be an urgent objective of the therapeutic plan | 1,13,16,42,49,51,58,135,136,141-146 |
| DYSFUNCTION BETWEEN THE INTESTINAL BARRIER AND THE IMMUNE SYSTEM | Data from clinical studies in animal models indicate that the composition of the gut microbiota plays an essential role in modulating immune responses to vaccines, but the mechanisms by which the gut microbiota modulates immunogenicity to different vaccines in various populations are not clear. The addition of natural adjuvants to enhance responses to vaccination is suggested.147-149 The intestinal barrier plays a role in protecting the intestinal mucosal layer tissues and circulatory system from exposure to pro-inflammatory molecules, such as micro-organisms, toxins, antigens and is vital for the maintenance of health and well-being. Gut barrier dysfunction has been implicated in several diseases such as food allergies, microbial infections, irritable bowel syndrome, inflammatory bowel disease, coeliac disease, metabolic syndrome, non-alcoholic fatty liver disease, diabetes, and septic shock96,148,150,151 | In the samples studied, the decrease in Bacteroides thetaiotaomicron and cellulosilyticus is very negative for maintaining the integrity of the intestinal wall (intestinal homeostasis), which favours an increase in intestinal permeability due to a deterioration of tight junctions, allowing the passage of intestinal contents into the bloodstream, with its serious consequences such as increased hepatic toxic load and increased antigenic load, among others.148-150 Ng et al. (2022) describe the composition of the gut microbiota by shotgun metagenomic sequencing in stool samples from 138 vaccinees (37 with CoronaVac and 101 with BNT162b2) in relation to immune responses and adverse effects in adults who received inactivated CoronaVac vaccine or the mRNA vaccine Pfizer's BNT162b2 BioNTech Comirnaty147 | They observe both spike and neutralising antibody levels 1 month after the first and second doses with Pfizer positively correlated with the efficacy of the mRNA vaccine. They suggest a longitudinal assessment of the gut microbiota profile and long-term (longer than 1 month) antibody response after inoculations. This study reinforces the theory of vaccine immune response in the context of HSR.1,147 The protective bacterial microbiota provides the microenvironment that prevents overgrowth of proteolytic bacteria and pathogens. The balance between the resident bacterial species confers stability to the overall microbial population. The barrier effect is due to the ability of certain bacteria to secrete anti-microbial substances (bacteriocins), which inhibit the proliferation of other bacteria, and to competition between bacteria for resources in the system, either nutrients or ecological space149,150 | Bifidobacterium adolescentis when abundant, is usually due to the use of probiotics of this bacterial species. Probiotics should always be used under the supervision of a physician. Some experiments have been reported where protein metabolites of Bifidobacterium adolescentis could cause hepatotoxicity in cells of human origin (THLE-2). Therefore, monitoring with liver function test would be advisable.148–151Bacteroid thetaiotaomicron degrades essential plant polysaccharides in the human gut, stimulates angiogenesis in the gut. It also mediates the formation of the intestinal mucosal barrier, which protects against pathogen invasion through regulation of the expression of species-specific antibiotic proteins150,151 | 1,96,147-151 |
| The genera Bifidobacterium, Enterococcus, and Lactobacillus are part of the mucosa-associated microbiota and play an important role in pathogen containment and protective function. Low levels of these genera may compromise their function.151Bifidobacteria reduce intestinal lipopolysaccharide (LPS) levels, decrease levels of pro-inflammatory cytokines, improve intestinal motility, and may reduce inflammatory status. When their abundance is decreased, it complicates this function.149,151 The genus Enterococcus comprises lactic acid bacteria (LAB) members of the commensal flora of the human colon. Enterococci can produce bacteriocins, antimicrobial compounds, which limit the proliferation of pathogens, although they may act as pathogens in certain diseases. Enterobacteriaceae have the ability to secrete LPS, which can act as an endotoxin, increasing inflammation in the gut149,151Collinsella aerofaciens at physiological levels can deconjugate bile acids and this ability to modify bile acids modulates the virulence and pathogenicity of enteric pathogens. Bile acids under physiological conditions are associated with inflammatory processes and carcinogenesis of the digestive tract147 | Bacteria of the genus Streptomyces (phylum Actinobacteria) can be found both in soil and in the gut. Although there are pathogenic species, it is also true that they produce anti-proliferative, anti-inflammatory, immunosuppressive, and antibiotic compounds. These substances are good allies against allergy and autoimmunity, as well as inflammatory bowel diseases.151 The study by Ng et al. (2022) suggests that the loss of the intestinal mucosa and the disruption of this immunomodulatory microbiota also make it possible for inflammatory processes to occur continuously over time in the intestinal tract, and situations of intestinal permeability may occur under conditions of an altered ecosystem (intestinal dysbiosis) and certain diseases. When levels are elevated in the sample, they may be producing a remarkably complex array of pro-inflammatory neurotoxins including surface lipopolysaccharides (BF-LPS) and toxic proteolytic peptides and pro-inflammatory cytokines. Factors released by intestinal epithelial cells (IECs), such as retinoic acid (RA) and transforming growth factor beta (TGF-β), promote the development of antigen-presenting cells (APCs), both DCs and macrophages, with tolerogenic properties, in the lamina propria.149,151 | APCs stimulated by signals such as flagellin release IL-23, which also promotes IL-22 production by innate lymphocytes type 3 (ILC3). IL-22 stimulates the release of RegIIIγ, an antimicrobial peptide (AMP) produced by IECs, which is retained in the mucus layer. Innate lymphocytes type 2 (ILC2) indirectly control microbiota colonisation by secreting IL-13, a cytokine that drives the differentiation of intestinal epithelial stem cells into goblet cells, which in turn produce mucin glycoproteins.149 These antimicrobial mechanisms are constitutively engaged by the immune system to prevent overgrowth of colonising microbes and to monitor the resident microbiota through immune homeostasis.149 Tolerance of the normal gut microbiota is crucial for gut homeostasis, which requires an extensive network of immune regulatory cells, including regulatory T cells (Tregs) and tolerogenic dendritic cells. Detection of commensal microbiota through TLR-MyD88 signalling is one method applied by the immune system to maintain host microbial homeostasis. The expression of pattern recognition receptors (PRRs) varies in different gut tracts, as does the composition of the resident microbiota. This results in cross-talk between the microbiota and the immune system.150,152 | Regional immuno-inflammatory activation has been demonstrated in the lungs, nervous system, gastrointestinal (GI) tract, and liver following administration of COVID-vaccines.1,16,35,41,86,124 As a consequence of SARS-CoV-2 GI infection, viral particles may infect the liver, causing hepatocellular damage and an immune-inflammatory response that may persist over time | 1,16,35,41,86,124,147,149-152 | |
| OTHER BIOMARKERS PREDICTING SYSTEMIC INFLAMMATION AND DRUG INDUCED INJURY | Lyoumi et al. (1998) and Vlasakova et al. (2022) evaluated 2 biomarkers of tissue remodelling and inflammation, α2-macroglobulin (A2M), and α1-acid glycoprotein (AGP) as potential biomarkers in blood of drug-induced tissue injury (DITI), drug-induced vascular injury (TIMP-1) and systemic inflammatory response (SIR). They observed an increase in hepatic acute-phase reactant proteins (AGP, A2M) with central nervous system (CNS) toxicity153,154 | Although plasma increases in AGP and A2M may have beneficial effects in relation to the systemic antimicrobial response, their inflammatory basis has causes that need to be investigated. Indeed, the hepatic response suggests the persistence of viral antigens in the GI tract with alterations in the permeability of the intestinal barrier and activation of pathophysiological responses with a neuro-immuno-inflammatory basis, such as dyspepsia and irritable bowel. In this regard, it should be recalled that the persistence of viral antigens in the GI tract leads to alterations of intestinal physiology, which in turn generate alterations in intestinal permeability, with neuroinflammatory and neurocognitive consequences in the patient's brain via the hepato-pulmonary pathophysiological response1,4,16,51,86,153,154 | On the other hand, pathophysiological communication through the brain–hepatopulmonary axis is bidirectional and involves the parasympathetic nervous system, represented by the vagus. As a mixed interoceptive nerve, with afferent (80%) and efferent (20%) fibres, it constantly monitors gut health, sensing metabolites from the microbiota and regional immune system, whose information it transmits centrally to activate an anti-inflammatory cholinergic response that dampens GI inflammation and modulates the composition of the microbiota, helping to regularise altered intestinal permeability. However, when abnormal brain activation via the vagal route coincides with an alteration of the blood–brain barrier, excessive activation of microglia occurs, which has neuroinflammatory effects, in turn altering neuronal signalling pathways with possible neuropsychiatric and neurocognitive consequences that must be prevented and treated1,4,16,51,86,153,154 | High levels of A2M interfere with the effects of leptin, contributing to hyperleptinaemia with consequences for the enteric nervous system.155 The mechanisms that may affect vaccination need to be assessed. They are complex and include factors related to COVID-19 vaccines (nature of the antigen, delivery system, adjuvants, and immunomodulators), the host immune system, and the gut microbiota. This “endogenous adjuvant potential” has been demonstrated in the significantly impaired antibody responses to these vaccines.1,2,13,25–29,31,35,38,39,43,46–52 Ng et al. (2022) offer a model to study how the immunogenic potential of the microbiota could be exploited to improve the immunogenicity of COVID-19 vaccines | 1,2,4,13,16,25-29,31,35,38,39,43,46-52,86,153-155 |
| SYSTEMS BIOLOGY EFFECTS OF mRNA VACCINES AGAINST SARS-CoV-2 | Hajjo et al. (2022) analyse the systems biology effects of COVID-19 mRNA vaccines to assess their safety and putative side effects. Large-scale simultaneous vaccination of the global population will reveal heterogeneity in immune responses, as well as predisposition to develop adverse effects post-vaccination, especially in vulnerable subjects. They apply a systems biology workflow, based on transcriptomics data for BNT162b2 from Pfizer-BioNTech in vaccinated subjects. No publications were available for Moderna mRNA-1273 at the time they evaluated these results. However, they generalise them to other mRNA vaccines based on evidence from the biomedical literature57,71,156,157 | They analyse the chemogenomic effects of vaccines and their composition at the systems biology level to generate testable hypotheses about the effects of vaccines on biological systems. Her group and others have successfully validated and used this theory to study the systems biology effects of small-molecule drugs and the various COVID-19 vaccines17,74,92,101,111,156,158-161 | The results show that the mRNA-based BNT162b2 vaccine affects immune response pathways related to interferon and cytokine signalling, which should lead to vaccine success, but may also produce adverse effects post vaccination. They also point to cardiac adverse effects related to mRNA vaccines, specifically, the effects of BT162b2 on calcium homeostasis. Their findings are significant for drug and medical device regulatory agencies, clinicians, policy makers, and the general public156 | They use multiple transcriptional gene signatures to study the pharmacological effects of mRNA vaccines. Their results indicate that BNT162b2 produces a strong immune response 21–22days after receiving the first dose and subsequent booster in vaccinated individuals.156 | 17,57,71,74,92,101,111,156-161 |
| The biological pathways stimulated are the immune response pathways necessary to induce the adaptive immune response triggered by vaccines. They highlight the role of innate and inflammatory immune mechanisms associated with IFN-gamma, interleukin, and protein C signalling. They also point to the clinical efficacy of the vaccine in stimulating the innate immune response, however, IFN-gamma signalling triggers inflammatory responses with perverse consequences including the involvement of female and male hormones in some of the inflammatory processes1,22,24,28,35,38,45,51,104,156 | These data should be used as testable hypotheses for the presence of putative functional links of the BNT162b2 vaccine. In turn, they compare vascular and cardiovascular diseases in COVID-19 patients with the top 50 enriched diseases by DEGs prioritised from single-cell transcriptomics data of BNT162b2, especially in the context of mRNA COVID-19 vaccines and myocarditis26,156 | Their results indicate that the selected 76 DEGs are involved in interferon signalling, cytokine signalling, interferon alpha/beta signalling, antiviral mechanisms by interferon-stimulated genes and interferon gamma signalling (Fig. 3). They point out that genes with a greater effect on transcription, such as transcription factors, or those with greater aberrations in response to treatment, exert greater effects on modulating the underlying biology, including the immune dysregulation shown. For example, IP10 and CCL2, both genes are linked to SARS-CoV-2 infection and thromboinflammation. They suggest that IP10 can be used as a biomarker for induced disease severity156 | In this study, they rank all DEGs according to their expression levels and generate 3 gene lists to identify compounds inducing transcriptional signatures similar to the BNT162b2 mRNA vaccine. If the query gene lists share few or no common genes they would be ranked in similar lists, this increases their confidence that the results are statistically significant and biologically relevant. Overall, they recognise 13 high confidence hits that belonged to 6 pharmacological classes: 4 ATPase inhibitors (38.46%), 3 protein synthesis inhibitors (30.76%), 1 Bruton's tyrosine kinase (BTK) (7.69%), 1 apoptosis stimulant (7.69%), 1 ribonucleoside reductase inhibitor (7.69%), and 1 guanylate cyclase activator (7.69%). They highlight that 12 of the top positive compound connections they report are known modulators of the immune system through their effects on innate immune pathways, such as stimulating apoptosis and NLRP3 inflammasome pathways156 | 1,22,24,26,28,35,38,45,51,104,156 | |
| OVEREXPRESSION OF TRANSMEMEMBRANE SERINE PROTEASE PROTEINS | Another finding to consider is the overexpression of several members of the transmembrane serine protease subfamily (TMPRSS) 21–22days after vaccination. TMPRSS proteins are required for haemostasis, promotion of some cancers, and entry of respiratory viruses into human cells. Activation of the SARS-CoV-2 spike protein by TMPRSS2 and TMPRSS4 has been studied. In this study TMPRSS4, TMPRSS6, TMPRSS7, and TMPRSS9 were overexpressed in response to the BNT162b2 vaccine. Therefore, they suggest that the adverse effects of BNT162b2 on TMPRSS expression may favour SARS-CoV-2 infection.156 | They note, if COVID-19 is characterised by an increased inflammatory response and a decreased immune response that trigger common vascular diseases, mRNA vaccines stimulate immune pathways to fight off SARS-CoV-2, leading to the production of particular antibodies and the induction of inflammatory responses, which could trigger rare vascular diseases. This study and others have validated these hypotheses, also finding that BT162b2 triggers immune responses similar to LPS-induced platelet activation4,156 | The role of platelets in haemostasis, host defence mechanisms and their modulatory effect on T- and B-cell communication for proper acquired immunity has been discussed previously. Platelets can recognise pathogens, select host defence peptides such as platelet factor 4 (PF4), a platelet-derived CXC chemokine, and together with leukocytes construct thrombotic immune complexes. Cases of vaccine-induced immune thrombotic thrombocytopaenia (VITT) have been reported following administration of adenovirus vector and mRNA vaccines1,75,156,162–168 | Therefore, this study indicates that mRNA COVID-19 vaccines can act as immunogens by encoding for the SARS-CoV-2 spike protein and also as adjuvants through their LNP content or through the intrinsic immunostimulatory properties of the modified and/or non-modified RNA | 1,4,76,156,162-168 |
| MORTALITY CAUSED BY COVID-19 VACCINATION | Murata et al. (2022) present 4 cases who died at home after receiving mRNA COVID-19 vaccine. Three cases vaccinated with 2 doses of Moderna mRNA, and 1 case received 2 doses of Pfizer-BioNTech mRNA vaccine. The time from administration of the second dose to death was 1–10days.102 | In the case of the Pfizer-vaccinated subject, post-vaccination adverse effects such as headache and fever were not observed on the day before death, whereas in the other cases, they were observed the day before death. The post-mortem interval inferred from post-mortem phenomena and the coroner's rectal temperature measurements estimated high body temperatures for all cases at the time of death. They use RNA sequencing and identify genes that were differentially expressed in the post-vaccination cases and the control group that died from blood loss and strangulation102 | They identify 390 up-regulated genes associated with neutrophil degranulation and cytokine signalling and 115 genes were down-regulated in post-vaccination cases compared to controls. They suggest causality of death with temporal occurrence due to immune dysregulation after vaccination, systemic immune response syndrome (SIRS).102 SIRS must meet at least 2 of the following criteria: fever <38°C or hypothermia <36°C, tachycardia >90 beats/min, tachypnoea >20 breaths/min, leukocytosis >12×109/l, or leukopaenia <4×109/l.19 SIRS has been reported to be induced by several factors, such as infection (including COVID-19 infection), ischaemia, surgery, and trauma.169 | Autopsies of these patients did not reveal cause of death in any patient and pathological analysis showed findings of sudden death, such as primary organ congestion without hypoperfusion, and no information about cause of death, including myocarditis. In this study, they estimate the cause of death by sequencing RNA obtained from peripheral blood. They obtain an average of 39.1150-base pair (bp) paired-end (PE) reads per sample. Of the 43751 genes with non-zero total read counts, 505 genes were significantly differentially expressed between individuals with unknown cause of death (vaccinated subjects) and the control group.102 They define COVID-19 vaccination as pseudoinfection with SARS-CoV-2 and as an inducer of SIRS. In the 4 deceased patients, immune function was sensitised by the first dose and the second dose caused SIRS and death in the more susceptible patients. They warn of the need to detect this aberrant cytokine response, such as the analysis of single nucleotide polymorphisms to prevent such cases 1,13,16,37,39,52,82,102,114,170 | 1,13,16,19,37,39,52,82,102,114,169,170 |
| Ittiwut et al. (2022) reported 13 cases of sudden unexplained death (SUD) following COVID-19 vaccination in Thailand, aged 23–72years; 10 (77%) were male and 3 (23%) female, 12 of Thai origin and 1 Australian. They reported underlying diseases in 5 patients, without arrhythmia. No case had a history of ultrasensitive troponin UST in family members. Eight (61%), 4 (31%), and 1 (8%) died after the first, second, and third vaccine doses, respectively. SUD was triggered after all types of COVID-19 vaccines administered in Thailand, 7 (54%), 2 (15%), 2 (15%), 2 (15%), 1 (8%), and 1 (8%) deaths after receiving ChAdOx1 nCoV-19 (AstraZeneca), BBIBP-CorV (Vero Cells) from Sinopharm (Beijing), CoronaVac (Sinovac), BNT162b2 (Pfizer/BioNTech), and mRNA-1273 (Moderna), respectively171 | Eight (61%) died after receiving the first dose of AstraZeneca's ChAdOx1nCoV-19 vaccine.171 Fever was self-reported in 3 cases. Ten (77%) and 11 (85%) died within 24–72h after vaccination. In 6 cases (46%), autopsy identified no explainable causes of death, they recorded another 6 cases with cardiac symptoms, including coronary atherosclerosis in 4 cases (31%), arrhythmogenic right ventricular dysplasia in one case (8%), and one case of dilated cardiomyopathy (DCM) (8%), the remaining case of the sample had thalassaemia (haemochromatosis and liver cirrhosis). Whole exome sequencing analysis identified 5 cases with SCN5A gene variants, coincident in patients diagnosed with Brugada syndrome, with a frequency of 38% (5 out of 13 cases)171 | A significantly higher rate compared to recorded cases of SUD in Thais occurring after vaccination with COVID-19 (between 8 and 30days), in a Thai SUD cohort studied before the pandemic (12%, 3 of 25 cases) and in their in-house exome database (12%, 386 of 3231 cases). All cases tested negative for SARS-CoV-2 by reverse transcriptase polymerase chain reaction (RT-PCR). Patients confirmed to have died naturally were referred for genetic studies171 | The authors suggest the hypothesis of an association between SCN5A variants and SUD within 7days after COVID-19 vaccination, 13 variants in 7 genes in 11 of 13 cases (85%). They show that the vaccine had a direct effect and causes myocarditis in the right ventricular outflow tract area, with pre-existing aberrant conduction in patients with SCN5A and leads to death. Regardless of vaccine type, number of doses and the presence of underlying diseases, or post-vaccine fever171 | 171 | |
| NEOPLASTIC EFFECTS OF mRNA-BASED COVID-19 VACCINES | Çinar et al. (2022) discuss the role of COVID-19 itself and the spike protein produced endogenously by mRNA vaccines and their immunological, microenvironmental, prothrombotic, and neoplastic effects. They show 4 cases of myeloid neoplasms following administration of Pfizer's BNT162b2 mRNA-based COVID-19 vaccine172 | They have used immunophenotyping to understand the impact of B-cell dysfunction on the serological response to COVID-19 vaccination in primary antibody deficiencies172 | They reinforce the theory of probable pathobiological mechanisms and causalities of spike protein-related toxicity and clonal myeloid disorders172 | They call for objective pharmacovigilance-based follow up to establish the clinical–biological correlation between spike-mRNA vaccines and leukaemogenesis. Meanwhile, all haematological adverse events must be closely monitored and reported.172 | 172 |
| IMMUNE RESPONSES IN THE HUMAN FEMALE REPRODUCTIVE TRACT AND POTENTIALLY HARMFUL EFFECTS ON FERTILITY AND LONG-TERM HEALTH IN WOMEN FROM COVID-19 VACCINES | Finally, the systematic review by Nazir et al. (2022) summarises the main predictors and rates of menstrual cycle changes after COVID-19 vaccination, including 78138 patients who received mRNA-based vaccines (Pfizer-BioNTech [BNT162b2] and Moderna [mRNA-1273]), inactivated whole virus vaccines (Sinopharm/BBIBP-CorV and Sinovac Biotech) and recombinant adenoviral vector vaccines (Johnson & Johnson/Janssen/Ad26.COV2.S, Oxford-AstraZeneca/ChAdOx1nCov-19 and Sputnik V). Of this sample, 39759 (52.05%) participants had menstrual abnormalities (menorrhagia, oligomenorrhoea, polymenorrhoea, dysmenorrhoea, and metrorrhagia) following COVID-19 vaccination, a significant sample, indicating a large heterogeneity in the occurrence of these adverse effects. The overall rate of adverse effects following COVID-19 inoculations in this study varied from 83% to 90.9%. There are few studies reported in the literature addressing the causal relationship between SARS-CoV-2 vaccination status and menstrual irregularities172 | They point to 3 studies that establish increase in female age (ages 18–30) as a causal predictor of menstrual problems. The authors add other predictors such as: history of pregnancy, first dose and subsequent boosters, systemic side effects of COVID-19, and smoking. Most of the cases reported were cross-sectional, questionnaire-based studies and had to be associated with the above predictors. Notably, 10 retrospective studies report a proportion of women with menstrual irregularities that could be associated with COVID-19 vaccination, for example, in a Norwegian cross-sectional survey based on 5756 women recruited through random sampling, 39%–41% reported these adverse events.173,21 They were most frequently experienced after the second dose, with 2 studies recording 3–6 menstrual cycles following vaccination. Symptoms reported are heavier bleeding, dysmenorrhoea, longer and/or shorter than normal cycles, and unexpected breakthrough bleeding, also in patients receiving contraceptive treatment.20,21,173,174 Pre-vaccination infection with SARS-CoV-2 also resulted in menstrual disturbances 103,173,175 | These findings allude to a cumulative immune effect due to repeated antigen exposure, as discussed above evidenced by the “spike effect”. They also recognise the need to follow more cycles for longer periods to study the long-term consequences, as well as the likelihood of pregnancy.173 They found gynaecological disorders such as menorrhagia, endometriosis, polycystic ovarian syndrome, fibroids, and adenomyosis that caused participants to have increased menstruation after vaccination mostly of short duration, but suggest that it may predispose to these changes. Most studies published to date conclude and repeat that the effect of COVID-19 vaccines on the menstrual cycle is temporary, mild, and without long-term consequences. However, it is known that the particular antigen that elicits this immune response is debatable, both the spike protein shared with SARS-CoV-2 and the adjuvants used in vaccination have been found to cause the intense immune response after administration of COVID-19 vaccines, and similarly after SARS-CoV-2 infection as a potential stressor that changes the activity of the hypothalamic–pituitary–ovarian axis that regulates the onset and duration of the menstrual cycle113,173 | The menstrual cycle and restoration of the endometrium are mediated by innate immune cells that reside in the endometrium. Activation of these immune cells would trigger heavier, irregular, and unexpected bleeding over time. Hormone levels involved in the menstrual cycle could be affected by vaccine-induced immune dysfunction.173,176 Other possibilities attributable to vaccination could occur through changes in thyroid and VITT, effects already observed in other vaccines (measles, diphtheria, and hepatitis).173,177–179 Another finding to consider is the 2-dose dosing schedule (21 and 28days) for Pfizer-BioNTech and Moderna, respectively; the first dose would be administered in the early follicular phase. Thus, it would affect the development of the dominant follicle during the follicular phase and consequently the cycle length.1,98,156,173 A contradictory finding that could permanently disturb the endocrinological homeostatic regulation of the menstrual cycle would be a severe and chronic systemic disease like long COVID, an adverse effect to be evaluated in the context of COVID-19 vaccination.173 This study evidences the complex interplay between inflammatory markers and hormonal disruptions due to COVID-19 infection, including mRNA-based vaccination, warning of its potential for damaging the long-term health and fertility of women, especially those diagnosed prior to vaccination.176,177,180,181 Tolerability and safety must be ensured for better acceptance of COVID-19 vaccination. Further cohort studies are needed to identify the temporal link between menstrual cycle changes. The authors call for population-based studies, which record variance in epigenetic findings due to genetic polymorphisms in ethnic and at-risk communities 173 | 1,20,21,98,103,113,156,173-181 |
| Data on the safety of COVID-19 vaccines administered during, before, and after pregnancy are scarce. Moro et al. (2022) evaluated Vaccine Adverse Event Reporting System (VAERS) reports of 3462 adverse event reports in pregnant women who received a dose of COVID-19 vaccines: 1831 (52.9%) with BNT162b2, 1350 (39.0%) with mRNA-1273, and 275 (7.9%) with Ad26.COV2.S, and 6 of unknown manufacturer. Reports indicating that vaccination was given before the last menstrual period or during the post-partum period were excluded. Eight maternal deaths and 12 neonatal deaths were reported. Six hundred and twenty-one (17.9%) reports were coded as serious. They included: 878 (25.4%) spontaneous abortions (<20weeks) (SAB), 101 (2.9%) episodes of vaginal bleeding, 76 (2.2%) preterm deliveries (<37weeks), 62 (1.8%) stillbirths (≥20weeks), and 33 (no calculated rate) cases of babies with major birth defects or a chromosomal abnormality, 18 of the 33 cases with BNT162b2 vaccine, and 15 of the 33 cases with mRNA-1273182 | They recorded 107 infant conditions including all 12 neonatal deaths, indicating prematurity as the cause of death. The crude reporting rates for preterm deliveries and stillbirths, as well as maternal and neonatal mortality rates were compared with the background rates of published sources. They (did not) note disproportionate reporting. They estimated 3173387 live births in the USA during their study period (December 2020–October 2021)182 | According to the National Centre for Health Statistics methods referenced include foetal deaths at 20weeks' gestation or more determined by obstetric estimate, among women aged 15–44years, excluding foreign residents. They report 18945 foetal deaths during the study period. Most SABs, (81.3%) 490 out of 603 cases with gestational age data, occurred at <12weeks gestation and (39.3%) 338 out of 859 cases the pregnant woman was aged ≥35years. Among the 849 SAB cases reporting onset interval (period of time from vaccination to presentation of adverse effects) were: 0–3days (26.4%), 4–7days (12.0%), 8–14days (15.5%), and ≥15days (46.1%)182 | They report the 8 maternal deaths as pulmonary embolism, amniotic fluid embolism, eclampsia with peripartum cardiomyopathy, and acute cerebellar intraparenchymal haemorrhage secondary to severe persistent thrombocytopaenia due to acute myelogenous leukaemia in 4 cases, respectively. Two others had sudden clinical deterioration leading to death. There were no medical reports for 2 of the 8 cases.182 All findings should be interpreted with caution | 182 | |
| In a recent study, Monin et al. (2020) discuss immune responses in the human female reproductive tract. They provide a detailed review of the current knowledge of the immune responses of the female reproductive tract (FRT) and their heterogeneity within the organ and between compartments. In the commensal-rich vagina, the immune system must allow the growth of beneficial microbes, whereas the purpose of the uterus is to allow the growth of the foetus183 | In both compartments, these objectives must be balanced with the activity of eliminating pathogens. This will not only facilitate interventions to prevent the spread of sexually transmitted diseases, but in the context of the “spike effect” will allow for improved fertilisation, implantation, and pregnancy outcomes for mothers and babies, as infections are among the common causes of infertility and late pregnancy failure183 | The authors point out that the uterine immune system also changes with the menstrual cycle and pregnancy. It is now beginning to be appreciated that cyclical changes also occur in the vagina and that these may have an impact on susceptibility to disease. They reiterate and indicate the composition of maternal immune cells (innate lymphoid cells and innate T cells in the vagina and uterus, in addition to APCs) in the blood and decidua, and prior to pregnancy, the frequency of immune cells in the endometrium varies during the menstrual cycle183 | In the female reproductive tract (FRT), antibodies contribute to pathogen clearance through mechanisms including pathogen neutralisation, opsonisation, and complement-driven lysis. IgG- and IgA-secreting plasma cells can be detected in the lamina propria (LP) of both the cervix and vagina, although compared to other mucosal sites, IgG, rather than IgA, is the predominant antibody isotope in the lower FRT183,184 | 183,184 | |
| Recently, Mansour et al. (2023) linking menstrual disturbances following COVID-19 vaccination and the role of immune and endocrine pathways (hypothalamic–pituitary–thyroid axis, hypothalamic–pituitary–adrenal axis, and hypothalamic–pituitary–ovary axis), focus on adverse effects in women of reproductive age. They repeat and evidence the detrimental effects of cleaved S protein in COVID-19 vaccines, including SARS-CoV-2 entry into target cells, endothelial damage, pro-inflammatory cytokine release, Toll-like receptor (TLR) activation, stimulation of microglia, molecular mimicry with chaperon and heat shock proteins (HSP), and activation of SARS-CoV-2S protein and its binding to angiotensin-converting enzyme 2 (ACE2)185 | It can also induce hyper-stimulation of the immune system and synthesis of multiple autoantibodies. Thus, they demonstrate that the normal menstrual cycle can be disrupted by impairment of any of these pathways. These findings suggest that disrupting the monocyte–endothelial–platelet axis may restore the immune dysregulation observed in all adverse effects secondary to administration of COVID-19 vaccines and could be a potential therapeutic alternative in the near future.27–29,46,49,185–187 Post-vaccination menstrual disorders are common and can occur with different vaccines, such as the human papillomavirus (HPV) vaccine 185,188 | During the follicular phase, vaccination may prolong the menstrual cycle and/or arrest/delay ovulation and disrupt the reproductive cycle. These adverse effects can be attributed to individual variation, and are very similar after the first and second dose.185,189 A specific profile could be defined in the female sex, young age (second dose with BNT162b2: SD=38.9years; third dose with BNT162b2: SD=45.9years) and without comorbidities as risk factors for vaccine-induced immune dysregulation.190 Incomplete inactivation of the X chromosome in females may be implicated in vaccine efficacy.185,190,191 | This inactivation is not uniform among all immune cells and there is a probability of biallelic expression of genes. X chromosomes come from different parents and offer more immune diversity in females. Females have a double copy of immune genes due to their XX chromosomes; this chromosome involves the immune-related genes in the human genome. The X chromosome regulates different components of the immune system such as TLR7, TLR8, CXCR3, and CD40L receptors, which are overexpressed in women and actively influence the response to vaccination.185,190 | 27-29,46,49,185-191 | |
| Women respond to vaccination through increased antibody production and a Th2-cell-dominant immune response, which produces anti-inflammatory cytokines.185,192 TLR7, a frequent TLR in females, is strongly associated with vaccine efficacy and sex differences in autoimmunity. It can escape X-chromosome inactivation and lead to increased expression of innate immune cell and T-cell associated genes, which induces production of type I IFN and activates the adaptive immune response. It would explain the higher frequency of adverse effects in women due to the stronger immediate response to the antigen, modulated through the innate immune system25,53,185,187 | Testosterone has a suppressive effect on innate and adaptive immune response, confirming that sex hormones are involved in the mechanism of increased vaccine reactogenicity in women. For example, genetic factors could also be involved in vaccine reactogenicity, such as the ACE2 gene and the angiotensin type 2 (Ang-II) receptor through interaction with sex hormones, a key peptide in vascular and renal damage131,187,193–195 | The differential activity of X-linked genes and polymorphisms of the ChrY (sex-differentiated immunity) gene, which are regulated by escape from X-chromosome inactivation and epigenetic mechanisms, respectively, also play a role. Thus, they may affect global gene expression in immune cells131,187,193–195 | Together, the sex chromosome complement, the activity of regulatory elements on sex chromosomes, and the expression of sex chromosome genes contribute to sex differences in immunity to viral infections.131,187,193–195 Intersectional approaches will be needed to better understand the adverse effects of COVID-19 vaccines, as well as treatments to decrease antiviral adaptive immunity, virus replication, and inflammation, taking into account that there are sex differences.193 | 25,53,131,185,187,192-195 |
The term “safety profile” refers to all adverse effects that could be caused, triggered, and/or worsened at any time after vaccination, such as anaphylactic reactions, diseases diagnosed after vaccination and autoimmune events.196 The WHO defines 5 types of adverse events following immunisation (AEFI), recorded in a 30-day window following vaccination, which are discussed in the current published literature and some reports of spontaneous adverse events: (1) vaccine product-related reaction; (2) vaccine quality defect-related reaction; (3) immunisation error-related reaction; (4) immunisation anxiety-related reaction; and (5) coincidental event.197 The first and third type of adverse effect are likely in parallel with COVID-19 vaccine technology, the current biomedical literature has evaluated more data for: (a) LNP components, (b) modified and unmodified mRNA, (c) spike protein produced by COVID-19 vaccines, not excluding their co-occurrence.
The present systematic review discusses, based on published evidence, the innate and adaptive immune stimulation induced by COVID-19 vaccines, based on the activation of biomarkers of inflammation and their effect on immunogenicity and reactogenicity will drive the design of effective approaches to manage the disease induced by these new vaccine platforms against SARS-CoV-2. To this end, we conducted a review of the published literature on the innate immune mechanisms of this new therapy. We also studied other variables such as unknown serious adverse effects and/or atypical variants of other post-viral syndromes, their clinical and epidemiological patterns, inflammatory signatures, and temporal occurrence associated with the presence of post-vaccine recognition antibodies such as anti-RBD IgG, anti-S1 IgG, anti-S2 IgG, and anti-N (nucleocapsid) in the context of infection by contagion. Clinical characteristics that, in principle, make this drug an ideal candidate to prevent the disease and/or the deleterious consequences as a secondary prevention system in the form of a vaccine.
ObjectivesThe research objectives were as set out below: to systematically review the literature on adverse effects following administration of COVID-19 vaccines to demonstrate their therapeutic potential in the treatment and/or prevention of the disease, and to demonstrate the association of causality and temporal occurrence.
The specific objectives were:
- 1.
To identify articles publishing information on the immunogenicity and reactogenicity of COVID-19 vaccines that induce long-lasting expression of the SARS-CoV-2 spike protein with systemic effect and that mimic long COVID.
- 2.
To identify and describe methodological aspects of work reporting potential inflammatory biomarkers that could predict cellular and humoral immune dysregulation.
- 3.
To assess the association between adverse effects and molecular mimicry, the production of particular autoantibodies, and the role of certain adjuvants.
- 4.
To demonstrate the mechanisms of exposure and subsequent dissemination to the cell line, integration into the human genome (genotoxicity), VEGF-mediated vascular cross-talk induced by the spike protein, and involvement of the gut microbiota composition.
- 5.
To verify the reactogenicity of COVID-19 vaccines with higher post-vaccination antibody levels.
A systematic search of the scientific literature reporting data on the adverse effects of vaccination against SARS-CoV-2.
The study was conducted in 2 phases: in the first phase, a systematic review was conducted of scientific articles published in the literature addressing the issue of immunogenicity and reactogenicity after vaccination.
The most relevant health science databases were used: MEDLINE (through PubMed and Web of Science); Web of Science, selects other bases: Web of Science (WOS) main collection, Current Contents Connect (CCC), Derwent Innovations Index (DIIDW), KCI - Korean Journal Database (JKD), MEDLINE, Russian Science Citation Index (RSCI), and SciELO Citation Index; Scopus (Elsevier).
The following MeSH (Medical Subject Headings) terms were used as search descriptors198: vaccine COVID-19, adverse events, as well as the same in Spanish, with their corresponding Boolean formulation (AND) and the truncation term for some of them. The search filters used for the PubMed-MEDLINE search platform were adapted to the Scopus and Web of Science databases. To locate articles more precisely, a search was conducted as to whether the term existed in the thesauri of the databases and when this was not possible, it was used as free text.
The limits of the search to select the documents in the databases were: documents in article format, published in scientific journals, without language restrictions, open access. It was limited to the titles of the articles and their abstracts.
The search period was taken as the last 3years of each database until 11 July 2023. In addition, a reverse search was conducted by analysing the literature references of the selected articles.
The inclusion criteria in selecting articles were that their objectives and hypotheses should address potential mechanisms of unknown and severe immune reactivity of COVID-19 vaccines. Also, the putative plausibility of molecular mimicry between viral spike peptides and the patient's own peptides resulting in an event (study design was considered for inclusion); articles published in the last 3years (2021–2023); provide primary data, i.e., data obtained directly by the authors.
Exclusion criteria were articles without available abstracts, editorials, conference papers, book reviews, correspondence, and social media, or unauthorised comments on COVID-19 vaccines, studies with methodological problems that condition the quality of the reported data, and articles reporting common spontaneous adverse events with a mild prognosis and no torpid course, poor reporting (under-diagnosis and/or half-diagnosis), no clear follow-up, laboratory results of positive nucleocapsid antibodies (SARS-CoV-2 anti-nucleocapsid IgG) that only appear from contact with the virus and not from vaccination, pre-existing medical conditions requiring immunosuppressive treatment at the time of vaccination and chronically due to immune-stimulatory underproduction.
Texts were initially selected based on the relevance of the title and abstract. For those that were selected in this way, the full text of the articles was analysed to be considered for inclusion in the review.
Articles included in the review had to meet the quality criteria established by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.170,199
The Spanish Critical Appraisal Skills Programme (CASPe)114,184 was used to assess the quality of the studies, and the methodological quality of the review was assessed according to the criteria established in the AMSTAR (Assessing the Methodological Quality of Systematic Review) tool.200
Information of interest for the study was extracted using an ad hoc form that collected information on the general characteristics of the studies, which analysed the main results.
The following variables were determined for each article: authors, year of publication, study design, study participants, sample size, time from vaccination to symptom onset (counted in days), recommendations (post-vaccination antibody titre, therapeutic alternatives/future treatments for adverse reactions caused), risk of bias-limitations (not peer-reviewed, sample size), biomarkers of inflammation-immune T-cell activation, clinical and epidemiological patterns.
Data were extracted and independently verified by the investigator.
For further analysis, the data were entered into a database table in Excel and then into a database created with SPSS Statistics IBM version 29.
In the second phase, the articles identified in the review were grouped and analysed according to the clinical and epidemiological characteristics associated with serious adverse events. Given the length of the systematic review, only the data referring to the inflammatory signature derived from reactogenicity are presented in this project.
For the variables included in the analysis, frequency measures for qualitative variables were calculated using SPSS Statistics IBM version.
After conducting the systematic review and establishing the existing evidence in the literature, it was decided whether the existence of causality and temporal occurrence derived from COVID-19 vaccines is valid and reliable for the prevention of the disease.
With regard to ethical aspects, the systematic review was conducted on based on the analysis of primary and secondary sources, in no case was there any experimentation with groups of patients or animals. The design and execution of the research was in accordance with the specified AMSTAR methodological criteria to guarantee the quality and veracity of the information collected, facilitating its replication in the future.
With the support of the PRISMA statement and the CASPe tool, a clear and organised structure was achieved for interpretation of the review. Inclusion and exclusion criteria were considered, providing rigour to the review.
ResultsThe systematic review identified a total of 2033 records which, after a screening process according to the inclusion criteria and the elimination of duplicate papers, papers with methodological problems, and papers without open access, were reduced to 58 articles, as described in the PRISMA flowchart of the review (Fig. 4); of these, 50 articles on human models and 2 on cellular models were identified. Tables A1 and A2 summarise the most relevant information from the articles/reports included in the review (study participants and time from vaccination to symptom onset counted in days, as well as the characteristics of all included studies).
Analysis of articles foundBased on the study participants, it can be seen that 50 articles (93.5%) are from human model studies; 2 (2.2%) from cell model studies.
Fig. 5 shows the temporal distribution of the articles according to the year of publication.
Graph 5 shows that the number of publications is not equal, in ascending order: 2 articles (3.4%) were found in 2021; 5 (8.6%) in 2023; 51 (87.9%) in 2022. Most of the articles are a year old.
A total of 58 research articles have been included, after discarding repeated or duplicate studies, 15 are descriptive studies (14 [15.1%] prospective and 1 [1.1%] retrospective), as well as 12 case reports (12.9%), 7 case series (7.5%), 1 case report with systematic review (1.1%), 1 systematic review with meta-analysis (1.1%), 8 systematic reviews (8.6%), 11 narrative reviews (11.8%), 1 randomised clinical trial (1.1%), and 2 experimental trials (2.2%), on human liver cell line Huh7 in vitro and in vivo, respectively. Forty-three articles record the sample size of each study, the smallest being one case report (12.9%) and the largest having 1057000 participants (1.1%).
The studies analysed serious adverse events based on histological analysis, human species, study participants, ethnically differentiated populations, time from vaccination to symptom onset (counted in days), clinical characteristics, follow-up period of the disease process, form of treatment, deaths, temporal association, identification of the inflammatory signature, design of the analyses performed in the studies, and measurement of outcomes.
Tables A1 and A2 summarise the common current evidence associated with adverse events induced after administration of COVID-19 vaccines, based on the spike effect of COVID-19 vaccines and the SARS-CoV-2 virus spike protein, as well as related variables.
The studies included in this review demonstrate the relevance of a systems biology approach to correlate the positive regulation of genes associated with innate immunity, cytokine production, and responses to virus infection, specifically IFN-inducible genes, with observed adverse effects. Most adverse effects were reported after vaccination with BNT162b2 (37 of 58 articles), ranking in descending order: 15 of 58 articles with mRNA-1273, 12 of 58 articles with ChAdOx1, 7 of 58 articles with Ad.26.COV2.S, 4 of 58 articles with CureVac, 3 with Sinovac, 1 with AZD1222, and 1 case with BBIBP-CorV.
Table A1 shows that the number of days to symptom onset varied greatly, in descending order: 31 articles (70.8%) were found between 0 and 365days; 8 articles (8.8%) between 0 and 15days; 6 articles (6.4%) between 1 and 7days; 4 articles (4.3%) for 2days.
Here, we review the current understanding that COVID-19 vaccines induce innate and adaptive immune activation. We discuss the innate immune recognition of mRNA vaccines and iLNP technology at the cellular level and consider the contribution of mRNA and LNP components to their immunogenicity. In this context, they act as adjuvants for this type of vaccine platform.3Table 2 summarises the immunological effects of adverse events caused by SARS-CoV-2 vaccines.
Summary of immunological effects of adverse events resulting from SARS-CoV-2 vaccines.
| REVERSE TRANSCRIPTION | Two studies looking at the in vitro effect of BNT162b2 mRNA vaccine in the human liver cell line (Huh7) Aldén et al. (2022) and Mouliou et al. (2022) confirm the rapid entry of BNT162b2 into cells and subsequent intracellular reverse transcription of BNT162b2 mRNA into DNA. It is likely that liver cells also present the vaccine-derived SARS-CoV-2 spike protein, explaining that liver cells are targets for pre-primed spike protein reactive cytotoxic T cells. The authors suggest in vivo models in future studies to better understand the effects of BNT162b2 on liver function. They show that BNT162b2 is reverse transcribed into DNA in the Huh7 cell line, they observe that BNT162b2-derived DNA integrates into the host genome and affects the integrity of genomic DNA, leading to genotoxicity as an adverse effect | It is unclear whether DNA reverse transcribed from BNT162b2 is integrated into the cellular genome, including whole genome sequencing of cells exposed to BNT162b2, as well as systemic distribution in tissues from human subjects who received BNT162b2 vaccination, which is of great concern. Different expression of genes and proteins has also been demonstrated, in addition to certain up-regulated proteins involved in RNA metabolism | Aldén et al. (2022) using a carcinoma cell model to verify effective retrotransposition of LINE-1, with active DNA replication which differs from non-dividing somatic cells, report active cell proliferation in several tissues, such as bone marrow or basal epithelial layers and during embryogenesis, as well as in non-dividing, terminally differentiated cells, e.g., human neurons. As discussed, the advantage of mRNA-iLNP platforms is that they do not require the addition of adjuvants to produce protective immune responses against pathogens, however, mRNA-iLNP vaccines are not immunosilent | (Aldén et al. 2022, Mouliou et al. 2022) |
| RECOGNITION OF VACCINE ANTIGENS AS A POTENTIAL PATHOGEN CAUSING ADVERSE EVENTS | Vaccine antigens are recognised as potential pathogens causing autoimmune reactions by the mechanisms of molecular mimicry and T-cell activation in 26 articles | We must remember that innate and adaptive immune responses vary between individuals due to their cellular and biochemical components. The SARS-CoV-2 spike protein in COVID-19 vaccines is confirmed to contain the sequence and structural motif of a superantigen, causing the hyperinflammatory syndrome that involves direct T-cell stimulation and excessive cytokine production in certain subjects | – | (Saluja et al. 2022, Garrido Suárez et al. 2022, Mouliou et al. 2022, Castaldo et al. 2022, Verbeke et al. 2021, Morikawa et al. 2022, Lagousi et al. 2022, Zagorec et al. 2022, Piras et al. 2022, Abu Serhan et al. 2022, Mohseni Afshar et al. 2023, Lee et al. 2022, Hoffmann et al. 2022, Magen et al. 2022, Pisani et al. 2022, Ekobena et al. 2022, Caliskan et al. 2022, Aliasin et al. 2022, Ahmed et al. 2022, Finsterer et al. 2021, Mingot-Castellano et al. 2022, Watanabe et al. 2022, Chow et al. 2022, Zlotnik et al. 2022, Ameratunga et al. 2022, Duijster et al. 2023) |
| PEAK EFFECT IN THE CONTEXT OF CUMMULATIVE EFFECT AND LOSS OF RECEPTOR ACTIVITY OF ACE2 | In addition, 19 studies observed the “spike effect”, known as “spike intoxication “, in the context of saturation and subsequent impairment of angiotensin II-converting enzyme receptor (ACE2) function | They propose the mechanisms: (1) cross-reaction between foreign antigens and self-antigens, (2) over-activation of antigen-presenting cells and subsequent autoimmune response, and (3) polyclonal activation of B cells or bystanders leading to cytokine synthesis and activation of autoreactive T cells. Studies show that spike proteins produced after vaccination have the native-like mimicry of the SARS-CoV-2 spike protein's receptor binding functionality and prefusion structure. | COVID-19 vaccines increase endogenous synthesis of SARS-CoV-2 spike proteins. Once synthesised, the free-floating spike proteins released by the destroyed cells previously targeted by the vaccines circulate in the blood and interact with ACE2 receptors (a zinc metalloproteinase) expressed by other cells, promoting ACE2 internalisation and degradation, mimicking the pathological features of SARS-CoV-2, a mechanism known as the spike effect of COVID-19 vaccines | (Talotta 2022, Lee et al. 2022, Castaldo et al. 2022, Saluja et al. 2022, Namiki et al. 2022, Levy et al. 2022, Piras et al. 2022, Abu Serhan et al. 2022, Mohseni Afshar et al. 2023, Lee et al. 2022, Tamborska et al. 2022, Aliasin et al. 2022, Azzolini et al. 2022, Ahmed et al. 2022, Kim et al. 2022, Angelini et al. 2022, Zlotnik et al. 2022, Ameratunga et al. 2022, Duijster et al. 2023) |
| RELATIONSHIP WITH HYPOTHESES ON MOLECULAR MIMICRY, INFLAMMATION SIGNATURE, SYSTEMIC DISTRIBUTION, PERSISTENCE, AND CONTINUED HIGH AVAILABLITY OF ANTIBODIES POST-VACCINATION | Six studies relate testable hypotheses on molecular mimicry, inflammatory signals attributed to post-vaccination reactogenicity, their systemic spread, temporal persistence, and the possibility of a “cumulative effect” of the spike protein with post-vaccination IgG anti-S and IgG anti-RBD antibodies, suggesting post-COVID-19 mimicry | These features offer high and sustained antigen availability, favouring more robust antibody responses. Both spike-encoding mRNA and spike protein are detectable 60days after the second dose of BNT162b2 and mRNA-1273 in vaccinated humans | It is therefore possible that SARS-CoV-2 vaccines composed of viral and recombinant glycoprotein vectors deliver more attenuated inflammatory signals or by other mechanisms that contribute to innate immune activation, not just mRNA encapsulated in lipid nanoparticles | (Mouliou et al. 2022, Levy et al. 2022, Pisani et al. 2022, Azzolini et al. 2022, Ahmed et al. 2022, Kim et al. 2022) |
| ENDOGENOUS ADJUVANT MECHANISM INVOLVED IN INNATE IMMUNE ACTIVATION | Endogenous adjuvant potential can be observed in significantly impaired antibody responses with these vaccines | – | – | (Mouliou et al. 2022, Verbeke et al. 2021, Morikawa et al. 2022, Namiki et al. 2022, Levy et al. 2022, Lee et al. 2022, Magen et al. 2022, Pisani et al. 2022, Azzolini et al. 2022, Abbasi et al. 2022) |
| PATHOGENIC HYPOTHESIS ASSOCIATED WITH THE NEOPLASTIC, INFLAMMATORY, NEURO-IMMUNE/VASCULAR EFFECTS PRODUCED BY SARS-CoV-2 | The “pseudoinfection” proposed in this review is more associated with lymphoproliferative, inflammatory disorders and neuromyelopathies, including neuroinflammatory alteration with alteration of the blood–brain barrier, which explains the development of the adverse effects cited above | – | – | (Mouliou et al. 2022, Castaldo et al. 2022, Mohseni Afshar et al. 2023, Hoffmann et al. 2022, Kim et al. 2022, Tamborska et al. 2022, Magen et al. 2022, Pisani et al. 2022, Ekobena et al. 2022, Caliskan et al. 2022, Aliasin et al. 2022, Ahmed et al. 2022, Umezawa et al. 2023, Zlotnik et al. 2022) |
| CELLULAR MECHANISMS AND PATHOGENIC/VIRAL, AUTOINFLAMMATORY, AUTOINMUNE AND PARANEOPLASTIC HYPOTHESIS ASSOCIATED WITH ADVERSE EFFECTS IN RECIPIENTS OF SARS-cov-2 VACCINES | Studies show the cytokine storm to be caused by uncontrolled infection (pathogenic/viral hypothesis, e.g., neurotropism), autoantibodies or autoreactive T cells associated with predisposing germline mutations (autoimmune hypothesis), germline mutations in genes that regulate inflammation (autoinflammatory hypothesis) and/or somatic mutations in monoclonal lymph node cells resulting in ectopic cytokine secretion (paraneoplastic hypothesis) in certain cases | Most of the included articles verify potent innate immune activation in reports of local and systemic adverse effects of post COVID-19 vaccination in humans, 56 articles (97.8%) and 46 articles (87%) for molecular mimicry | – | (Mouliou et al. 2022, Hajjo et al. 2022, Verbeke et al. 2022, Castaldo et al. 2022, Saluja et al. 2022, Chen et al. 2022, Morikawa et al. 2022, Lagousi et al. 2022, Pirani et al. 2022, Zagorec et al. 2022, Levy et al. 2022, Piras et al. 2022, Abu Serhan et al. 2022, Mohseni Afshar et al. 2023, Lee et al. 2022, Hoffmann et al. 2022, Wong et al. 2022, Magen et al. 2022, Pisani et al. 2022, Ekobena et al. 2022, Caliskan et al. 2022, Aliasin et al. 2022, Ahmed et al. 2022, Finsterer et al. 2021, Umezawa et al. 2023, Hetland et al. 2022, Mingot-Castellano et al. 2022, Watanabe et al. 2022, Abbasi et al. 2022, Chow et al. 2022, Zlotnik et al. 2022, Ameratunga et al. 2022) |
| BIOMARKERS THAT RECOGNISE AND DIAGNOSE SEVERE FORMS OF ADVERSE REACTIONS CAUSED BY COVID-19 VACCINES | It can be seen that several biomarkers were used for the recognition and diagnosis of some severe forms of SARS-CoV-2 vaccine adverse reactions, the most representative being: 3 studies used vascular endothelial growth factor (VEGF) (Talotta 2022, Mouliou et al. 2022, Abu Serhan et al. 2022); 9 studies used human type 1 interferons: the proteins IFN-α, IFN-β, IFN-γ, MDA5-IFN- α (Verbeke et al. 2022, Garrido Suárez et al. 2022, Hajjo et al. 2022, Mouliou et al. 2022, Shafiq et al. 2022, Watanabe et al. 2022, Szebeni et al. 2022, Castaldo et al. 2022, Morikawa et al. 2022) | Five studies used plasmacytoid dendritic cells (pDCs), such as: CD123+y BDCA2+(Verbeke et al. 2022, Watanabe et al. 2022, Szebeni et al. 2022, Castaldo et al. 2022, Morikawa et al. 2022); 14 studies used B-cells: immunoglobulin specific (IgG anti-S and IgG anti RBD) post-COVID-19 vaccination (Mouliou et al. 2022, Levy et al. 2022, Pisani et al. 2022, Azzolini et al. 2022, Ahmed et al. 2022, Kim et al. 2022, Verbeke et al. 2022, Mohseni Afshar et al. 2023, Hoffmann et al. 2022, Finsterer et al. 2021, Ameratunga et al. 2022, Szebeni et al. 2022, Castaldo et al. 2022, Morikawa et al. 2022); | Eleven studies used draining lymph nodes and a set of cell types, T cells: monocytes, macrophages, dendritic cells (DC), follicular helper T cells (Tfh), cytotoxic T lymphocyte (CTL), natural killer (NK) cells and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Verbeke et al. 2022, Mohseni, Mouliou et al. 2022, Mohseni Afshar et al. 2023, Hoffmann et al. 2022, Magen et al. 2022, Ekobena et al. 2022, Finsterer et al. 2021, Ameratunga et al. 2022, Szebeni et al. 2022, Castaldo et al. 2022, Morikawa et al. 2022) | Eleven studies used the cytokines: tumour necrosis factor alpha (TNF-α) and the interleukins IL-1, IL-1β, IL-4, IL-5, IL-6 (Zlotnik et al. 2022, Garrido Suárez et al. 2022, Ameratunga et al. 2022, Szebeni et al. 2022, Castaldo et al. 2022, Morikawa et al. 2022, Verbeke et al. 2022, Mohseni Afshar et al. 2023, Piras et al. 2022, Hoffmann et al. 2022, Umezawa et al. 2023); neuropilin-1 (NRP-1) (Talotta 2022); transmembrane serine protease 2 protein (TMPRSS2) (Aliasin et al. 2022) |
| Four studies used angiotensin converting enzyme 2 (ACE2) receptors (Castaldo et al. 2022, Piras et al. 2022, Aliasin et al. 2022, Ameratunga et al. 2022); calprotectin in blood (Hetland et al. 2022); other proteins: syndecan-1 and histone H3-neutrophil extracellular trap (NET)- (H3-NETS) (Hetland et al. 2022); natriuretic peptides (NT-proBNP) (Lee et al. 2022); prostaglandin (PG) E2 (Zlotnik et al. 2022) | Specific antibodies: 2 studies used antibodies to anti-aquaporin 4 (AQP4-IgG) (Umezawa et al. 2023, Caliskan et al. 2022), antibodies against myelin oligodendrocyte glycoprotein (MOG-IgG) (Umezawa et al. 2023), 3 studies used antibodies against platelet factor 4 (anti-PF4) (Mouliou et al. 2022, Mingot-Castellano et al. 2022, Abbasi et al. 2022), antibodies against leucine-rich glioma inactivated 1 (anti-LGI1) (anti-LGI1)) (Zlotnik et al. 2022), antibodies against cyclin citrullinated peptide (CEP) (Morikawa et al. 2022) and anti-ganglioside antibodies (Mohseni Afshar et al. 2023) | Two studies used C-reactive protein (Morikawa et al. 2022, Hajjo et al. 2022); γ-aminobutyric acid (GABA) neurotransmitter (Garrido Suárez et al. 2022); 6 studies used Toll-like receptors: TLR3, TLR7, and TLR8 (Verbeke et al. 2022, Watanabe et al. 2022, Szebeni et al. 2022, Castaldo et al. 2022, Morikawa et al. 2022, Rahimi Mansour et al. 2023); other laboratory findings suggestive of VITT, such as low platelets, low fibrinogen, and high D-dimer, together with elevated anti-PF4 titres, are classical findings (Abbasi et al. 2022). | These cytokines interact with complement systems which, in addition to producing inflammation and cross-talk between innate-adaptive immunity, activate the coagulation system and increase the permeability of the endothelial layer in a time- and dose-dependent manner, in turn promoting their distribution to the systemic circulation, resulting in elevated inflammation. (Umezawa et al. 2023, Pisani et al. 2022, Abu Serhan et al. 2022, Mouliou et al. 2022, Levy et al. 2022) | |
| Several studies propose platelets as a biomarker of inflammation, we know that both LNP mRNA and spike protein activate platelets. The identification of VEGF in plasma samples accurately measures the actual concentrations of this mediator in peripheral blood, like other viral infections, alterations in VEGF cause changes in circulating pro-inflammatory mediators such as TNF-α, IL-1β, IL-6, and IFN-γ-inducible protein 10 (IP-10 | Studies show that the concentration of VEGF in serum samples could be affected by the number of platelets, platelets being a major source of VEGF | Three studies identify elevated plasma levels of VEGF in SARS-CoV-2 vaccinees responsible for diffuse neurological and microvascular damage. The authors test the hypothesis that the adverse neurological and cardiovascular effects of COVID-19 vaccines could be caused by both direct viral damage and hyperactivation of the immune response. In turn, they mediate the prolonged symptoms of adverse events | (Talotta 2022, Verbeke et al. 2022, Abu Serhan et al. 2022) | |
| HYPERSENSITIVITY RESPONSES TO COVID-19 VACCINES | Also, non-anaphylactic allergic reactions, mostly HSR types III and IV classified by timing of onset and symptoms, can be observed, and were reported in the context of IMAE to SARS-CoV-2 vaccines, especially with mRNA vaccines | Ten studies confirm alterations in platelets together with activation of the complement system in mediating anaphylaxis, CARPA, and HSR through the release of biologically active molecules (ATP, thromboxane, and chemokines) and lipid inflammatory molecules such as PAF, which triggers degranulation of perivascular mast cells and release of thromboxane and serotonin, also causing inflammatory responses and tissue injury | They include systemic and local symptoms affecting the skin, gastrointestinal, respiratory, and cardiovascular systems | (Yoshida et al. 2023, Szebeni et al. 2022, Granados Villalpando et al. 2022, Mouliou et al. 2022, Morikawa et al. 2022, Piras et al. 2022, Pisani et al. 2022, Ahmed et al. 2022, Son et al. 2022, Ameratunga et al. 2022) |
| GUT MICROBIOTA AND IMMUNE SYSTEM DYSFUNCTION AND DAMAGE | Three studies assess immunomodulatory gut microbiota with COVID-19 vaccines | They suggest intestinal mucosal damage and inflammation and regional immune system and neuro/myenteric dysfunction system leading to gastrointestinal disorders, persistence of viral antigens in the gastrointestinal tract with alterations in the permeability of the intestinal barrier and activation of pathophysiological responses with neuroinflammatory and neurocognitive consequences | This hypothesis reinforces the theory of the immunogenicity-mediated role of HSR triggered by the vaccine and how it may induce indirect neurotoxicity through immune-mediated pathogenesis and gastrointestinal infection | (Ameratunga et al. 2022, Ng et al. 2022, Chen et al. 2022) |
| COEXISTENCE OF ADVERSE EFFECTS CAUSED BY COVID-19 VACCINES WITH THE ABSENCE OF ORGAN DAMAGE | Three studies confirm the absence of organ damage producing symptoms following administration of COVID-19 vaccines | This has been studied in inflammatory disorders that produced impairment without accompanying structural disease. But the evidence in favour of these findings is sparse given the lack of published data | – | (Zlotnik et al. 2022, Chen et al. 2022, Finsterer et al. 2021) |
| ASSOCIATION OF GENES INVOLVED WITH TRANSCRIPTIONAL EFFECTS OF mRNA VACCINES (Pfizer's BNT162b2) | Six articles evaluate genes associated with the cell cycle and transcription, they look at diseases related to genes involved in the transcriptional effects of BNT162b2, such as: immune system diseases, viral diseases, autoimmune diseases, musculoskeletal, rheumatological, joint, skin and connective tissue, connective tissue, lacrimal apparatus, xerostomia, and Sjögren's syndrome (Hajjo et al. 2022) | Magen et al. (2022) performed partial mapping of the BNT162b2 vaccine spike protein mRNA sequence in a patient's sample, showing an unusual pattern of vaccine mRNA expression in blood cells, namely, “chopped” parts of the mRNA vaccine molecules from the BNT162b2/Pfizer vaccine, indicating that the exogenously expressed mRNA was stable enough to persist over time | Lagousi et al. (2022) adding the hypothesis that in susceptible individuals the immune response to mRNA may not reduce, they associated younger age with greater alterations in monocyte, inflammatory response, and platelet-related gene expression shortly after the second dose of the BNT162b2 vaccine. It is likely that the amount of mRNA antigen contained in each vaccine formulation may also be a factor in mRNA reactogenicity | (Murata et al. 2022, Mouliou et al. 2022, Morikawa et al. 2022, Lagousi et al. 2022, Magen et al. 2022, Hajjo et al. 2022) |
| Çinar et al. (2022) encourage undertaking expanded immunophenotyping studies to detect B- and T-cell dysfunction in the serological response to COVID-19 vaccination | – | – | – | |
| MORTALITY CAUSED BY COVID-19 VACCINES | Data on mortality are limited, this review includes 3 articles. Murata et al. (2022) define COVID-19 vaccination as SARS-CoV-2 pseudoinfection and as inducing SIRS. They present 4 patients who died after COVID-19 vaccines. They identify 390 up-regulated genes associated with neutrophil degranulation and cytokine signalling; 115 genes were down-regulated in post-vaccination cases compared with controls. They note the need to detect this aberrant cytokine response, as well as analysis of single nucleotide polymorphisms to prevent such cases | Ittiwut et al. (2022) describe 13 cases of sudden unexplained death (SUD) following COVID-19 vaccination | Moro et al. (2022) reported 8 maternal deaths and 12 neonatal deaths, 621 (17.9%) reports were coded as serious. They included: 878 (25.4%) spontaneous abortions (<20weeks) (SAB), 101 (2.9%) episodes of vaginal bleeding, 76 (2.2%) premature deliveries (<37weeks), 62 (1.8%) stillbirths (≥20weeks), and 33 (no calculated rate) cases of babies with major birth defects or a chromosomal abnormality, which were 18 of 33 cases with BNT162b2 vaccine and 15 of 33 cases with mRNA-1273. They recorded 107 infant conditions including the 12 neonatal deaths, indicating prematurity as the cause of death | (Murata et al. 2022, Ittiwut et al. 2022, Moro et al. 2022) |
| ASSOCIATION OF CELL APOPTOSIS AND LEUCEMOGENESIS CAUSED BY VACCINATION AGAINST SARS-CoV-2 | Five studies comparing the endogenous adjuvant potential in vaccination associated with apoptotic cells and leukemogenesis are noted (Verbeke et al. 2021, Mouliou et al. 2022), such as: malignant neoplastic events related to the pathogenic role of the spike protein in COVID-19 vaccines (clonal myeloid disorders) (Çinar et al. 2022), uterine fibroids (non-malignant) (Nazir et al. 2022), lymphoproliferative disorders associated with neuropilin (NRP-1), this mediator may disrupt physiological pathways involved in angiogenesis, nociception, embryogenesis, carcinogenesis, inflammatory disorders and/or neuromyelopathies, and alter the blood–brain barrier (Talotta 2022) | – | – | (Verbeke et al. 2021, Mouliou et al. 2022, Çinar et al. 2022, Nazir et al. 2022, Talotta 2022) |
| DIFFERENTIATED IMMUNITY WITH THE HIGHEST INCIDENCE OF ADVERSE EVENTS IN FEMALES | Sex-differentiated immunity is observed in 6 articles, the higher frequency of adverse effects in women could be explained by the stronger immediate response to the antigen, modulated through the innate-adaptive immune system | Differential activity of X-linked genes and ChrY gene polymorphisms are regulated by escape from X-chromosome inactivation and epigenetic mechanisms, respectively. Testosterone depresses the innate and adaptive immune response, confirming that sex hormones are involved in the mechanism of increased vaccine reactogenicity in women | The articles included confirm that women respond to vaccination through increased production of antibodies and anti-inflammatory cytokines | (Moro et al. 2022, Green et al. 2022, Namiki et al. 2022, Rahimi Mansour et al. 2023, Nazir et al. 2022, Mouliou et al. 2022) |
| DRUG TREATMENTS TRIED AFTER VACCINATION AND OTHER THERAPEUTIC OPTIONS | Immunosuppressive and/or immunomodulatory treatment was administered after vaccination in 19 articles: oral systemic steroids in intermittent short courses (Saluja P et al. 2022); intravenous methylprednisolone (1g/24h) for 5days, with gradual tapering (Chen et al. 2022); intravenous methylprednisolone 1000mg/24h for 5days, followed by a tapering dose of oral prednisone for the next 10days (Pirani et al. 2022); ramipril and methylprednisolone pulses for 2months (Zagorec et al. 2022) | Intravitreal anti-VEGF (n=39 cases, 30.4%), followed by corticosteroids, 18 cases (14.2%). They include other treatments (thrombolytic, antiplatelet, or anticoagulant) in addition to surgery (Abu Serhan et al. 2022); antivirals and steroids have been frequently tried as treatment and accelerate recovery in Bell's palsy and transverse myelitis, intravenous immunoglobulin and plasmapheresis can be considered effective treatment for Guillain-Barre syndrome. High-dose methylprednisolone (1g/24h for 3–7days) started immediately in all cases of transverse myelitis improved neurological function and accelerated recovery (Mohseni Afshar et al. 2023) | Oral prednisolone 0.3mg/kg/day (20mg), colchicine (0.6mg/12h), and low-molecular weight heparin (enoxaparin 40mg/day) were started. Prednisolone was tapered over 2months after discontinuation of all medications and colchicine was given for 3months. Colchicine may be helpful in treating multisystem inflammatory syndrome (MIS) or preventing the recurrence of hyperinflammation with moderate doses of systemic corticosteroids. At 2-month follow-up laboratory results were in the normal range (Lee HJ et al. 2022); interleukin-6 blockade with siltuximab. Six months after completion of treatment, the patient experienced no signs of idiopathic multicentric Castleman disease (iMCD) or inflammation (Hoffmann et al. 2022) | The same treatment is proposed for adverse effects related to other drugs or inflammatory processes (steroids, antivirals, acetylsalicylic acid, and/or cochlear implant) (Pisani et al. 2022); same treatment for other adverse effects related to COVID-19 vaccines (steroids, antivirals, antihistamines) (Ekobena et al. 2022); partial clinical improvement after treatment with intravenous methylprednisolone, scheduled long-term treatment with rituximab (Caliskan et al. 2022). The immediate use is recommended of steroids for sudden onset tinnitus following COVID-19 vaccination due to its underlying immunosuppressive mechanism (Ahmed et al. 2022). |
| The case showed improvement 28days later after treatment with high-dose glucocorticosteroids: 2cycles of therapy, each of 1000mg intravenous methylprednisolone for 3days (first cycle was started 21days after vaccination, the second cycle was started at 28days), continued for 16days with 40mg oral prednisone and a tapering dose for 2 more weeks (Umezawa et al. 2023). Intravenous immunoglobulins (IVIG) (n=13 patients), steroids (n=3 patients) or no therapy (n=3 patients). Two patients required plasmapheresis because IVIGs were ineffective, 6 patients required artificial ventilation, 1 case received pregabalin for dysaesthesia. Only 1 case recovered completely and partially recovered in 9 cases (still disabled). Outcome was not reported in 9 patients (Finsterer et al. 2021). | Case 1: topical steroids and oral antihistamines. Case 2: high-dose intravascular immunoglobulin therapy and intravenous methylprednisolone (Watanabe et al. 2022); apixaban as anticoagulant and intravenous immunoglobulin (IVIG) (1g/kg for 2days), platelets improved. For long-term treatment, apixaban was prescribed for 6months and recanalisation of previously thrombosed veins (Abbasi et al. 2022); prednisone (Chow et al. 2022) | Initially treated with high-dose methylprednisolone (1g/24h for 5days), with favourable response, the case continued treatment with oral prednisolone (1mg/kg), showing marked improvement in temporal orientation, short-term memory, and language, although executive skills remained impaired. Serum sodium levels normalised within 3weeks of symptom onset. While continuing to improve gradually 4months later, the patient still has some difficulties with executive skills (Zlotnik et al. 2022). All cases receiving immunosuppressive treatment with systemic corticosteroids showed clinical improvement, but not complete cure | Patterson et al. (2023) propose that disruption of the monocytic–endothelial–platelet axis with maraviroc 300mg/12h and pravastatin 10mg/24h to restore the immune dysregulation observed in post-acute sequelae of COVID (PASC) could be a therapeutic alternative in the near future for COVID-19 vaccine adverse events. This provides the framework for a future randomised, double-blinded, placebo-controlled trial to further investigate the efficacy of immunosuppressive drugs, especially antiviral drugs, statins, and corticosteroids compared to other immunomodulators such as colchicine | |
This systematic review has met the initial objectives set out to assess knowledge of the topic under investigation.
The findings of this review are relevant to the general public, clinicians, drug and medical device regulatory agencies, and policy makers. The main enriched biological pathways are the immune response pathways, which are essential for inducing adaptive immune response. The results of enrichment in process networks highlight inflammatory and innate immune mechanisms associated with signalling of IFN-γ, cytokines, VEGF, platelet activation, B cells (specific immunoglobulins IgG anti-S and IgG anti-RBD following COVID-19 vaccination), the set of cell types (T cells: monocytes, macrophages, dendritic cells [DC], follicular helper T cells [Tfh], cytotoxic T lymphocyte [CTL], NK cells, and granulocyte-macrophage colony-stimulating factor [GM-CSF]), procalcitonin, and C-reactive protein.3,15,22,46,107,156,201 This confirms that vaccines produce the desired effect of stimulating the immune response, however, some of the stimulated innate immune processes can trigger inflammatory processes and cause adverse effects following COVID-19 vaccination, mainly IFN-γ signalling. The results confirm that COVID-19 vaccines induce a strong immune response in subjects vaccinated within the first 4h (i.e., on the same day of receiving the first and/or booster dose) for up to 365days (70.8%), compared to reactogenicity responses assessed by clinical and gene expression at 0, 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 13, 14, 15, 17, 20, 21, 22, 30, 37, 39, 42, 60, 62, 90, 117, 120, and 180days.
These results should be seen as testable hypotheses for the presence of putative functional links between COVID-19 vaccines and the diseases caused, and should not be considered proof that what the vaccine will produce will depend on the individual's particular immunity. Vascular and cardiovascular diseases are considered known risk factors in patients infected with COVID-19. Hajjo et al. (2022) identified 50 diseases enriched by DEGs from single-cell transcriptomics data of BNT162b2, the authors associate the functional link between mRNA COVID-19 vaccines and cardiovascular effects.
It can be observed that mRNA vaccines, especially BNT162b2 and mRNA-1273, induce immune responses similar to platelet activation triggered by lipopolysaccharide (LPS). Platelets, in addition to mediating communication between T and B cells (acquired immunity), regulate haemostasis and mechanisms of pathogen recognition and recruitment of host defence peptides, such as platelet factor 4 (PF4), a platelet-derived CXC chemokine. In turn, platelet activation may develop thrombotic adverse effects, such as VITT following administration of adenovirus vector vaccines and the less common mRNA vaccine involving antibodies against platelet factor 4 (anti-PF4). Platelets and leukocytes build immune thrombotic complexes, a classic sign of disseminated intravascular coagulation.47,49,156
Much of the innate immune activation depends on the iLNP component of nucleoside-modified iLNP-mRNA vaccines, as empty iLNPs stimulate a variety of cytokines (TNF-α, IL-1, IL-1β, IL-4, IL-5, IL-6, GM-CSF) and chemokines.9,16,55,56,76 In relation to the mRNA component, modification of the nucleoside m1ψ would improve the tolerability of mRNA-iLNP vaccines in humans by attenuating innate immune recognition that would promote translation efficiency and adaptive immunity, including licenced SARS-CoV-2 mRNA vaccines, capable of inducing a type 1 IFN response, raising the possibility of complementary, even synergistic, innate immune activation by the mRNA component.9,16,87 NK cells and T cells express a progressive and continuous increase in the presentation of genes associated with cell cycle and transcription. Another observation to note is the MDA5-IFN-α signalling pathway, which is required for T-cell responses, e.g., CD8+, may have implications for the development of mRNA vaccines for other infectious diseases and cancer, where CD8+ T cells are critical for effective immunity.3 The up-regulation of these immune pathways appears to underlie immune-mediated diseases, especially in young women, due to overexpression of X-linked genes by the required antiviral response and stimulation of the immune system by immune and endocrine pathways (hypothalamic–pituitary–thyroid axis, hypothalamic–pituitary–adrenal axis, and hypothalamic–pituitary–ovarian axis); the focus is on adverse effects in women of reproductive age.1,182,185
Cationic LNPs, similar to those used in mRNA vaccines, have been shown to act as adjuvants, can activate Toll-like receptors (TLR2, TLR3, TLR4, TLR5, TLR7, and TLR8) and NLRP inflammasome pathways.9,156 This finding confirms that COVID-19 mRNA vaccines produce immunogenicity by encoding the SARS-CoV-2 spike protein and act as adjuvants because of their LNP content and/or the intrinsic immunostimulatory characteristics of unmodified RNA (used in CureVac) and nucleoside-modified RNA (BNT162b2, mRNA-1273). This explains the differences in immunogenicity and reactogenicity between the COVID-19 vaccines. Prolonged duration of biopersistence of adjuvants, or the ability of adjuvant particles to move and accumulate slowly in lymphoid organs and other tissues are important safety concerns.1
A recent study confirmed that BNT162b2 is rapidly taken up in the human liver cell line Huh7 in vitro, causing changes in LINE-1 expression and distribution after exposure to BNT162b2, the mRNA of BNT162b2 is intracellularly reverse transcribed into DNA from 4 to 24h, the regulation of LINE-1 activity in response to BNT162b2 mRNA vaccines should be studied. Questions arise as to whether vaccine-derived mRNA can integrate into the human genome, which would cause genotoxicity.1,59
These features not only offer high and sustained antigen availability, but also favour stronger antibody responses, a condition that resembles a vaccine overdose, under specific circumstances. Reactogenicity has been associated with higher levels of antibodies following administration of COVID-19 vaccines, which reinforces the cumulative effect hypothesis, i.e., the relationship between elevated post-vaccination antibody levels (anti-S IgG, anti-RBD IgG) and their temporal persistence, which seems to mimic that of post-COVID-19. These data associate them with the cumulative immune effect due to repeated exposure and sensitisation to the antigen with each vaccine boost.25,36,40,41,43
Mediators have been identified in ACE2 and TMPRRS2 that affect the expression of receptors related not only to PASC but also in the adverse effects of COVID-19 vaccines.121,156 The spike effect of COVID-19 vaccines is recognised, evidencing that the SARS-CoV-2 spike protein contains the sequence and shares the native-like structural and functional mimicry of the antigen, causing the inflammatory syndrome involved in direct T-cell stimulation and excessive cytokine production in some subjects. These cytokines interact with complement systems which, in addition to producing inflammation and cross-talk between innate–adaptive immunity, activate the coagulation system and increase the permeability of the endothelial layer in a time- and dose-dependent manner, in turn promoting their distribution to the systemic circulation, which causes elevated inflammation and regulates persistent symptoms of adverse effects. The resemblance of vaccine antigens as potential pathogens causing adverse reactions is verified by the mechanisms of molecular mimicry and T-cell activation.
The production of the spike protein could be studied in liver cells, the persistence of viral antigens in the GI tract causing alterations in the permeability of the intestinal barrier and the activation of neuro-immuno-inflammatory pathophysiological responses, with neuroinflammatory and neurocognitive consequences, could occur through the hepato-pulmonary pathophysiological response, which could in turn alter neuronal signalling pathways. Mouliou et al. (2022) associate the cross-reactivity of the SARS-CoV-2 spike protein with strong interactions with transglutaminases, myelin basic proteins, mitochondria, nuclear antigens, myosin, collagen, claudin 5/6, and S100B, among other proteins, including proteins involved in synaptic vesicle trafficking, endocytosis, axonal transport, neuronal transmission, thrombosis, inflammation, the blood–brain barrier, and mitochondrial barrier, as well as protein growth factors.
The cross-talk between the human gut microbiota and the immune system has been demonstrated in recent years, and the context of COVID-19 vaccines is no exception, it can also modulate the immune response to vaccination and microbial disturbance and has an impact on the immune response to COVID-19 vaccines. Microbial imbalance, a state called “dysbiosis”, predisposes the body to disease. Many studies highlight the role of the gut microbiota in inflammatory bowel diseases, Clostridium difficile infection, metabolic and neuropsychiatric disorders, autoimmune diseases, and of the non-intestinal microbiota in diseases such as central nervous system disorders, chronic obstructive pulmonary and periodontal disease, cystic fibrosis, and chronic rhinosinusitis. Currently, understanding dysbiosis is challenging because of the intravariability and dependent intervariability of the human microbiota that make it difficult to identify distinct abnormal and normal microbial communities. Understanding the underlying dynamics of the interactions between the microbiota and the immune system is necessary to clarify the potential implications for human health following vaccination.147–152 The immune system is built by a set of innate and adaptive immune mechanisms aimed at enhancing microbiota containment, barrier immunity, and tissue repair unlinked to inflammation. Tolerance of the normal gut microbiota is essential for gut homeostasis, which requires a large network of immune regulatory cells, including regulatory T cells (Tregs) and tolerogenic dendritic cells. Detection of the commensal microbiota through the TLR-MyD88 signalling pathway is one strategy used by the immune system to maintain host microbial homeostasis.147–152 Combined approaches will identify the gene expression pattern, gene function, regulation, and physiology of bacteria in a community to improve characterisation of the microbiome. DNA sequencing-based diagnostics do not distinguish between live and metabolically active, damaged, or dead bacterial cells, or free DNA, giving an incomplete picture of the functional profile of a microbial community. RNA sequencing analyses can identify living and metabolically active cells and inform about the transcriptional activity of the community. A detailed understanding of the transcriptome of a given organism will make it easier to recognise cellular processes and proteins that may or may not be expressed and/or produced under current culture assay conditions.150
Due to the increasing number of cases with symptoms following COVID-19 vaccination and the lack of approved studies and therapies, many of those affected have received different therapies. This is reflected in case series participants and single cases who had received immunosuppressive and immunomodulatory treatments, such as antiviral drugs, statins, systemic corticosteroids, and colchicine. This suggests that the pathophysiology of adverse effects may be the monocyte–endothelial–platelet axis.186 Most manifest in the brain, colon, heart, lungs, along with endothelial cells and astrocytes. Stimulated platelets also release RANTES, which bind to endothelial cells and stimulate monocyte adhesion to inflamed endothelial tissues and function as a chemotactic agent for inflammatory cells. Activated platelets and endothelial cells also secrete VEGF which induces microvascular hyperpermeability and angiogenesis. VEGF is a known diagnostic biomarker for pro-inflammatory, prothrombotic, and vasculitic neuropathy. Patterson et al. (2023)186 target treatment for vascular endothelialitis and effects associated with PASC consistent with the effects caused by SARS-CoV-2 vaccines that require assistance with activities of daily living (neurological symptoms, severe headache, cognitive impairment, neurological symptoms): severe headaches, cognitive impairment, neuropathy and weakness; autonomic dysfunction such as postural orthostatic tachycardia syndrome [POTS] consisting of unexplained tachycardia, dizziness, light-headedness, fainting, and abdominal pain, along with sensitivity to light; cardiorespiratory complaints causing exertional intolerance and limitation of physical activity [these include basic activities for daily living]; fatigue along with myalgias. They use the combination of maraviroc's protective role on the endothelium with pravastatin, targeting CCR5 and fractalkine receptors. Their hypothesis has been studied, it is described how CD14+ and CD16+ monocytes transmigrate across the blood–brain barrier and play an important role in central nervous system (CNS) immune surveillance, these monocytes act as reservoirs of human immunodeficiency virus (HIV) in the CNS causing neuroinflammation, neuronal damage, and cognitive impairment. Both maraviroc and statins cross the blood–brain barrier, and maraviroc specifically has been proposed as a treatment for Parkinson's disease, stroke, and cognitive impairment. The authors reported improved neurological function and capacity. Their findings correlated with a statistically significant decrease in VEGF (r=0.4, p=.02) and sCD40L (r=0.42, p=.01), suggesting that treatment targeting cytokines associated with vascular endothelialitis correlated with improvement in neurological symptoms. It is possible that antagonism of these receptors may also inhibit autonomic effects, suggesting that pro-inflammatory macrophage activation may trigger vascular endothelialitis, the authors associated elevations in sCD40L, already associated with sympathoadrenal activation. Many patients with cardiac, neurological, and pulmonary symptoms have been studied with electrocardiogram, echocardiogram, stress test, pulmonary function tests, cerebrospinal fluid puncture, computed tomography, and magnetic resonance imaging, which have not detected structural abnormalities, or disease. Symptoms have only been treated with anti-arrhythmics, bronchodilators, alpha-adrenergics, anti-inflammatory drugs, and anti-depressants, rather than treating the underlying cause. The proposed treatment improved exercise tolerance, mobility, and respiratory symptoms. Both maraviroc and statins may reduce cardiovascular risk by protecting the endothelium from infiltration by pro-inflammatory macrophages (IL-2, IL-8, and TNF-α).
Clinical trials will need to be conducted to understand the variation in length of treatment and adapt them to the personal clinical conditions of each patient. CD4/CD8 ratios and the amount of post-vaccine antibodies should also be studied before and after treatment.
It should be noted that COVID-19 vaccines do not contain live virus and therefore should not cause disease in the vaccinated person without prior exposure to the virus. Paradoxically, the results of this study show that COVID-19 vaccines may expose some people to an increased risk of immune dysregulation. This likelihood is confirmed by recent evidence from the published biomedical literature linking immune dysregulation, the spike effect of COVID-19 vaccines, and the temporal occurrence with the adverse effects caused. These findings are testable, not confounded by SARS-CoV-2 infection, concurrent or prior autoimmune diseases, or effects of host genetic background on susceptibility to reactogenicity. However, cases of systemic adverse reactions have been reported in patients with allergic diseases, such as asthma, hay fever, allergic rhinitis, atopic dermatitis, food allergies, and/or intolerances, who are potentially susceptible to COVID-19 and worsening of their chronic diseases after vaccination with BNT162b2, such as intestinal dysbiosis.115 Some reports posit the onset autoimmune diseases after vaccination ruling out that autoimmune conditions were diagnosed at baseline or recurred after vaccinations, including infections. In post-mortem investigations of post-vaccination deaths, the causal association was established, all sudden deaths should be disclosed and investigated to determine clear evidence about the death following COVID-19 vaccination,102,171 and maternal and neonatal mortality rates should be compared with baseline rates from actual published reports. Close and continuous surveillance for adverse effects in pregnant women who received SARS-CoV-2 vaccines should be performed because of the risk of spontaneous abortion, foetal growth retardation, and congenital abnormalities caused.
Most of the studies included have small sample sizes, some were single case reports, this could be related to a limited observation period as well as inconsistent population adherence to mass vaccination. The most complete data on adverse events were published in follow-up reports obtained from online questionnaires, pharmacovigilance systems, or case series, some of these reports might not be clinically verified as they are subject to recording and reporting biases by pharmacovigilance systems.
It is concerning that a vaccine may cause immune dysregulation that can lead to the disease against which it was developed, this observation warrants further investigation by vaccine manufacturers, the Centres for Disease Control and Prevention (CDC), and the Spanish Agency for Medicines and Health Products (AEMPS). Cellular and humoral immune dysregulation following SARS-CoV-2 vaccination is likely to be predicted.
This analysis proposes that personalised studies should be undertaken using patient-specific genetic signatures for future vaccine genomics research. The application of systems biology methods to analyse the pharmacological responses of COVID-19 vaccines strengthens the understanding of adverse events following vaccination.156 They provide testable hypotheses alongside clinical data on adverse events.
ConclusionsThis study provides a framework for future clinical trial designs to further investigate the efficacy, utility, and especially the potential cure of adverse events caused by COVID-19 vaccines.
A better understanding of the nature of reactogenicity will allow the design of next generation COVID-19 vaccines to stimulate more tolerable adaptive immune responses while mitigating or eliminating serious adverse reactions experienced by those affected by the vaccine. The final suggestion is that further, more accurate, laboratory diagnostic tests should be performed, with clinical evidence establishing the correct causality of case reports. Clinical and laboratory information should be used to assess the current and past medical condition of each case to understand the adverse events.
FundingThis research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interestThe author declares no conflict of interest related to the topic of the article.
I would like to thank my family, who have supported me throughout the process.
Variables identified from reports included in the systematic review (time from vaccination to symptom onset [days], future recommendations and limitations related to COVID-19 vaccines).
| Report | Year of publication | Study participants | Sample size | Time from vaccination to onset of symptoms (days) | Recommendations | Limitations |
|---|---|---|---|---|---|---|
| Verbeke et al.3 | 2022 | – | – | In pre-clinical studies, the peak is reached 4–24h after vaccine administration and gradually wanes, lasting from several days, weeks or months. Detectable in axillary LNs up to 60days after the second dose of BNT162b2 or mRNA-1273 in vaccinated humans, although antigen expression may be higher | Improve the next generations of mRNA-iLNPs, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Potential alternative for gene therapy and treatment of autoimmune diseases | Many variables that can affect host-dependent reactogenicity and immunogenicity of mRNA vaccines. Limited knowledge of interactions of mRNA-iLNP components with innate immune mechanisms |
| Aldén et al.59 | 2022 | Cell model. Carcinoma cell line, with active DNA replication | – | 6h | Improve the next generations of mRNA-iLNPs, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Potential alternative for gene therapy and treatment of autoimmune diseases | Many variables that can affect host-dependent reactogenicity and immunogenicity of mRNA vaccines. Different gene and protein expression, activated in several tissues in both cell proliferation and terminally differentiated cell processes. No data available on effect on placental transfer, however, it occurs during embryogenesis |
| Szebeni et al.15 | 2022 | – | – | In pre-clinical studies, peak 1–4days after intramuscular vaccine administration in mice detectable in liver, 10days detectable at the injection site, may vary according to type III and/or IV HSR. After the first dose with mRNA-iLNP (BNT162b2 or mRNA-1273), although antigen expression might be higher | Improve the next generations of mRNA-iLNPs, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Potential alternative for gene therapy and treatment of autoimmune diseases | Many variables that can affect host-dependent reactogenicity and immunogenicity of mRNA vaccines. Limited knowledge on immune mechanisms and pharmacokinetics of BNT162b2 and mRNA-1273 vaccines |
| Yoshida et al.115 | 2023 | Human model. From 5 to 11years of age | 421 participants | 1–7days after the first and second administration of the BNT162b2 vaccination | Improve the next generations of mRNA-iLNPs, they must offer improved tolerability, safety, and efficacy to ensure clinical success | Limited study, need to investigate long-term adverse reactions to BNT162b2 vaccine based on the presence or absence of allergic diseases in the sample of 5–11-year-olds |
| Granados Villalpando et al.116 | 2022 | Human model. Both sexes, aged between 18 and 65years, residents of Tabasco and Mexico, were admitted following a survey. Pregnant and lactating women were excluded | 443 participants | 1h after first, second, third, and fourth dose | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success | Long-term adverse reactions to COVID-19 vaccines need to be investigated. In most studies, the association between younger age and higher prevalence of adverse effects is strong, in this study associating older age with higher prevalence of adverse effects could be a confounding variable. Better distribution by age and sex is desirable Larger cohort study |
| Talotta R.107 | 2022 | – | – | – | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Further research is needed to offer treatment | Long-term adverse reactions of COVID-19 vaccines need to be investigated. Many variables that can affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines |
| Chen et al.132 | 2022 | – | – | – | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Further research is needed to offer treatment. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone | Long-term adverse reactions to COVID-19 vaccines need to be investigated. Assessing potential risks in frail populations. Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines. Currently available treatments can only control infection, change the severity and duration of pain |
| Garrido Suárez et al.133 | 2022 | – | – | – | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Further research is needed to understand immunogenicity. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone | Long-term adverse reactions of COVID-19 vaccines need to be investigated Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines Pharmacovigilance should consider future studies to understand possible long-term inflammatory mechanisms of these new vaccines |
| Ng et al.147 | 2022 | Human model. Both sexes were included, aged 18years or older with no history of SARS-CoV-2 infection and had received 2 doses of inactivated vaccine or 1 dose of mRNA, following a standardised questionnaire | 138 participants | – | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Further research is needed to understand immunogenicity. Adding natural adjuvants is suggested to improve responses to vaccination | Long-term adverse reactions of COVID-19 vaccines need to be investigated Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines Should compare adverse effects of vaccination with RNA sequencing analyses, they could identify live, metabolically active cells, reporting on population transcriptional activity |
| Hajjo Ret al.156 | 2022 | Cell model. Systems biology analysis for COVID-19 mRNA vaccines using transcriptomics datasets | – | 21 to 22days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical successAdverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered aloneFurther research is needed to offer treatment, personalised recommendations using patient-specific genetic signatures | Long-term adverse reactions of COVID-19 vaccines need to be investigated Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines Limited studies on vaccine genomics, more in-depth studies needed |
| Murata et al.102 | 2022 | Human model. Analysis of single nucleotide polymorphisms in 4 deceased subjects following COVID-19 vaccination with Moderna and Pfizer | 6 participants | 1–10days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone Further research is needed to prevent death, personalised recommendations using patient-specific genetic signatures, RNA sequencing of each subject | Long-term adverse reactions of COVID-19 vaccines need to be investigated Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines. Limited vaccine genomics studies, more research needed |
| Ittiwut et al.171 | 2022 | Human model. Whole exome sequencing, SCN5A gene | 13 participants | 1–7days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone Further research is needed to prevent death, personalised recommendations using patient-specific genetic signatures, RNA sequencing of each | Long-term adverse reactions of COVID-19 vaccines need to be investigated Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines Limited vaccine genomics studies, more research needed |
| Çinar O et al.172 | 2022 | Human model. Pathobiological mechanisms and causalities of spike protein-related toxicity and clonal myeloid disorders after BNT162b2 vaccination | 4 participants | 30–37days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone Further research is needed to offer treatment, personalised recommendations using patient-specific genetic signatures | Long-term adverse reactions of COVID-19 vaccines need to be investigated Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccine Limited published studies on immunophenotyping of vaccines, further research is needed |
| Nazir et al.173 | 2022 | Human model. Women aged 18–30. Most of the surveys included in the analysis were conducted in the USA, UK, and Norway, countries belonging to the ‘Global North’ | 78138 participants | ≥14days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone Personalised recommendations using patient-specific genetic signatures. Assess the condition of spike toxicity, the so-called ‘spike effect’ | Long-term adverse reactions of COVID-19 vaccines need to be investigated Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines. Limited published studies on adverse effects of vaccines that change the hypothalamic–pituitary–ovarian axis, further research is needed |
| Moro et al.182 | 2022 | Human model. Women aged 15–44years. US residents, excluding foreign residents | 3462 participants | 0–≥15days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. Personalised recommendations using patient-specific genetic signatures. Assess the condition of spike toxicity, the so-called ‘spike effect’ | Long-term adverse reactions of COVID-19 vaccines need to be investigated. Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines. Limited published studies on adverse effects that modify the hypothalamus–pituitary–ovary axis and, immune responses in the female reproductive tract and their heterogeneity within the organ and between compartments due to COVID-19 vaccines, there needs to be further research |
| Rahimi Mansour et al.185 | 2023 | Human model. Women of reproductive age experience menstrual disturbances following vaccination | – | – | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. Personalised recommendations using patient-specific genetic signatures. Assess changes in the immune and endocrine pathways | Long-term adverse reactions of COVID-19 vaccines need to be investigated Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines Limited published studies on adverse effects that change the hypothalamic–pituitary-ovarian/adrenal and hypothalamic–pituitary–adrenal axes |
| Green et al.187 | 2022 | Human model. First and second dose with Pfizer: 923 participants, ≥30years; third dose with Pfizer: 266 participants, between 20 and 65years | 923 participants | 1 day | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. Personalised recommendations using patient-specific genetic signatures. Assess changes in the immune and endocrine pathways | Long-term adverse reactions of COVID-19 vaccines need to be investigated. Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines. Notable and consistent excess adverse event rates in females after immunisation with Pfizer-BioNTech COVID-19, across all age groups. Gender-specific factors influencing vaccine response are suggested. Different vaccine doses for males and females should be explored, also gender differences in the phenotype of mast cell (MC)-associated diseases, established in childhood |
| Mouliou et al.1 | 2022 | Human model. Address adverse effects published in monitoring reports prepared by pharmaceutical agencies or obtained from online questionnaires | – | 1–60days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. To achieve optimal, long-term vaccination outcomes, more well-designed, mutation-based studies are needed to identify epitopes with minimal heterogeneity. The design is called for of more adjuvants that stimulate innate immunity rather than hypersensitivity and inflammation. More studies are called for with laboratory and clinical diagnostic evidence using specific genetic signatures Adverse effects of vaccination should be compared with post-COVID-19 adverse outcomes rather than considered alone | These reports are not clinically verified, are subject to increased bias and would not reflect an honest clinical condition. Poor reports or underdiagnosis prevents the scientific community from making pure associations and assessments. Repeated booster doses may lead to cumulative toxicity and innate immune overstimulation contributing to excessive inflammation Benefits/risks appear to be unbalanced. Many variables that may affect the reactogenicity and immunogenicity of COVID-19 vaccines are host-dependent. Investigate long-term adverse reactions to COVID-19 vaccines |
| Castaldo et al.201 | 2022 | Human model. There are 9338 records, 94% received BNT162b2 or ChAdOx1 | 1057000 participants | 1–7days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. Investigate long-term adverse reactions to COVID-19 vaccines | It was not possible to predict the exact timing of the onset, duration, disappearance, and/or worsening of post-vaccination headache The benefits/risks appear to be unbalanced. Many variables that may affect the reactogenicity and immunogenicity of COVID-19 vaccines are host-dependent |
| Verbeke et al.202 | 2021 | – | – | 2days | Improve the next generations of iLNP-mRNAs, they must ensure greater tolerability, safety, and efficacy to achieve clinical success. Potential alternative for gene therapy and treatment of autoimmune diseases. Investigate long-term adverse reactions to COVID-19 vaccines | Many variables that may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines It was not possible to predict the exact timing of the duration, disappearance, and/or worsening of adverse effects |
| Saluja et al.203 | 2022 | Human model. Case report | One participant | 2days | Investigate long-term reactions to COVID-19 vaccines. Assess the putative plausibility of the COVID-19 vaccine in further studies. Further research is needed to understand immunogenicity | It was not possible to predict the exact timing of the duration, disappearance, and/or worsening of adverse effects. Limited vaccine genomics studies, more research needed |
| Chen et al.120 | 2022 | Human model. Case report | One participant | 2days | Investigate long-term adverse reactions to COVID-19 vaccines Assess the putative plausibility of the COVID-19 vaccine in further studies. Further research is needed to understand immunogenicity | It was not possible to predict the exact timing of the duration (follow-up of 45days without full recovery), remission and/or worsening of adverse effects. Limited vaccine genomics studies, more research needed |
| Morikawa et al.22 | 2022 | Human model. Case report | One participant | 3days | Investigate long-term adverse reactions to COVID-19 vaccines. Prospective clinical studies are needed to understand the immunological reactions after COVID-19 vaccination. In addition, the correlation between rheumatoid arthritis (RA) and eosinophils needs to be considered in the clinical setting. Improve the next generations of iLNP-mRNAs, they must ensure greater tolerability, safety, and efficacy | In this case, vaccination with BNT162b2 induces RA-related cytokine production triggering early-stage disease. Limited vaccine genomics studies, close monitoring of individual/population immunophenotyping is necessary. Better age and sex distribution is desirable. Larger cohort study |
| Chen et al104 | 2022 | Human model. Pharmacovigilance analysis using VAERS | 14956 reports | – | Facilitate access to results on the association between COVID-19 vaccines and adverse effects on the auditory organ. Prospective clinical studies are needed to understand immunological reactions after COVID-19 vaccination. Establish the correlation between hearing dysfunction and COVID-19 mRNA and virus vector vaccines. Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy | Limited vaccine genomics studies, individual/population immunophenotyping should be closely monitored Larger cohort study. Personalised studies using patient-specific genetic signatures |
| Namiki et al.23 | 2022 | Human model. Survey-based study with female healthcare workers (doctors, nurses) and undergraduate medical students at an affiliated hospital in Japan | 424 participants | 14–≥30days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. Personalised recommendations using patient-specific genetic signatures. Assess changes in the immune and endocrine pathways. Assess the condition of spike toxicity, the so-called ‘spike effect’ | Limited vaccine genomics studies, antibody responses with BNT162b2 vaccine should be closely monitored at individual/population level. Larger cohort study. Personalising patient-specific genetic signatures. Gender-specific factors influencing vaccine response are suggested. Different vaccine doses for males and females should be explored |
| Lagousi et al.109 | 2022 | Human model. Summarises studies showing the incidence of myocarditis after COVID-19 vaccination in different age groups (≤18–39years) | – | 42–120days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Precision vaccinology will be able to identify inflammatory biomarkers that can predict the hyperactivity of new vaccines. Urgent pre-clinical evaluation of COVID-19 vaccines in different population groups is called for. Long-term cohort studies would be needed Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. The distinctive profile of immunity and inflammation observed after natural infection has also been observed after COVID-19 vaccination | These studies did not have the statistical power to reveal the serious effects of vaccine-associated myocarditis among young men. Previous studies have demonstrated the relevance of a systems biology approach to correlate positive regulation of genes associated with innate immunity, cytokine production and responses to virus infection, particularly IFN-inducible genes, with adverse effects observed in human trials. It will facilitate a better understanding of the underlying mechanisms of side effects |
| Pirani et al.106 | 2022 | Human model. Case series | 2 participants | 6–8days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Long-term cohort studies would be needed Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. The distinctive profile of immunity and inflammation observed after natural infection has also been observed after administration of COVID-19 vaccines | Limited vaccine genomics studies, antibody responses with BNT162b2 vaccine should be closely monitored at individual/population level Further research is indicated to clarify the pathophysiology of post-vaccination optic neuritis with Pfizer BNT162b2 and the interaction between environmental exposure and genetic predisposition in post-vaccination optic neuritis needs further investigation |
| Zagorec et al.24 | 2022 | Human model. Case series | 2 participants | 1 day | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Long-term cohort studies would be necessary for future monitoring of adverse effects. The authors call for reaction if new-onset signs of immune-mediated disease appear after COVID-19 vaccination | Limited vaccine genomics studies, antibody responses with BNT162b2 vaccine should be closely monitored at the individual/population level It was not possible to predict the exact timing (follow-up of 65–160days with partial or full recovery), remission and/or worsening of all adverse effects |
| Levy et al.25 | 2022 | Human models. In health workers | 831 participants | 7days | Improve the next generations of iLNP-mRNAs, they must ensure greater tolerability, safety, and efficacy to achieve clinical success. Long-term cohort studies would be needed for future monitoring of adverse events. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. The distinctive profile of immunity and inflammation observed after natural infection has also been observed after administration of COVID-19 vaccines | Limited vaccine genomics studies, antibody responses with BNT162b2 vaccine should be closely monitored at the individual/population level It was not possible to predict the exact timing of the duration (follow-up of 4months), remission and/or worsening of all adverse events. Further research is called for to clarify the pathophysiology of adverse events following vaccination with BNT162b2Larger cohort studies are needed, personalising patient-specific genetic signatures |
| Piras et al.26 | 2022 | Human model. Case report and in-depth review of 110 reports published up to July 2022 | 111 participants | 5–13days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Long-term cohort studies would be needed for future monitoring of adverse effects. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. Personalised recommendations using patient-specific genetic signatures. Assess the condition of spike toxicity, the so-called ‘spike effect’ | Limited vaccine genomics studies, close monitoring of individual/population immunophenotyping is necessary. Previous studies have demonstrated the relevance of a systems biology approach to correlate the positive regulation of genes associated with innate immunity, cytokine production and responses to virus infection, particularly IFN-inducible genes, with adverse events observed in human trials. This will facilitate a better understanding of the underlying mechanisms of these side effects |
| Abu Serhan et al.27 | 2022 | Human model | 130 participants | 5–10days | The available data show persistent symptoms in most patients, being a common clinical feature in most vascular–ocular effects, the authors call for further long-term follow-up research. The problem of vascular-ocular adverse effects after COVID-19 vaccination is an important cause of blindness that deserves more attention. More studies are needed to determine the incidence, risk factors, prognosis, and treatment of vascular-ocular effects following COVID-19 vaccination | More research is needed into the underlying pathophysiology of these effects, their risk factors and possible methods of prevention and treatment. People at risk should be counselled about these effects before COVID-19 vaccination, the visual prognosis appears guarded. They highlight the lack of diagnostic information in many cases, the lack of outcome assessment in those affected and the inability to statistically analyse relative risk due to insufficient data |
| Mohseni Afshar et al.28 | 2023 | Human model | – | ≤3–30days | Future studies should determine the real risk of these adverse effects following COVID-19 vaccination. Assess changes in immune pathways and the condition of peak intoxication, the so-called ‘spike effect’. Adverse effects of vaccination should be compared with post-COVID-19 adverse outcomes rather than considered alone. The distinctive profile of immunity and inflammation observed after natural infection has also been observed after administration of COVID-19 vaccines | The authors stress that any neurological symptoms following vaccination with COVID-19 may be potentially damaging and must be carefully evaluated. A systems biology approach to correlate positive regulation of genes associated with innate immunity, cytokine production and responses to virus infection, particularly IFN-inducible genes, with adverse events observed in human trials. This will facilitate a better understanding of the underlying mechanisms of these side effects |
| Lee et al.29 | 2022 | Human model | One participant | 20–62days | During the COVID-19 pandemic, colchicine was one of the drugs explored for repurposing as a potential anti-inflammatory drug based on its mechanism of action. They hypothesise that colchicine may be helpful in treating MIS or preventing the recurrence of hyperinflammation with moderate doses of systemic corticosteroids. Adverse effects of vaccination should be compared with post-COVID-19 adverse outcomes rather than considered alone. Immune pathway changes and the spike effect should be assessed. To detect and treat these adverse effects, vaccine safety monitoring and investigation of any adverse effects should continue. Clinicians should take a complete history, and perform physical and laboratory examination to rule out other concurrent diseases. If clinical suspicion of MIC-A is high, treatment should not be delayed | Current understanding of the pathophysiology of MIS-C/A is growing, but it remains unclear. There are currently no established protocols for the management of MIS-C/A. General supportive care and close monitoring for possible compromise are crucial. High-dose steroids have also been used as anti-inflammatory agents in the majority of MIS-A cases reported after COVID-19 vaccination. International research collaboration is essential for a standardised protocol for the best treatment actions for any adverse effects |
| Chen et al.30 | 2022 | Human model | 112 participants | 4–10days | Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. The distinctive profile of immunity and inflammation observed after natural infection has also been observed after administration of COVID-19 vaccines. Immune pathway changes and the spike effect should be assessed. Helpful with other vaccine platforms to study vascular properties | Study limited by the proportion of subjects with cardiovascular events (small sample), to strengthen the reliability of the pulse–variability score analysis, more participants are needed |
| Hoffmann et al.31 | 2022 | Human model | One participant | 14days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success Personalised recommendations using patient-specific genetic signatures. Personalised recommendations using patient-specific genetic signatures. Assess changes in immune pathways and the condition of spike toxicity, the so-called ‘spike effect’ | Limited vaccine genomics studies, antibody responses with COVID-19 vaccines should be closely monitored at individual/population level. Previous studies have demonstrated the relevance of a systems biology approach to correlate positive regulation of genes associated with innate immunity, cytokine production and responses to virus infection, particularly IFN-inducible genes, with adverse events observed in human trials. It will facilitate a better understanding of the underlying mechanisms of side effects |
| Wong et al.32 | 2022 | Human model | 62679 participants | 0–365days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Adverse effects of vaccination should be compared with post-COVID-19 adverse outcomes rather than considered alone. Personalised recommendations using patient-specific genetic signatures. Assess the condition of spike toxicity, the so-called ‘spike effect’ | The authors warn of its potential for long-term health damage to women. They highlight the need for population-based studies that record epigenetic variation due to genetic polymorphisms in ethnic communities, at-risk populations, following more cycles over longer periods to study long-term consequences, not assessing risk to pregnancy. Many variables may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines. Limited published studies on adverse events modifying hypothalamic–pituitary–ovarian axis and immune responses in the female reproductive tract and their heterogeneity within the organ and between compartments due to COVID-19 vaccines should be further investigated |
| Kim et al.33 | 2022 | Human model | 4290 participants | – | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success Investigate long-term adverse reactions to COVID-19 vaccines. Assess the putative plausibility of the COVID-19 vaccine in further studies | It was not possible to predict the exact timing of the duration, follow-up, remission, and/or worsening of adverse effects. Limited vaccine genomics studies, Further research is needed to understand immunogenicity |
| Tamborska et al.34 | 2022 | Human model | 70 participants | ≤12–90days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success Investigate long-term adverse reactions to COVID-19 vaccines. Assess the putative plausibility of the COVID-19 vaccine in further studies and the spike effect. Clinicians and surveillance agencies should be alert to this adverse event and its atypical variants | They could not ensure that all patients had the same microbiological, electrophysiological, and anti-ganglioside antibody study. They report only one patient positive for anti-ganglioside antibodies, possibly because the tests were analysed in routine diagnostic services rather than in a specialised laboratory. The surveillance system is subject to verification and case reporting bias, as well as under-reporting. Comparison of age distribution is limited by case ascertainment and is not adjusted for confounding factors, e.g., administration of different vaccines at various ages |
| Magen et al.35 | 2022 | Human model. Case report | 1 participant | 4days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. They highlight the need for further studies to understand the autoimmunity caused by modified mRNA vaccines. Adequate clinical trials are needed to design new COVID-19 vaccines without risk of adverse events, in different population groups (with autoimmune diseases—allergies, different ages, different ethnicities | The mechanisms that may affect vaccination need to be assessed. They are complex and include factors related to the COVID-19 vaccines (nature of the antigen, delivery system, adjuvants and immunomodulators), the host immune system, and the gut microbiota. They have demonstrated this ‘endogenous adjuvant potential’ in significantly impaired antibody responses with these vaccines. |
| Pisani et al.36 | 2022 | Human model. Includes 11 scientific articles on audiovestibular disorders COVID-19 vaccine | – | 3–42days | There is a lack of systematisation and standardisation of clinical information due to under-reporting of adverse events, as well as the follow-up and treatment of those affected, hindering the interpretation of results. Long-term adverse reactions to COVID-19 vaccines should be investigated | Absence of RT-PCR tests that could rule out SARS-CoV-2 infection in these patients. There is reasonable suspicion if symptoms are due to COVID-19 infection, also characterised by high neural tropism with potential damage to the inner ear, even in mild forms |
| Ekobena et al.37 | 2022 | Human model | 4 participants | ≥12h–15days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone | Long-term adverse reactions to COVID-19 vaccines need to be investigated. It was not possible to predict the exact timing of the follow-up, remission and/or worsening of adverse events. Many variables may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines. Limited published studies on otorhinolaryngological adverse events, also characterised by high neural tropism with damage to the inner ear, even in mild forms, more research is needed |
| Caliskan et al.38 | 2022 | Human model. Case report | 1 participant | 1 day | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone | They highlight the need to continue to monitor these autoimmune adverse events after BNT162b2 vaccination and to investigate the mechanisms of immune system activation in the development of mRNA vaccine-related central nervous system autoimmunity. It was not possible to predict the exact timing of the duration, follow-up, remission, and/or worsening of adverse effects |
| Aliasin et al.39 | 2022 | Human model | – | – | Larger studies are needed with larger populations. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. It is also strongly recommended to search for certain modifiable and non-modifiable risk factors that have predisposed those affected to overreact to COVID-19 vaccines and develop serious adverse events. For example, they show that the rate of post-vaccination GBS increases in women aged 30–49years, as well as an increased risk of cerebral venous sinus thrombosis (CVST) triggered by vaccine-induced thrombotic thrombocytopaenia (VITT) in persons vaccinated with adenoviral vectors. Different types of mRNA vaccine-related cranial nerve lesions are postulated. Information gathered from vaccine surveillance reports can help healthcare systems compare different platforms and determine tolerability in different populations. This can also guide policy makers to make the right decisions and take the right actions | Study is suggested of the following mechanisms: cross-reactivity between foreign antigens and self-antigens, over-activation of antigen presenting cells and subsequent autoimmune response, and activation of polyclonal B cells or bystanders leading to cytokine synthesis and activation of autoreactive T cells. This approach will make it easier for experts to determine the exact contraindications and precautions that must be considered to treat or avoid adverse effects of COVID-19 vaccines. Studies should also focus on the different characteristics of various vaccine platforms. Some adverse outcomes have been shown to be more likely to occur after certain vaccine platforms |
| Azzolini et al.40 | 2022 | Human model. Prospective cohort study on healthcare professions of a tertiary hospital in North Italy. IgG-COVID study | 4156 participants | 10days | Long-term adverse reactions of COVID-19 vaccines need to be investigated. There is a lack of systematisation and standardisation of clinical information due to under-reporting of adverse events, hindering the interpretation of results. Long-term adverse reactions to COVID-19 vaccines should be investigated. Personalised recommendations using patient-specific genetic signatures. Assess immune pathway changes and ‘spike effect’ | Self-reported data may not estimate well the correlation between adverse events and BNT162b2 vaccine, the incidence of adverse events should be obtained from similar studies with a longer follow-up period. The age of this population may not reflect the general population, the tolerability profile in older subjects and the IgG response may differ significantly. Representation of signs and symptoms may be less reliable and representative. The authors note that they do not have the medical history of the subjects enrolled and also cannot rule out the presence of ongoing immunosuppressants or coexisting comorbidities in the determination of adverse events or the serum response to vaccination |
| Ahmed et al.41 | 2022 | Human model | 4 participants | ≥5h–6days | The association between vaccines and other vascular dysregulation should be investigated, they may disrupt laminar blood flow and cause pulsatile tinnitus. The possibility that one or more vaccine components exert toxic effects cannot be discounted and requires attention. Assess long-term adverse reactions to COVID-19 vaccines, not just the transient nature of these reactions | Limited vaccine genomics studies, antibody responses with COVID-19 vaccines should be closely monitored at individual/population level Previous studies have demonstrated the relevance of a systems biology approach to correlate positive regulation of genes associated with innate immunity, cytokine production and responses to virus infection, particularly IFN-inducible genes, with adverse events observed in human trials. This will facilitate a better understanding of the underlying mechanisms of side effects |
| Shafiq et al.204 | 2022 | Human model | 61 participants | 3–14days | Systematic characterisation of adverse events is recommended following vaccination. It will facilitate early identification and treatment, as well as recording of evidence on the incidence of neurological adverse events. The putative plausibility of the COVID-19 vaccine in further studies and the spike effect. Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Investigate long-term adverse reactions to COVID-19 vaccines. Clinicians and surveillance organisations must be alert to this adverse event and its atypical variants | Under-recording of adverse effects after administration of other vaccines. The incidence may not be real. Comparison of age distribution is limited by case ascertainment and is not adjusted for confounding factors, e.g. administration of different vaccines at various ages |
| Son et al.42 | 2022 | Human model | 256994 participants | ≥5h–15days | It is recommended to collect information on adverse effects of various SARS-CoV-2 vaccines. Detect unusual, rare, or new adverse events; monitor and track reporting trends (duration, remission vs. cure and worsening); identify potential risk factors for particular adverse events; detect administration errors and vaccine safety issues (limited published knowledge on the safety of COVID-19 vaccines). Assess long-term adverse reactions to COVID-19 vaccines, not just the transient nature of these reactions | Under-recording of adverse events after administration of other vaccines. The incidence may not be real. Representation of signs and symptoms may be less representative. Comparison of age distribution is limited by case ascertainment and is not adjusted for confounding factors, e.g., administration of different vaccines at various ages, but does not include persons under 20years of age |
| Kim et al.43 | 2022 | Human model | 497 participants | 0–117days | Research and evaluation of the effectiveness vs. reactogenicity of COVID-19 vaccines is needed to develop future vaccination policies. Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Investigate long-term adverse reactions to COVID-19 vaccines Clinicians and surveillance organisations must be alert to this adverse event and its atypical variants | There is no strict control of the period since vaccination. Therefore, there is no concordance between assays. They could not measure the precise amount of antibodies because of the parametric limits of the laboratory. They did not assess the long-term reactogenicity of the vaccines (they assess up to 4months post vaccination) |
| Finsterer et al.44 | 2021 | Human model | 19 participants | ≥3h–39days | These cases were collected by passive surveillance and were not revised cases. According to the WHO Global Advisory Committee on Vaccine Safety (GACVS) subcommittee on reports of Guillain-Barre syndrome following adenovirus vector COVID-19 vaccines, only rare cases of SCoVaG have been reported. The committee indicates that more rigorous studies using alternative data sources and robust study designs and comparison of vaccinated and unvaccinated populations are warranted. Information gathered from vaccine surveillance reports can help healthcare systems compare different platforms and determine tolerability in different populations. This can also guide policy makers to make the right decisions and take the right actions | Studies should also focus on the different characteristics of various vaccine platforms. It has been shown that adverse events are more likely to occur after certain vaccine platforms Guillain-Barre syndrome was not diagnosed according to the validated Brighton criteria, without diagnosis by cerebrospinal fluid (CSF) cell counts and without using nerve conduction studies (NCS) in all patients. It should be remembered that the absence of organ damage can also occur in these inflammatory disorders resulting in impaired neurological function without accompanying structural disease |
| Umezawa et al.45 | 2023 | Human model | 1 participant | 1–17days | No local inflammation reactions occurred in her left arm (vaccinated arm), although this feature may be only a coincidence, it needs to be discussed in terms of pathophysiology. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone | Further research is called for into the pathophysiology of neuromyelitis optica spectrum disorder following vaccination with Pfizer's BNT162b2. It was not possible to predict the exact timing of the duration, follow-up, total/partial remission, and/or worsening of adverse effects |
| Hetland et al.46 | 2022 | Human model | 50 participants | 0–10days | Possible emerging side effects should be monitored, NETs analysis could also be used after vaccination with the COVID-19 Janssen vaccine, which also is an adenoviral vector vaccine and has been reported to trigger VITT. A strength to be considered for future studies is the comparison of results after ChAdOx1 vaccination with non-vaccinated healthy controls from a similar population, who were from the pre-COVID-19 era (2015), and thus not sensitised to the SARS-CoV-2 virus. They reiterate examining relevant assays for inflammation, as demonstrated by the high degree of correlation between their results and degree of side effect severity after the vaccinationLarger observational studies are also needed | There are few reports on these inflammatory biomarkers following COVID-19 vaccination with ChAdOx1 in relation to clinical outcome, research in this context contributes to the understanding of the serious adverse effects of vaccine-induced immune thrombotic thrombocytopaenia (VITT). Statistical power is affected, limited number of study participants, subject to variability of adverse events |
| Mingot-Castellano et al.47 | 2022 | Human model | – | – | Rapid and accurate diagnosis is essential to initiate treatment and to continue surveillance and publication of cases, the real incidence of these adverse events needs to be known. Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Long-term adverse reactions of COVID-19 vaccines need to be investigatedClinicians and surveillance organisations must be alert to this adverse event and its atypical variants | It was not possible to predict the exact timing of the duration, follow-up, remission, and/or worsening of adverse effects. Many variables may affect host-dependent reactogenicity and immunogenicity of COVID-19 vaccines. Limited vaccine genomics studies, more research needed to understand immunogenicity. Pharmacovigilance should consider future studies to understand the possible long-term inflammatory mechanisms of these new vaccines |
| Watanabe et al.48 | 2022 | Human model | 2 participants | 2days | They recommend further investigation of plasmacytoid dendritic cells (p-DC) in drug eruption or viral exanthems to determine whether rashes are cased by vaccination. Further cases are needed to establish the clinical significance of p-DC biomarkers. Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Further research is needed to offer treatment | Long-term adverse reactions of COVID-19 vaccines need to be investigated. Many variables that may affect the reactogenicity and immunogenicity of COVID-19 vaccines are host-dependent |
| Abbasi et al.49 | 2022 | Human model | 1 participant | 15days | Rapid and accurate diagnosis is essential to initiate treatment and to continue surveillance and publication of cases, the real incidence of these adverse effects needs to be known Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Investigate long-term adverse reactions to COVID-19 vaccinesClinicians and surveillance agencies should be on the lookout for these adverse events and their symptoms (headache, abdominal pain, and neurological signs) in the first 5–24days post-first dose vaccination. Diagnosis and treatment are vital to prevent morbidity and mortality | It was not possible to predict the exact timing of cure and/or worsening of adverse events. Many variables that may affect the reactogenicity and immunogenicity of COVID-19 vaccines, host-dependent. Limited vaccine genomics studies, more research needed to understand immunogenicity. Pharmacovigilance should consider future studies to understand the possible long-term inflammatory mechanisms of these new vaccines. Study of suspected cases includes coagulation profile, D-dimer, fibrinogen, and anti-PF4 levels |
| Chow et al.50 | 2022 | Human model | 1 participant | 7days | Systematic characterisation is necessary of adverse events following vaccination. This will facilitate early identification and treatment, and the recording o evidence on the incidence of adverse events. The putative plausibility of the mRNA-1273 vaccine must be assessed in further studies and the spike effect. Improve the next generations of COVID-19 vaccines, they must offer better tolerability and safety to ensure clinical success. Investigate long-term adverse reactions to mRNA COVID-19 vaccines. Clinicians and surveillance organisations must be alert to this adverse event and its atypical variants | Many variables that may affect the reactogenicity and immunogenicity of COVID-19 vaccines, host-dependent Different gene and protein expression, activated in various tissues in both cell proliferation and terminally differentiated cell processes. Adverse effects of vaccination should be compared with RNA sequencing analyses can identify live, metabolically active cells, reporting on the transcriptional activity of the population |
| Zlotnik et al.51 | 2022 | Human model | 1 participant | 14–21days | Improve the next generations of COVID-19 vaccines, they must offer improved tolerability, safety, and efficacy to ensure clinical success. Further research is needed to understand immunogenicity. Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone | Pharmacovigilance should consider future studies to understand the possible long-term inflammatory mechanisms of these new vaccines |
| Ameratunga et al.52 | 2022 | Human model | 1 participant | 1 day | Future studies should determine the real risk of these adverse events following COVID-19 vaccination. Assess changes in immune pathways and the condition of spike intoxication, the so-called ‘spike effect’. Further studies are needed to understand the autoimmunity caused by modified mRNA vaccines. Adequate clinical trials are needed to design new COVID-19 vaccines without risk of adverse events, in different population groups (with autoimmune diseases - allergies, different ages, different ethnicities). Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alone. The distinctive profile of immunity and inflammation observed after natural infection has also been observed after administration of COVID-19 vaccine | The mechanisms that may affect vaccination are complex and include factors related to COVID-19 vaccines (nature of the antigen, administration system, adjuvants – immunomodulators, and the host immune system). They have demonstrated this ‘endogenous adjuvant potential’ in antibody responses was significantly impaired with these vaccines. Single-cell RNA profiling will facilitate cell identification, but was not possible in this case due to RNA degradation post-mortem. Many variables that may affect the reactogenicity and immunogenicity of COVID-19 vaccines are host-dependent. Pharmacovigilance should consider future studies to understand the possible long-term inflammatory mechanisms of these new vaccines |
| Duijster et al.53 | 2023 | Human model | 16929 participants | 30–≥180days | Improve the next generations of COVID-19 vaccines, they must offer better tolerability and safety to ensure clinical success Adverse effects of vaccination on the nervous system should be compared with post-COVID-19 adverse outcomes rather than considered alonePersonalised recommendations using patient-specific genetic signatures. Assess the condition of spike toxicity, the so-called ‘spike effect’ | Long-term adverse reactions of COVID-19 vaccines need to be investigated. Many variables may affect reactogenicity and immunogenicity of COVID-19 vaccines, host dependent. Limited published studies on adverse effects that modify the hypothalamus-pituitary-ovary axis and, immune responses in the female reproductive tract and their heterogeneity within the organ and between compartments due to COVID-19 vaccines, there needs to be further research |
Variables identified from the reports included in the systematic review (design, immunogenicity/reactogenicity and clinical characteristics related to COVID-19 vaccines).
| Report | Design and study objectives | Clinical–epidemiological situation |
|---|---|---|
| Verbeke et al. 20223 | Narrative review. The mechanisms of innate immune sensing of mRNA vaccines at the cellular and intracellular levels are discussed and the contribution of mRNA and LNP components to their immunogenicity and reactogenicity are identified. Detailed mechanistic understanding of how innate immune pathways modulate adaptive immunity in mRNA vaccine responses | Limited. Recent data gained from descriptive and hypothesis-driven studies on the dynamics and activation of innate immune cells. Experimental studies that evaluate protein production in vitro or in vivo in animal and human models. Systemic distribution |
| Aldén et al. 202259 | Experimental study, in the human liver cell line Huh7 in vitro. The uptake of BNT162b2 in the human liver cell line Huh7, changes in LINE-1 expression and distribution are investigated. Evidence is presented that BNT162b2 mRNA is reverse transcribed intracellularly into DNA after BNT162b2 exposure in ≤6h. The intracellular accumulation of BNT162b2 antigen in human liver cell line Huh 7 cells is investigated | The mRNA vaccine can distribute non-specifically to several organs such as the liver, spleen, heart, kidney, lung, brain, bone marrow, basal layers of epithelia, ovaries, and testicles. The concentration in the liver is 100 times lower than that of the intra-muscular injection site. May potentially mediate genotoxicity and carcinogenicity |
| Szebeni et al. 202215 | Narrative review. The objective was to evaluate the mechanisms of HSR in mRNA SARS-CoV-2 vaccines. Demonstrates that the same components of LNP-mRNA vaccines have immunostimulatory effects of effector and target cells, and biochemical pathways (complement and coagulation system) essential for clinical success | Limited. Recent data from descriptive and experimental studies on innate immune cell dynamics and activation related to HSR and other types of IMAE due to the wide inter-individual variability of notified adverse events (cytokine levels, presence of anaphylatoxins, complement split products, tryptase, histamine, heparin, PAF, induced and/or pre-existing antibodies, and spectrum of inflammatory mediators) in immune responses. Potential mediator in genotoxicity and immunodeficiency. Systemic distribution |
| Yoshida et al. 2023115 | Descriptive cohort study (duration of 3months) using a paper-based questionnaire. The type and frequency of adverse reactions in healthy and allergic disease individuals aged 5–11years over 7days following the first and second BNT162b2 vaccination is studied. | Ishikawa district (4 municipalities of 1536 people aged from 5 to 11years. Of the 806 children recruited, 421 (52.2%) agreed to participate in the study. The mean age was 8.8 ± 1.9years, 216 (51.3%) were male, 216 (51.3%) had allergic disease and 190 (45.1%) experienced systemic adverse reactions. Allergic disease profile of the patients: 162 had hay fever, 71 allergic rhinitis, 54 atopic dermatitis, 46 asthma, 16had food allergies. Of the 216 participants with allergic disease, 45 (20.8%) experienced worsening of their chronic diseases after the first BNT162b2 dose, 41 (19%) after the second BNT162b2 dose, and 54 (12.8%) reported worsening of their chronic diseases after the first and/or second BNT162b2 vaccination. The frequency of worsening of chronic diseases (p<.001), fatigue (p=.002), and nausea (p=.038) after the second BNT162b2 vaccination was significantly higher among individuals with allergic diseases |
| Granados Villalpando et al. 2022116 | Descriptive cross-sectional study (duration of 3months). They associated the adverse effects between COVID-19 vaccines and allergic reactions between categories: sex, age and absence or presence of comorbidities. Assessing their risk factors | Epidemiological typing and classification, including sex, age, vaccines administered, post-vaccination COVID-19 infection, comorbidities (especially allergies and asthma), number of doses (1272), adverse effects, and allergic reactions. BNT162b2 vaccines 50.62% (644 doses) and ChAdOX1 41.9% (533 doses) are strongly associated with the occurrence of allergic reactions, with ORs of 1.6 (95% CI, 1.18–2.3) and 1.87 (95% CI, 1.35–2.6), respectively. Higher odds of developing adverse effects and/or allergic reactions associated with COVID-19 vaccines in females 68.2% (p<.001, OR 3.1), older age, and prevalence of comorbidities (p=.06, OR 7.63), in the context of the need for medical care after vaccination (95% CI, 0.63–92.15). Mean age of all participants: 25.93years (SD±11.29years) |
| Talotta 2022107 | Narrative review. Assessing impaired VEGF-A-mediated neurovascular cross-talk induced by SARS-CoV-2 spike protein (by antagonising the docking of VEGF-A to NRP-1). Causal association of the side effects of COVID-19 and the pathogen | Neurological and cardiovascular side effects mimicking long COVID-19 have been reported in recipients of COVID-19 vaccines. The S protein has a pathogenic role, it can bind to neuropilin (NRP-1). It also disrupts the physiological pathway involved in angiogenesis and nociperception. It is involved in embryogenesis and carcinogenesis (lymphoproliferative disorders), inflammatory disorders and/or neuromyelopathies, altering the blood–brain barrier. It is suggested that VEGF could be a potential biomarker to detect the damage caused by COVID-19 vaccines |
| Chen et al. 2022132 | Systematic review. They discuss the impact and involvement of the nervous system after vaccination against SARS-CoV-2 and related adverse effects | Neuroinvasion and neuroimmune cross-talk are indicated as inducing the neurological symptoms caused by vaccination against SARS-CoV-2. They posit 2 mechanisms: on the one hand, the shared SARS-CoV-2 pathogen may infect the brain directly, through haematogenous propagation and retrograde axonal transport, except for the olfactory neuron pathway. On the other hand, it can induce indirect neurotoxicity through immune-mediated pathogenesis and gastrointestinal infection |
| Garrido Suárez et al. 2022133 | Narrative review. The objective was to assess the orexinergic system linked to inflammatory signalling that promotes sleepiness after peripheral activation of the innate immune system, caused by COVID-19 vaccines | They verify the mechanistic link between reactogenic inflammatory parameters and hypothalamic circuits involved in the sleep–wake cycle. They propose that pro-inflammatory cytokines especially INF-γ, TNF-α, IL-1β, may propagate a peripheral inflammatory response after vaccination. A subset of perifornical LHA Nts-expressing GABAergic neurons are activated as well as inhibitory neurons from sleep-promoting areas providing an inhibitory input on wake-promoting Ox neurons. The adenosinergic modulation of sleep–wake signals could also be implicated. They propose identifying biomarkers linked to the reactogenicity of the COVID-19 vaccines |
| Ng et al. 2022147 | Descriptive, cohort study (duration from 3 to 10weeks after the 2 doses) They describe the composition of the gut microbiota through metagenomic sequencing in stool samples of 138 vaccinees (37 with CoronaVac and 101 with BNT162b2) in relation to immune responses and adverse effects in adults who received CoronaVac inactivated virus vaccine and Pfizer-BioNtech mRNA BNT162b2 Comirnaty | They identify specific gut microbiota markers in association with immune response and adverse effects following administration of COVID-19 vaccines. They propose gut microbiota-based interventions to mediate in the immunogenicity produced by its inflammatory role. They highlight the loss of the intestinal mucosa and the alteration of this immunomodulatory microbiota, with consequences in the intestinal tract, causing inflammatory processes continuously over time, and situations of intestinal permeability (intestinal dysbiosis) may occur |
| Hajjo et al. 2022156 | Experimental study. Analysing the systems biology effects of COVID-19 mRNA vaccines to assess their safety and putative side effects | They apply a systems biology workflow, based on transcriptomics data for BNT162b2 for Pfizer-BioNTech in vaccinated subjects. Their results verify that BNT162b2 induces a strong immune response 21–22days after receiving the first dose and subsequent booster dose. The triggered immune and inflammatory pathways are associated with IFN-gamma, interleukin, and protein C signalling. They suggest 76 DEGs involved in interferon signalling, cytokine signalling, interferon alpha/beta signalling, antiviral mechanisms by interferon-stimulated genes, and interferon gamma signalling as potential biomarkers to detect damage caused by COVID-19 vaccines |
| Murata et al. 2022102 | Case report. RNA sequencing of each case of death to establish cause of death due to vaccination in the context of time of death. Evaluating SIRS after vaccination | Four cases who died at home after receiving the mRNA COVID-19 vaccine. Three cases were vaccinated with 2 doses of Moderna mRNA, and one case received 2 doses of Pfizer-BioNTech mRNA vaccine. Time from receipt of the second dose to death was 1–10days. Three hundred and ninety genes were found to be upregulated, genes involved in neutrophil degranulation and cytokine signalling, and 115 genes were downregulated in post-vaccination cases compared to controls. They suggest time-dependent cause of death by immune dysregulation following vaccination, systemic immune response syndrome (SIRS) |
| Ittiwut et al. 2022171) | Case series. Studying the genetic basis of SUD after COVID-19 vaccination in Thailand | Thirteen cases of sudden unexplained death (SUD) following COVID-19 vaccination in Thailand, aged 23–72years; 10 (77%) were male and 3 (23%) female, 12 of Thai origin, and one Australian. They reported underlying diseases in 5 patients, without arrhythmias. No case had a history of ultrasensitive troponin T (UST) in relatives. Eight (61%), 4 (31%), and 1 (8%) died after the first, second, and third vaccine doses, respectively. SUD was triggered after all types of COVID-19 vaccines administered in Thailand, 7 (54%), 2 (15%), 2 (15%), 2 (15%), 1 (8%), and 1 (8%) deaths with ChAdOx1 nCoV-19 (AstraZeneca), BBIBP-CorV (Vero Cells) from Sinopharm (Beijing), CoronaVac (Sinovac), BNT162b2 (Pfizer/BioNTech), and mRNA-1273 (Moderna), respectively. Association with SUD within 7days after COVID-19 vaccination with SCN5A variants, 13 variants in 7 genes in 11 of 13 cases (85%) |
| Çinar et al. 2022172 | Case series. Relating the emergence of myeloid neoplasms following BNT162b2 mRNA-based COVID-19 vaccination | The authors highlight the risk/benefit ratio of SARS-CoV-2 spike protein mRNA-based vaccines, and indicate potential malignant neoplastic adverse events. Undertaking extended immunophenotyping studies is proposed to detect B-and T-cell dysfunction in the serological response to COVID-19 vaccination |
| Nazir et al. 2022173 | Systematic review. Comprehensive interpretation of menstrual cycle changes after COVID-19 vaccination to assess possible safety issues with the vaccine | Menstrual cycle abnormalities after vaccination are assessed. Patient-level study characteristics, type of study, sample size, administered vaccines and menstrual abnormalities were summarised. A total of 78138 vaccinated women were included from 14 studies. Of these, 39759 (52.05%), aged between 18 and 30years, had menstrual problems after vaccinationThe overall rate of menstrual abnormality ranged from 0.83% to 90.9%. Menorrhagia, oligomenorrhoea, dysmenorrhoea, and metrorrhagia were the most commonly observed problems, in addition to gynaecological disorders such as endometriosis, polycystic ovary syndrome, fibroids, and adenomyosisThe authors warn of a potentially harmful effect on fertility and long-term health for women. They stress the need for population-based studies, recording: epigenetic variation due to genetic polymorphisms in ethnic communities, at-risk populations, following more cycles over longer periods to study long-term consequences (fertility-embryogenesis) |
| Moro et al. 2022182 | Systematic review. To evaluate and summarise reports to the Vaccine Adverse Event Reporting System (VAERS), in pregnant persons who received a COVID-19 vaccine to assess for potential vaccine safety problems | Of the 3462 reports of adverse events in pregnant persons who received one dose of the COVID-19 vaccine: 1831 (52,9%) after BNT162b2, 1350 (39.0%) after RNA-1273, and 275 (7.9%) after Ad26.COV2.S, and 6had unknown manufacturer. Reports indicating that COVID-19 vaccination was administered prior to the last menstrual period or during the postpartum period were excluded. Eight maternal deaths and 12 neonatal deaths were recorded. Six-hundred and twenty-one (17,9%) reports were coded as serious. Outcomes included: 878 (25.4%) spontaneous abortions (< 20weeks) (SAB), 101 (2.9%) episodes of vaginal bleeding, 76 (2.2%) preterm deliveries (< 37weeks), 62 (1.8%) stillbirths (≥ 20weeks) and 33 (no rate calculated) infants with major birth defects or chromosomal abnormalities: 18 of 33 cases after the BNT162b2 vaccine and 15 of 33 cases after the mRNA-1273 vaccine. One hundred and seven infant conditions were reported, which included the 12 neonatal deaths, prematurity was noted as cause of death |
| Rahimi Mansour et al. 2023185 | Narrative review. To analyse the endocrine and immune changes following COVID-19 vaccination and possible mechanisms of vaccine-related menstrual disturbances | Detrimental effects of the shared S protein in COVID-19 vaccines include SARS-CoV-2 entry into target cells, endothelial damage, pro-inflammatory cytokine release, Toll-like receptor (TLR) activation, microglia stimulation, and molecular mimicry with chaperon and heat shock proteins (HSP) and activation of the SARS-CoV-2S protein. Hyper-stimulation of the immune system and synthesis of multiple autoantibodies can also be induced |
| Green et al. 2022187 | Descriptive, cross-sectional study. Duration: December 2019 to June 2021. To observe gender differences in adverse effects following Pfizer-BioNTech COVID-19 vaccine administration | Almost all adverse events classified as moderate or severe (systemic) were more frequent among females (F:M RR 3.43), following both the first dose and the second. For example, for pain and sensitivity over the injected hand –F:M RRs were 7.03 and 4.13 after the first and second doses, respectively. For shivering – F:M RRs were 8.77 and 3.87 after the first and second dose, headache - F:M RRs were 9.15 and 3.28 for the first and second doses, fatigue – F:M RRs were 3.32 and 2.27 and difficulty in standing, which was reported almost exclusively in femalesA possible mechanism has been proposed for the increased reactogenicity of vaccines in females, due to a stronger immediate response to the antigen, modulated through the innate immune system. They include epigenetic mechanisms that regulate X chromosome-linked genes and ChrY gene polymorphisms (gender-related differences in immunity) |
| Mouliou et al. 20221 | Narrative review. To illustrate the nature of all current post-vaccination adverse events' reports and related existent hypotheses. To discuss the innate immune mechanisms triggered by mRNA COVID-19 vaccines, especially Pfizer's BNT162b2 | They recognise vaccine antigens as potential pathogens causing autoimmune reactions by the mechanisms of molecular mimicry and T-cell activation. They verify the hypotheses on molecular mimicry, reverse transcription in the human liver cell line Huh7 (causing alterations in LINE-1 expression and distribution), inflammatory signals attributed to post-vaccination reactogenicity, their systemic dissemination, endothelial damage, and the ‘cumulative effect’, i.e., the relationship between elevated antibody levels (anti-S IgG, anti-RBD IgG) post vaccination and their temporal persistence, suggesting post-COVID-19 mimicry. They indicate the extended duration of biopersistence of the adjuvants discussed above and their ability to move and slowly accumulate in the lymphoid organs or other tissues.The add genetic predisposition in susceptible individuals, including: HSR, history of previous allergy, and overexpression of X-linked genes (sex-differentiated immunity).They propose the hypothesis of mRNA overtranslation and consequent spike protein overproduction resulting in immune dysregulation and potential toxicity (genotoxicity, hepatotoxicity, neurotoxicity). The pathogenesis of these diagnoses is mainly vascular, neurological, digestive, respiratory, cardiac, HSR/spontaneous allergic reactions, skin lesions, endocrine, renal, auditory, and ocular events, paraneoplastic, and with effects on fertility/embryogenesis and menstrual disorders |
| Castaldo et al. 2022201 | Systematic review and meta-analysis. To describe the mechanism of post-vaccination headache, and discuss the mechanisms of spread and impact of SARS-CoV-2 vaccines on the human body | Headache was the third most common adverse effect. Headache was detected in 22% (95% CI, 18%–27%) of cases after the first dose and in 29% (95% CI, 23%–35%) after the second dose, with extreme heterogeneity. They observed no differences between different vaccines or mRNA-based vaccines against COVID-19 versus traditional vaccines. The clinical picture is characterised by a migraine with pulsating quality, phono, and photophobia, in 40%–60% of cases aggravated by activity. The authors propose the hypothesis of headache secondary to a systemic immunological reaction (SIR). They postulate several routes for spread in the human body: 1) use of the bloodstream with subsequent neuronal dissemination, infection of endothelial cells within the blood–brain barrier or blood-cerebrospinal fluid barrier, 2) use of transsynaptic pathways after infection of nerve endings (forward or retrograde transport), crossing the blood–brain barrier as a result of leukocyte infection (Trojan horse mechanism), or 3) through the lymphatic system.They confirm the ACE2 receptor mechanism, including ACE2 expression outside lung tissue, was confirmed in neurons, astrocytes, oligodendrocytes, olfactory bulbs, substantia nigra, brainstem, posterior cingulate cortex, striatum, and hypothalamus.They demonstrate SARS-CoV-2 as a neurotropic virus (ability to infect and replicate in cultures of neuronal cells and brains) and its potential as an endogenous adjuvant in vaccination. |
| Verbeke et al. 2021202 | Narrative review. To compare design-activity differences between 3 mRNA vaccines (BNT162b2, mRNA-1273, and CureVac). Detailed overview of the composition and (pre) clinical performance of mRNA vaccines, of their structural design and how innate immune pathways modulate adaptive immune responses to mRNA vaccinesThe mechanisms of innate immune sensing of mRNA vaccines at the cellular and intracellular levels are discussed and the contribution of mRNA and LNP components to their immunogenicity and reactogenicity are identified | In addition to the insights into the mode of action of mRNA vaccines that they highlight, they also point out likely hypotheses and unknowns that require further investigation and optimisation in future mRNA vaccine developmentExperimental studies assessing protein production in vitro or in vivo in animal models and humansSystemic distribution. Potential endogenous adjuvant in vaccination, associated with apoptotic cells. They verify the high similarity to SARS-CoV-1S protein, making it the main factor to investigate in the development of mRNA vaccines. They also mention T-cell and antibody responses correlating to milder disease, whereas an uncoordinated response frequently failed to control the disease. In addition, previous experience with the closely related SARS-CoV-1 showed that CD8+ and CD4+ memory T cells were detectable up to 17years after infection, while neutralising antibody titres had waned substantially by one year after infection |
| Saluja et al. 2022203 | Case report. They describe the clinical features and treatment outcomes of bilateral optic neuritis developing after administration of the first dose ChAdOx1_nCoV-19 (Covishield) vaccine in a previously healthy young immunocompetent male | A healthy 35-year-old male, with sudden decrease in vision progressive in nature. Symptoms developed two days following the first dose of the ChAdOx1_nCoV-19 vaccineDiagnosis of vaccine-associated optic neuritis was based on the temporal association between the administration of vaccine and the development of ocular symptoms and ruling out other immune or infectious aetiologiesGood response to oral systemic steroid pulse therapy |
| Chen et al. 2022120 | Case report. They describe a case of concurrent vasculitis in spine and acute partial transverse myelitis (APTM) after the second dose of inactivated COVID-19 vaccine CoronaVac (Sinovac) | Case of concurrent vasculitis in vertebral bodies and acute transverse myelitis (ATM) following COVID-19 vaccinationA 33-year-old man presented with weakness of left lower limb and aberrant sensation of right (left) lower trunk and limb (from level T9 to toes) for 2days following vaccination, no inflammatory change on cranial MRI scan. On the fifth day a demyelinating lesion was shown at T7 spinal cord on 3.0T MRI. Vertebral bodies of T3-T7 presented a high signal in T-2 (T2WI) accompanied by multiple sites of flowing void effect, indicating vasculitis. Oligoclonal band was positive in cerebrospinal fluid (CSF), but negative in sera. Conventional laboratory tests including blood cell counts, coagulation, liver function, kidney function, electrolytes, plasma glucose, lipids, C-reactive protein, and procalcitonin were normal.His limb weakness and aberrant sensation improved and he as able to walk unaided after treatment: intravenous methylprednisolone (1g/24h) for 5days, with subsequent dose tapering |
| Morikawa et al. 202222 | Case report. To report the eosinophilic and rheumatoid reactions as an adverse event after BNT162b2 COVID-19 vaccination | Eosinophilic inflammation is reflected in an anti-inflammatory reaction in the initial phase of rheumatoid arthritis (RA)An 88-year-old woman diagnosed with RA and chronic eosinophilic pneumonia (CEP) after vaccination. Respiratory and joint symptoms gradually worsened. Laboratory examinations showed increased rheumatoid factor, anti-cyclin citrullinated peptide antibody (CEP), C-reactive protein, serum IL-6, and absolute eosinophil count and eosinophil tissue infiltration. Ultrasonography showed synovitis. Respiratory and joint symptoms improved after oral methylprednisolone pulse therapy (40mg/24h), RA and CEP stabilised.They confirm that cytokine storm is the main cause of lung injury and several immune factors, such as IL-6, JAK1/2, and GM-CSF, were common signalling cascades, like those in R. In this case, the authors report that the immune response was caused by SARS-CoV-2 vaccination rather than infection. They also report on cytokine release syndrome(CRS). They conclude that cytokine reactions against SARS-CoV-2 spike proteins were similar to those against COVID-19 infection and its endogenous adjuvant potential in vaccination. Both innate immune and Th cell adaptive reactions to COVID-19 might be a trigger of RA, subsequently inducing activation of disease activity of CEP. This might induce the M2-phenotype reaction |
| Chen et al. 2022104 | Descriptive, cross-sectional study. Duration: January 2020 to November 2021. To link hearing impairment to COVID-19 vaccines | They identified increased risk for hearing disorder following administration of mRNA and virus vector COVID-19 vaccines compared to influenza vaccination. They consulted the Vaccine Adverse Event Reporting System (VAERS), the disproportionality pattern for hearing impairment of COVID-19 vaccine by calculating the reporting odds ratio (ROR) and proportional reporting ratio (PRR). A further subgroup analysis based on the type of COVID-19 vaccine and the doses administered was performed. The disproportionalities for hearing dysfunction between influenza and COVID-19 vaccines were compared. A total of 14956 adverse event reports linking hearing dysfunction to COVID-19 vaccination and 151 to influenza vaccination during the VAERS analysis period were identifiedThe incidence of hearing disorder following COVID-19 vaccination was 6.66 per 100000.The results of the disproportionality analysis show that side effects of hearing impairment following administration of COVID-19 vaccines were significantly more reported: (ROR 2.38, 95% CI 2.20–2.56; PRR: 2.35, X2. 537.58) for both mRNA (ROR 2.37, 95% CI 2.20–2.55; PRR: 2.34, X2529.75) and virus vector vaccines (ROR 2.50, 95% CI 2.28–2.73; PRR: 2.56, X2 418.57). In comparison, the disproportionate level of hearing dysfunction in influenza vaccine was lower (ROR 0.36, 95% CI 0.30–0.42; PRR: 0.36, X2172.24) |
| Namiki et al. 202223 | Descriptive, cohort study. Investigating gynaecological adverse events in a Japanese female population after BNT162b2 mRNA COVID-19 vaccination | A total of 424 women were surveyed, 309 surveys were deemed appropriate for analysis. The frequencies of abnormal bleeding were 0.6%, 1.0% and 3.0% for the first, second and third doses with Pfizer's BNT162b2, respectively. An irregular menstrual cycle was more common than abnormal bleeding: 1.9%, 4.9%, and 6.6% for the first, second, and third doses. Both abnormal bleeding and irregular menstrual cycle were associated with the spike effect, suggesting the cumulative immune effect due to repeated antigen exposure with vaccine booster. Reinforce the hypothesis of ‘endogenous adjuvant potential’, although they do not report antibody responses in this vaccine |
| Lagousi et al. 2022105 | Narrative review. Reviewing the epidemiological data on myocarditis following mRNA COVID-19 vaccination (Pfizer's BNT162b2) and describing the mechanisms involved that explain sex-related differences and mRNA immune reactivity | They confirm the already proposed ‘mRNA immune reactivity’ as a mechanism for systemic adverse reactions and myocarditis post-immunisation with SARS-CoV-2 mRNA vaccines. They point out that exogenous mRNA is intrinsically immunostimulatory and is recognised by a variety of cytosolic, endosomal, and cell surface innate immune receptors and can elicit an exacerbated immune response. It triggers a cascade of hyperinflammation causing unfavourable systemic reactions with detrimental effects on different organs, including the myocardium. They add the hypotheses that in susceptible individuals the immune response to mRNA may not be turned down; younger age was associated with greater changes in monocyte, inflammatory response, and platelet-related gene expression shortly after the second dose of BNT162b2 vaccine; the quantity of mRNA antigen contained in each vaccine formulation may also be a factor in mRNA reactivity, as most reported cases of myocarditis have been associated with formulations containing the highest mRNA loads per dose; and molecular mimicry between the SARS-CoV-2 spike protein and cardiac self-antigens resulting in cross-reacting auto-antibodies targeting the myocardium (generalisable to systemic distribution) following immunisation. Also, the autoantibodies found in the peripheral blood of patients with symptomatic myocarditis are the result of myocardial inflammation and injury, and are the product and not the cause of myocarditis.In this study it is apparent that younger age (≤18–39years) and male sex contribute as independent factors to susceptibility to mRNA immune hyperactivity |
| Pirani et al. 2022106 | Case series. The aim of the study was to report two cases of optic neuritis following Pfizer-BioNTech COVID-19 vaccination in patients with autoimmune diseases (Hashimoto thyroiditis and ankylosing spondylitis) | They confirm the causal link between vaccine-induced immunisation and autoimmune reaction at the level of the optic nerve, it is not only temporal but also causal, especially in genetically susceptible individuals. They confirm that the reactogenicity of COVID-19 mRNA vaccine in individuals suffering from immune-mediated diseases have a pre-existent dysregulation of the immune response. They support the hypothesis that patients who are already affected or predisposed to autoimmune or autoinflammatory disorders should be evaluated for the benefits and risks of vaccinationThey associate molecular mimicry, the production of particular autoantibodies, and the role of certain vaccine adjuvants with the manifestation of these autoimmune phenomena. In both cases no pathological findings were found after neurological examination and in immunological and serological tests, including antibodies against aquaporin-4 and myelin oligodendrocyte glycoprotein. MRI of the brain, orbits, and spinal cord did not reveal lesions of demyelination within the brain or spinal cord while T1-weighted MRI of brain and orbits and post gadolinium contrast did. Both women, aged 31 and 46years, were treated with intravenous methylprednisolone 1000mg/24h for 5days, followed by a tapering dose of oral prednisone over the next 10days. There was improvement of retinal sensitivity after visual field testing |
| Zagorec et al. 202224 | Case series. The aim of the study was to report 2 cases of immune-mediated disease linked to the Pfizer-BioNTech COVID-19 vaccine | They attribute immune-mediated disease following SARS-CoV-2 vaccination to the mechanisms of molecular mimicry and cross-reactivity between the viral spike protein and viral and host endothelial antigens. They report a case of de novo immunoglobulin A nephropathy (IgAN) in a 26-year-old man and a case of cutaneous vasculitis in a 68-year-old woman, both after the second dose of BNT162b2. In both diseases, deposition of immune complexes activates the inflammatory response with end-organ damage and deposition in vessel walls, causing vasculitis. None of the patients had a history of adverse reactions to vaccines or autoimmunity. In the second case, the involvement of medium-sized arteries warned of systemic involvement due to vasculitis, but in extensive diagnostic work-up, including kidney biopsy, no systemic distribution was observed. They suggest a causal mechanism by temporal association between vaccination and disease development in the absence of other possible intercurrent inciting events.In both cases, immunosuppressive treatment (ramipril and methylprednisolone pulses) for 2months was warranted to prevent disease progression and partially or completely resolve immune-mediated disease after vaccination with BNT162b2 |
| Levy et al. 202225 | Descriptive, cohort study. Duration: 4months. To determine the correlation between immunogenicity and reactogenicity of the BNT162b2 vaccine, to evaluate the ADRs and antibody titres after each dose of the vaccine | They identified BNT162b2 mRNA COVID-19 vaccine reactogenicity, adverse effects correlate with higher post-vaccination antibody levels (anti-S IgG, anti-RBD IgG) and were independently associated with younger age, female sex, and antibody levels.They included subjects without prior SARS-CoV-2 infection who participated in active surveillance after receiving the BNT162b2 vaccine. They excluded immunocompromised healthcare workers and those with autoimmune diseases. Participants reported adverse drug reactions (ADRs) using questionnaires administered by text message after receiving each dose of vaccine, scoring based on reactogenicity type, duration of ADRs, anti-receptor binding domain (RBD) levels, and neutralisation assays were performed 7–21days and 7–38days after the first and second vaccine doses, respectively. A total of 831 healthcare workers were included. The mean age was 46.5years (SD=±11.8years) and 75.5% were women (n=627 women). Associations between ADRs and antibody levels were assessed by Spearman correlations, multivariable logistic regression analyses were used to identify factors associated with ADR (significant correlation between systemic ADR score and anti-RBD-IgG titres (R=0.366, p<.0001) and a weaker correlation with neutralising antibodies (R=0.283, p<.005) after adjustment for age, gender, and days after the second dose). Thirty-three percent and 83.2% had at least one systemic ADR after the first and second dose, respectively. Being female, being younger than 55years, and having high anti-RBD IgG levels were significantly and independently correlated with an increased risk of adverse reactions (OR=2.86, 95% CI 1.6–5.1, p=.0004); (OR=3.18, 95% CI 1.83–5.52, p<.0001); (OR=1.36, 95% CI 1.33–1.39, p=.0029), respectively.They state that systemic reactogenicity results from spillage of inflammatory mediators or products into the blood and via immune system activation by the spike protein used as the antigen. They propose the spike effect through the cumulative immune effect due to repeated exposure to the antigen with each vaccine booster. This reinforces the hypothesis of ‘endogenous adjuvant potential’ and systemic distribution |
| Piras et al. 202226 | Case report. They describe a typical example of myopericarditis occurring in a 16-year-old adolescent after the second shot of the Pfizer mRNA vaccine and analyse 110 case reports published up to July 2022 with related features and outcomes | Report of a clinical case of myocarditis, given the lack of concurrent risk factors, recent vaccination, anti-COVID-19 IgG positivity, and onset of high fever, the authors propose the suggested mechanisms: hyper immune or inflammatory reaction after exposure to spike protein, mRNA strand, late onset of hypersensitivity (HSR), eosinophilic myocarditis, hyperreactivity to vaccine excipients, reaction to vaccine adjuvants (LNP), self-immunity through molecular mimicry; other pathways such as: release of anti-idiotype antibodies against certain regions of antigen-specific antibodies, activation of pre-existent dysregulated immune pathways in predisposed subjects, antibody-dependent amplification of immunity or other forms of immune intensification with re-exposure to the virus after vaccination, direct cell invasion through the interplay of the spike protein and ACE2, hyperviscosity-induced cardiac problem and effort induced secretion of pro-inflammatory interleukin 6.Extensive analysis focusing on a significant number of single case reports, including articles excluded from systematic reviews and meta-analyses on adverse effects of myocarditis and/or pericarditis induced by mRNA (Pfizer-BNT162b2 and Moderna mRNA-1273) and vector (AstraZeneca and Janssen) vaccines. They analysed 110 reports identifying the clinical features of the disease: 12 cases of pericarditis (10.9%), 20 cases of perimyocarditis/myocarditis (18.2%) and 78 cases of myocarditis (70.9%). The latter were prevalent compared to pericarditis and perimyocarditis combined (p<.00001). They confirm that most cases occurred in males after the second mRNA vaccine. Recent cases are reported that not only children and adolescents (aged 12–18years) but also older people, especially women, were affected by this adverse effect. For male patients, the mean age of onset was 31.7years (SD=±15.5years), in females it was older 51.6years (SD=± 7.4years). This was statistically significant (p<.00001). Notably, pericarditis cases were balanced between genders (7 males vs. 5 females, p=ns).The majority of cases were attributed to Pfizer-BioNTech 60 out of 110 cases (54.5%), 37 out of 110 cases with Moderna (33.7%), 8 out of 110 cases with AstraZeneca (7.3%), and 5 out of 110 cases with Janssen (4.5%). |
| Abu Serhan et al. 202227 | Systemic review. To investigate the current evidence regarding the association between COVID-19 vaccination and ocular vascular events | A total of 49 studies with 130 ocular vascular cases in persons receiving COVID-19 vaccines were included. Venous occlusive events were the most common (54.3%), mostly occurring after the first dose (46.2%) and within 5days after vaccination (46.2%, p=.095). Ocular adverse events were reported more with the Pfizer and AstraZeneca vaccines (81.6%), and mostly presented unilaterally (73.8%), Pfizer-BioNTech was the most reported vaccine (n=56 cases, 43.1%), AstraZeneca the second most reported (n=50 cases, 38.5%). The remaining 24 cases (18.6%) were reported with other types of vaccines: Moderna, CoronaVac, Janssen, one case of non-available data on the vaccine, and one case with a non-specific mRNA vaccine. The most frequently reported treatment was intravitreal anti-VEGF (n=39 cases, 30.4%), followed by corticosteroids, 18 cases (14.2%). They include other treatments (thrombolytic, antiplatelet, or anticoagulant) in addition to surgery. Most patients (90.1%) showed improvement (p=.321) or persistence (p=.414) in the final best corrected visual acuity (BCVA). Improvement was grouped into 3 categories: improved, persisted, and deteriorated. There was no significant difference between improvement, persistence, or worsening between groups (p=.369, p=.516, and p=.34, respectively). The age of the patients varied widely, ranging from 20 to 96years, with a mean (± SD) of 58.92 (±17.57), and the population was almost equally distributed between genders (51.5%).They classified the symptoms into 3 categories: A) visual disturbances (decreased visual acuity, floaters, light flashes, photopsia, curtains obstructing vision, visual field defects, and greyish spots accounted for 68.5% of the symptoms reported by patients) and B) other (proptosis, red eye, scalp tenderness, ophthalmoplegia, retrobulbar pain, temporal headache, uveitis, blurred vision, etc.).They claim that immune-mediated mechanisms cause thrombosis through activation of platelets, immune cells, and hypercoagulability factors. They propose the mechanisms of molecular mimicry, protein contaminants, and adenovirus vector proteins. They also point out that there is an increased likelihood of vascular adverse effects after administration of the first dose due to the production of higher spikes of immunoglobulin after the first exposure. They indicate the temporal association attributed to vaccine-related antibodies |
| Mohseni Afshar et al. 202328 | Narrative review. To investigate the current evidence on the association between COVID-19 vaccination and neurological adverse events | They link durability and debilitating sequelae following immunisation which may exacerbate or induce new-onset neuroimmunological diseases such as myasthenia gravis (MG), Guillain-Barre syndrome (GBS), seizures, varicella-zoster virus reactivation, stroke, Bell's palsy, transverse myelitis (TM), longitudinally extensive transverse myelitis (LETM), acute encephalopathy, acute disseminated encephalomyelitis (ADEM), small vessel vasculitis, narcolepsy, small fibre neuropathy, neuroleptic malignant syndrome (NMS), and multiple sclerosis flare-ups. They develop each of these adverse events. Common neurological symptoms are experienced as headache, anosmia, dysgeusia, myalgia, paraesthesia, weakness, dizziness, tremor, diplopia, tinnitus, dysphonia, delirium, syncope, cognitive impairment, and altered consciousness that are significant. Diagnosis is made by typical clinical evidence of bilateral/unilateral sensory, motor, or autonomic deficits, and may be without organ damage but with inflammation. These effects have been observed with several types of vaccines: Pfizer-BioNTech, Moderna, Janssen, AstraZeneca, and Sinovac and CureVac. However, a higher risk of developing these neurological diseases has been estimated with mRNA vaccines than with other COVID-19 vaccine platforms.Antiviral agents and steroids have been frequently tried as treatment and hasten recovery (Bell's palsy, TM), intravenous immunoglobulin and plasmapheresis may be considered effective treatment for GBS. Although there is insufficient evidence, high-dose methylprednisolone (1g every 24h for 3–7days) should be started immediately in all TM cases to improve neurological function and accelerate recovery.The mechanisms discussed above are proposed: hyperimmune or inflammatory reaction after exposure to the spike protein; activation of APCs; mRNA chain; autoimmunity through molecular mimicry; other pathways such as: release of anti-idiotype antibodies against certain antigen-specific antibody regions; activation of pre-existing dysregulated immune pathways in predisposed subjects; amplification of antibody-dependent immunity or other forms of immune enhancement with re-exposure to virus following vaccination; direct cell invasion through spike protein interaction; hyperviscosity-induced stroke. As a result of forming antibodies to gangliosides and ganglioside complexes, MAC and macrophages are activated, attacking, and destroying the myelin. In the GBS condition, such demyelination decreases the speed of action potential transmission through these nerves, causing inflammatory ascending polyradiculopathy. Acute encephalopathy has also been attributed to toxins, infections, and vaccines.They indicate that COVID-19 vaccines can induce activation of T cells, B cells, and changes in the parameters of several key inflammatory mediators, including IL-6 |
| Lee et al. 202229 | Case report. The study objective was to detect and respond to the development of MIS-C/A after SARS-CoV-2 vaccination | They present a patient diagnosed with MIS-C/A (diagnostic certainty level: definitive case) after BNT162b2 mRNA vaccination, on the basis of age, fever, clinical features (conjunctivitis, vomiting, hypotension, and headache), elevated inflammatory markers and NT-proBNP, neutrophilia, lymphopenia, and pericardial effusion. SARS-CoV-2 PCR was repeatedly negative. He received 2 doses of COVID-19 mRNA vaccine, 62days and 20days prior to symptom onset and had no personal history of COVID-19 and no close contact with known COVID-19 cases within 12weeks.He was successfully treated with medium-dose steroids and colchicine. He was started on oral prednisolone 0.3mg/kg/day (20mg), colchicine (0.6mg/12h), and low molecular weight heparin (enoxaparin 40mg/day). Prednisolone was tapered for 2months after discontinuation of all medication and colchicine was given for 3months. At the 2-month follow-up the laboratory results were within normal range, the patient was in good condition. The adverse event has not recurred after the end of treatment.In February 2021, the Brighton Collaboration created the standardised case definition for MIS-C/A as an adverse event after immunisation with 3 levels of diagnostic certainty: definitive (level 1), probable (level 2), and possible case (level 3). The Brighton Collaboration's case definition of MIS-C/A could facilitate the diagnosis of MIS-C/A following administration of COVID-19 vaccines and could be extrapolated worldwide.Colchicine is an anti-inflammatory agent that inhibits neutrophil activation, degranulation, and migration and disrupts the inflammatory cycle. It is recommended in combination with other anti-inflammatory agents for all patients with acute idiopathic or viral pericarditis because of its remission rates and lower risk of recurrence.The hypothesis is reinforced of spike effect due to cumulative immune effect due to repeated antigen exposure with each vaccine booster and that of ‘endogenous adjuvant potential’, also, systemic distribution |
| Chen et al. 202230 | Descriptive study. Combining non-invasive pulse measurements and frequency domain analysis to determine if the Pfizer-BioNTech COVID-19 (BNT162b2) vaccine and its cardiovascular side effects induce changes in arterial pulse transmission and waveform | Significant effects on the pulse harmonic indices were noted in group V (only vascular side effects) after following BNT162b2 vaccination. Pulse-variability score analyses and machine learning (ML) can aid discrimination among subjects with cardiovascular side effects. The areas under receiver operating characteristic curve (AUC) of the score analysis was 0.94 and 0.75 for vascular and cardiovascular adverse effects, respectively. The findings presented show that the combination of non-invasive pulse measurement, frequency domain pulse waveform analysis and pulse variability, and ML score analyses can be a useful method to detect changes in vascular properties associated with cardiovascular side effects following vaccination.The cardiac sequelae included are heart failure, cardiomyopathy, acute coronary syndrome, and arrhythmia. They verify the mechanisms underlying vascular damage may include deep endotheliopathy and vascular thrombosis. Vasculitis can cause thrombosis, haemodynamic instability, and autonomic dysregulation, which may lead to impairments in vascular integrity and tissue inflammation. Cardiovascular events associated with mRNA COVID-19 vaccination range from inflammation to thrombosis and ischaemia. They report increased systemic reactogenicity and immunogenicity after BNT162b2 vaccination. |
| Hoffmann et al. 202231 | Case report. Reporting a case of idiopathic multicentric Castleman disease (iMCD), TAFRO syndrome (thrombocytopaenia, anasarca, fever, reticulin fibrosis, renal failure, and organomegaly) after mRNA SARS-CoV-2 vaccination | iMCD is a life-threatening systemic disease due to cytokine dysregulation. In this case, occurring in a previously healthy young man (20years old) shortly after mRNA SARS-CoV-2 vaccination, second dose with BNT162b2, responding to interleukin-6 blockade with Siltuximab. Six months after completion of treatment, the patient remained with no signs of iMCD or inflammation, the authors indicate a temporal trigger of the disease. It not only adds to the pathogenic spectrum of MCD, but also extends the clinical picture of adverse events following COVID-19 immunisation. No alternative disease trigger was identified, SARS-CoV-2 PCR was negative. The aetiology of the disease, the rapid response to treatment and the lack of disease recurrence for more than half a year of follow-up verifies causality and temporal occurrence.They set out the proposed mechanisms: pathogenic/viral hypothesis, paraneoplastic hypothesis, and autoimmune hypothesis.The cytokine storm that induces iMCD is caused by uncontrolled infection (pathogen hypothesis), auto-antibodies, or auto-reactive T cells associated with predisposing germline mutations (autoimmune hypothesis), germline mutations in genes regulating inflammation (autoinflammatory hypothesis) and/or somatic mutations in monoclonal lymph node cells that lead to ectopic cytokine secretion (paraneoplastic hypothesis).It is indicated that COVID-19 vaccines can induce activation of T-cells, B-cells, and changes in the parameters of several key inflammatory mediators, including IL-6 |
| Wong et al. 202232 | Descriptive, cohort study. Duration: 2years. To identify and classify reports of menstrual irregularities and vaginal bleeding after COVID-19 vaccination submitted to a voluntary active surveillance system (v-safe) | Menstrual irregularities were reported in 63815 surveys. The most frequently observed problems were menorrhagia, oligomenorrhoea, polymenorrhoea, dysmenorrhea, and metrorrhagia. They included 57997 female respondents (≥18–49years). The majority of respondents received BNT162b2 (33149 cases [51.9%]) and mRNA-1273 (26741 cases [41.9%]) vaccines. They identify by ethnicity a higher incidence in white women 49795 cases (78%) compared to 4866 cases (7.6% Black), 4929 (7.7%) cases were Asian, and 8116 cases (12.7%) were South American.Notifications were reported as: menses with unexpected timing, missed menstrual cycles, intermenstrual spotting, heavy flow, more pain than usual, prolonged bleeding, also using the descriptor “worse symptoms” for 56890 cases (67%). Mean age: 37years (30–40)The authors claim that the link between COVID-19 vaccination and menstrual irregularities is causal. They consider both the shared spike protein with SARS-CoV-2 and the adjuvants used in vaccination to cause the intense immune response after administration of COVID-19 vaccines, and similarly after SARS-CoV-2 infection as a potential inducer that changes the activity of the hypothalamic–pituitary–ovarian axis that regulates the onset and duration of the menstrual cycle |
| Kim et al. 202233 | Descriptive study, disproportionality analysis of CNS demyelinating diseases following COVID-19 vaccination. To investigate potential safety signals of CNS demyelinating disease with COVID-19 vaccines using the World Health Organisation pharmacovigilance database | They calculate the disproportionality of demyelinating disease by calculating the information component (IC) or the reporting odds ratio (ROR) compared to the database and the other viral vaccines. They identified 715 cases of optic neuritis, 515 cases of myelitis, 220 cases of acute disseminated encephalomyelitis (ADEM), and 2840 CNS demyelinating disease events for all adverse drug reactions from July 2020 to February 2022Disproportionality for mRNA and ChAdOx1 nCoV-19 vaccines was: (IC25=−0.93, ROR25=0.38; IC25=−0.76, ROR25=0.26) for optic neuritis: (IC25=−0.69, ROR25=0.50; IC25=−0.63, ROR25=0.53) for myelitis: (IC25=−1.05, ROR25=0.33; IC25=−1.76, ROR25=0.20) for ADEM (IC25=−0.66, ROR25=0.52; IC25=−1.31, ROR25=0.34) for overall CNS demyelinating disease events compared with other viral vaccinesThey verify the risk of developing CNS demyelinating disease associated COVID-19 vaccines |
| Tamborska et al. 202234 | Descriptive, cohort study. Duration: 6months. To investigate features of Guillain-Barre syndrome (GBS) following SARS-CoV-2 vaccines and evaluate for a cause link between the two | They captured cases of GBS after SARS-CoV-2 vaccination through the UK's open-access online national surveillance system. For each case, the certainty of GBS was graded using the Brighton criteria, and the relationship to the vaccine was examined using modified WHO Causality Assessment criteria. They compare the age distribution of cases with prepandemic GBS cases and clinical features with the International GBS Outcome Study (IGOS).They include 67 reports of GBS following the ChAdOx1 vaccine (65 first doses) and 3 reports following the BNT162b2 vaccine (all first doses). The causal association of the vaccines was classified as probable for 56 cases vaccinated with ChAdOx1 (80%), possible for 12 cases (10 with ChAdOx1 and 2 with BNT162b2, 17%) and unlikely for 2 cases (3%, one case with ChAdOx1). They report a greater proportion of cases in the age group 50–59years in comparison to prepandemic GBS. The median age of patients with GBS following SARS-CoV-2 vaccination was 59 (IQR 51–67) years, 36 (51%) were male, and 34 (49%) were female, all but one were white (99%). The age distribution of GBS after BNT162b2 vaccination was not calculated because there were only 3 cases.The most frequent clinical variants were sensory-motor GBS (n=55 cases, 79%) and facial diplegia with paraesthesias (n=10 cases, 14%). Ten percent (n=7 of 69 cases) reported a previous infection, compared with 77% (n=502 of 652 patients) of the IGOS cohort (p<.00001). Facial weakness (n=44 of 70 patients, 63%) compared to 36% (n=220 of 620 patients); p<.00001 and sensory dysfunction 93% (n=63 of 68 patients) versus 69% (n=408 of 588 cases); p=.00001.Overall, most reports of GBS were after the first dose, especially with the ChAdOx1 vaccine. The absence of alternative aetiologies, different than expected age distribution, and the presence of unusual clinical features support a causal link. They propose the mechanism of antibody cross-reactivity between nerve components and the adenovirus vector and/or the SARS-CoV-2 spike protein |
| Magen et al. 202235 | Case report. Clinical and molecular characterisation of a case of BNT162b2 mRNA COVID-19 vaccine-associated myositis | They report a case of myositis after inoculation of the first dose of BNT162b2 vaccine into the left deltoid muscle of a 34-year-old, healthy woman with no previous SARS-CoV-2 infection who presented with progressive proximal muscle weakness, progressive dysphagia, and dyspnoea with respiratory failure. One month after vaccination, they detected BNT162b2 vaccine mRNA expression in DNA and RNA samples from biopsy tissue from the right deltoid and quadriceps muscles and in the patient's blood.Partial mapping of the vaccine spike protein mRNA sequence in the patient's sample indicated an unusual pattern of vaccine mRNA expression in blood cells, namely the “chopped” parts of the mRNA vaccine molecules from the Pfizer vaccine. This was supported by the low level of anti-SARS-CoV-2 IgG detected, suggesting that the mRNA vaccine was not translated into the spike protein, resulting in the intense immune response.This result highlights that, although BNT162b2 vaccine mRNA was not properly expressed in blood cells 7days after the first dose, it was still expressed in muscle tissue distant from the vaccination site one month later. They propose that the unusual pattern of BNT162b2 mRNA expression observed in muscle cells is associated with the development of myositis, also, they indicate that the exogenously expressed mRNA was stable enough to persist over a long period of time. SARS-CoV-2-associated muscle inflammation is triggered by clonal expansion of T cells and production of pro-inflammatory cytokines, leading to muscle damage with severe bulbar weakness |
| Pisani et al. 202236 | Systematic review. To evaluate the consistency of reports and propose plausible supporting theories that identify a shared pathway for the management of these patients | They report sudden sensorineural hearing loss (SSNHL), new onset tinnitus, vestibular neuritis, dizziness, and vertigo as adverse effects that occurred in the first month after immunisation, requiring immediate attention. They propose the same treatment for adverse events related to other drugs or inflammatory processes (steroids, antivirals, acetylsalicylic acid, and/or cochlear implantation). In general, patients had a normal MRI and/or CT scan, few reports considered reverse transcription polymerase chain reaction (RT-PCR), reported as negative, they critically evaluate the reports without this essential information. The record audiological manifestations after COVID-19 vaccination with Pfizer, Moderna, AstraZeneca, and Sinovac vaccines.They suggest the relationship of several mechanisms characterised by high neural tropism: cross-reactivity due to molecular mimicry between SARS-CoV-2 antibodies and ear antigens, the self-adjuvating mechanism of the mRNA vaccine causing the mRNA to act as both antigen and adjuvant at the same time triggering autoimmunity, autoreactive lymphocyte activation, and a strong T- and B-cell response. They find concordance between the onset of symptoms and the development of post-vaccination immunoglobulin G (IgG) (10–14days). They report similar viral effects on the neural pathways of the sense of smell, vertigo, or hearing loss with known sequelae of other viruses, e.g., they show herpes simplex virus DNA in vestibular nerve fibres of patients diagnosed with vestibular neuritis. They also postulate dissemination through the blood circulation of the shared pathogen template, alteration of the blood–brain barrier, hypersensitivity reaction (HSR), dysregulation of cochlear blood flow due to altered plasma viscosity, cell and platelet aggregability, red blood cell deformability, and endothelial function leading to localised inflammation that damages inner ear microvessels. Finally, they reiterate the role of the SARS-CoV-2 spike protein as an effective activator of the complement alternative pathway, which may contribute to endothelial damage and is an enhancer of platelet aggregation, leading to thrombus formation |
| Ekobena et al. 202237 | Case series. The study aim was to report the clinical course and ENT adverse effects after vaccination with an mRNA SARS-CoV-2 vaccine | They report 4 clinical cases of sudden sensorineural hearing loss (SSNHL), new onset tinnitus with hearing loss, and vestibular neuritis, which occurred after administration of 2 BNT162b2 vaccines from Pfizer-BioNTech and 2 mRNA-1273 vaccines from Moderna (first dose and boosters). Hearing loss was unilateral in all cases and recovered partially, gait instability was associated in 2 cases (one month and 7months after vaccination, respectively).They propose the same treatment for other adverse events related to COVID-19 vaccines (steroids, antiviral drugs, antihistamines). Physical examination of the 2 cases of vestibular neuritis showed persistence of gait instability (one at 7months) and rapid onset of symptoms of rotatory vertigo ≥48h, one of the patients had spontaneous horizontal nystagmus.Some reports refer to symptoms such as headache, dizziness, nausea, facial paralysis, or numbness suggesting involvement of other cranial nerves, such as the facial or vestibular nerve. They raise several hypotheses related to mRNA vaccines: cochleovestibular symptoms suggest a SARS-CoV-2 neurotropism for the cochleovestibular nerve; autoimmune processes involving autoreactive T lymphocytes and a transient or persistent break of immune tolerance mediated through vaccine antigen molecular mimicry could enhance inflammation of the vestibulocochlear or facial nerve; endothelial disorders with focal damage to cochlear vessels, IgG-mediated immune off-target reaction directed against the vestibulocochlear nerve; further assumptions include reactivation of latent viruses after immunisation. SARS-CoV-2 tropism is suggested for middle ear mucosa, vestibular, hair and Schwann cells, causing transient middle ear effusion, in addition to inner ear inflammation in other reported cases. Symptoms occurring soon after immunisation should be noted, especially in first-dose recipients without previous COVID-19 infection |
| Caliskan et al. 202238 | Case report. Identifying the BNT162b2 vaccine as a trigger for neuromyelitis optica spectrum disorder (NMOSD) | They report an optic neuritis attack 24h following vaccination with the second dose of COVID-19 mRNA vaccine BNT162b2, followed by a second transverse myelitis attack with an elevated anti-AQP-4 antibody titre, the diagnosis of neuromyelitis optica spectrum disorder was confirmed.They propose the current hypothesis of molecular mimicry between spike protein and host antigens, predisposing host immunity, and altered cytokine expression profile. The authors excluded rheumatological, infectious, and neoplastic aetiologies. Binding of AQP-4 antibody, an autoantibody against aquaporin-4 water channels, activates complement-mediated cytotoxicity or antibody-dependent cell-mediated cytotoxicity, resulting in astrocyte cell death with secondary demyelination.The case is a 43-year-old, Caucasian, previously healthy woman with a second-degree family history of systemic lupus erythematosus. She was discharged, achieving partial clinical improvement after treatment with intravenous methylprednisolone, scheduled long-term treatment with rituximab.They highlight the coincidental nature of the causal association between NMOSD with BNT162b2 vaccination, demographic data, and sex. Parallel increase in reports of NMOSD after COVID-19 infection and vaccination suggests shared mechanism, such as increased blood–brain barrier permeability, molecular mimicry, and bystander peripheral immune activation |
| Aliasin et al. 202239 | Narrative review. The aim is to provide an insight on and discuss the correlational or causal relationship between neurological adverse effects of COVID-19 vaccines and whether they can count as a threat to public health | The authors include in the overview of neurological adverse effects reported after SARS-CoV-2 vaccines: demyelinating diseases (Guillain-Barre syndrome, transverse myelitis, neuromyelitis optica, acute disseminated encephalomyelitis), cranial neuropathies (Bell's palsy, abducent nerve palsy, olfactory dysfunction, sensorineural hearing loss), cerebrovascular complications (cerebral venous sinus thrombosis, ischaemic stroke, haemorrhagic stroke), seizures, other rare neurological clinical conditions (Tolosa-Hunt syndrome, Parsonage-Turner syndrome, small fibre neuropathy) and functional neurological disorder (FND), the latter being defined as a neuropsychiatric condition. However, it is known that the absence of organ damage can also occur in these inflammatory disorders resulting in impaired neurological function without accompanying structural disease. There may also be symptoms such as paralysis, weakness, dysphagia, and psychogenic non-epileptic seizures (PNES), and hypothetical mechanisms which can lead to the reported side effects.Since adequate production of S antigen in the body is an important factor for COVID-19 vaccines to trigger an efficient immune response, the authors theoretically claim that these vaccines may cause the adverse effects cited. Another hypothesis they compare is the characteristic of the virus itself to produce neurological adverse effects following SARS-CoV-2 vaccines. SARS-CoV-2 uses the angiotensin-converting enzyme receptor 2 (ACE-2) and transmembrane serine protease 2 (TMPRSS2) to enter the target cell with the help of spike protein (S protein). They point to studies that show high concentrations of ACE-2 and TMPRSS2 are expressed in the central nervous system. For example, they discuss in detail the autoimmune reactions caused by cross-reactivity between vaccine epitopes and antigens located on myelin sheaths can induce GBS, can bind to sialic acid located on the gangliosides and glycoproteins of the cell membrane of neurons. Antibodies that attack S proteins also respond against the antigens of the myelin sheaths. They do not rule out systemic autoimmune disorders and drug toxicity, or incorrect administration of COVID-19 vaccines with severe neurological sequelae.The studies presented support the fact that individuals receiving SARS-CoV-2 vaccines are statistically more likely to develop neurological adverse effects. Among other clinical conditions discussed in this article, demyelinating disorders also lead to significant morbidity if left untreated. Immunotherapy could be considered in these conditions. |
| Azzolini et al. 202240 | Prospective, descriptive cohort study. Duration: 3months. The objective was to determine the incidence of adverse reactions to the BNT162b2 vaccine and the impact on IgG response | The study included 4156healthcare professionals who received the 2 doses of the BNT162b2 vaccine 21days apart and obtained 6113 online questionnaires on adverse events. The serum response was tested in 2765 subjects 10days after the second dose (SARS-CoV-2S1/S2 IgG) to determine the number of specific antibodies, IgG anti-S1 and anti-S2The multivariate analysis demonstrated that female sex (OR=1.95, 95% CI: 1.74–2.19), p<.001; younger age (OR=0.98 per year, p<.001, median age was 37years (IQR 27–48); second dose of the vaccine (OR=1.36, p<.001; previous COVID-19 infection (OR=1.41, p<.001) were independently associated with adverse events. IgG response was significantly higher in subjects with adverse events (1110AU/ml – IQR: 345–1630 than subjects who did not report any symptoms after vaccination 386AU/ml – IQR: 261–1350, p<.0001), and the association was more pronounced in subjects experiencing myalgia, fever, and lymphadenopathy. The authors demonstrate a more pronounced IgG response in subjects experiencing specific adverse events, and these are commonly reported by healthcare professionals after the BNT162b2 vaccine for SARS-CoV-2The authors suggest a spike effect due to a cumulative immune effect due to repeated antigen exposure with each vaccine booster. This reinforces the hypothesis of “endogenous adjuvant potential” and systemic distribution |
| Ahmed et al. 202241 | Systematic review. To evaluate any otological manifestation following COVID-19 vaccine administration, pathophysiology, clinical approach, and treatment of post-vaccination tinnitus | They report 4 cases of tinnitus following vector-based and mRNA SARS-CoV-2 vaccines (2 cases of first doses with BNT162b2). They report tinnitus as intermittent or continuous, unilateral, or bilateral, pulsatile, or non-pulsatile, acute, or chronic, and subjective or objective.They raise the mechanisms related to other adverse effects produced by COVID-19 vaccines: anti-spike antibodies can react with antigens anywhere along the auditory pathway and initiate an inflammatory reaction involving the tympanic membrane, ossicular chain, cochlea, cochlear vessels, organ of Corti, etc. Understanding the phenomenon of cross-reactivity and molecular mimicry will be helpful in potential treatment protocols not only for tinnitus but also for other otological adverse effects. Cochleovestibular symptoms suggest SARS-CoV-2 neurotropism for the cochleovestibular nerve; endothelial disorders with focal damage to cochlear vessels, IgG-mediated immune reaction directed against the vestibulocochlear nerve; they also include reactivation of latent viruses after immunisation. They suggest the deposition of circulating immune complexes and vestibule-cochlear antibodies in the incidence of inner ear autoimmune reactions. The antibodies form complexes with one or more antigens leading to a type III hypersensitivity reaction (HSR). In addition to inflammation of the inner ear, it predisposes patients to immune dysfunction and thus to abnormal immune responses.Numerous drugs and chemicals have been reported as ototoxic, causing damage to the auditory pathway and cochlear hair cells, and may cause tinnitus and other otological manifestations. The authors propose that mechanisms behind ototoxicity, not fully understood, involve chemical and electrophysiological alterations in the inner ear structures and the eighth cranial nerve. (Certain agents) cause these symptoms by inhibiting the endolymph production from the stria vascularis and/or direct toxicity to the hair cells of the organ of Corti.The immediate use of steroids is recommended for sudden onset tinnitus following COVID-19 vaccination, due to their underlying immunosuppressive mechanismSymptoms with rapid onset after vaccination are highlighted, especially in those affected after the first dose |
| Shafiq et al. 2022204 | Systematic review. To evaluate the causality between neurological adverse effects and COVID-19 vaccines | This review compiles clinical data from reports of neurological immune-related adverse events following COVID-19 vaccination, not including those relating to haematological abnormalities. The search yielded 18 results that met the criteria. Sixty-one patients (64 events) who had received a COVID-19 vaccination and experienced at least one neurological adverse event. The most reported event was facial nerve palsy (50% of all adverse events), Guillain-Barre syndrome (6.2%), demyelinating disease, including multiple sclerosis, acute disseminated encephalomyelitis, and transverse myelitis (6.2%), neuropathies such as neuritis, neuralgia, and peripheral neuropathy (10.9%), reactivation of herpes zoster (10.9%), and other reported effects 15.6%: delirium, periauricular vesicular rash, bilateral sensorineural hearing loss, visual disturbance, gait disturbance, serotonin syndrome, vestibular ataxia neuroleptic malignant syndrome. These adverse events were reported after BNT162b2, mRNA-1273, AZD1222, Janssen COVID-19, Sinopharm/BIBP and Covishield vaccination. The most affected age group that developed neurological adverse effects after vaccination was 35–54years with BNT162b2. The other vaccines did not have clear age profiles. Apart from AZD1222, female patients developed neurological adverse effects more frequently than male patients.Hypothetical mechanisms explaining causality of reported side effects: production of type 1 interferon by the vaccine leads to reduced tolerance of myelin sheath antigens; transient lymphopenia caused by the vaccine; immune dysregulation (hyperimmune or inflammatory reaction after spike protein exposure); mRNA chain; autoimmunity through molecular mimicry; other pathways such as: release of anti-idiotype antibodies against certain antigen-specific antibody regions; activation of pre-existing dysregulated immune pathways in predisposed subjects; amplification of antibody-dependent immunity or other forms of immune enhancement with re-exposure to virus after vaccination; direct cell invasion via spike protein interaction |
| Son et al. 202242 | Prospective, descriptive cohort study. Evaluating adverse events caused by COVID-19 vaccination and reported to VAERS over 7months, to confirm a causal relationship with COVID-19 vaccinations | They collected comprehensive data including 1122 AEFIs from 3 COVID-19 vaccines (Ad26.COV2.S, mRNA-1273, and BNT162b2) in 256994 affected individuals. They considered medical records: VAERS ID, age, sex, current illness, and medical history at the time of vaccination, vaccine type, manufacturer, vaccination date, and adverse event onset date, and recovery from vaccine-induced adverse events. They excluded 2 or more different COVID-19 vaccinations, individuals under 20years of age, data associated with the number of days (duration, persistence, remission, and worsening), cases reported after 2weeks and unreported recovery.The average age of each group was 50.01± 16.59years: 44.69 ±15.1 for Ad26.COV2.S; 51.45 ± 16.76 for mRNA-1273, and 49.4 ± 16.43 for BNT162b2. Total gender distribution was 718 (0.28%) unknown, 66280 (25.79%) men and 189996 (73.936%) women. The proportion of women was higher than that of men (66%–76%). Three underlying disabilities (hypertension [8.31%], asthma [4.87%] and diabetes [4.66%] were diagnosed before time of vaccination). The proportion was higher in the mRNA-1273 vaccine than the other 2 vaccinesThe adverse event onset interval for each COVID-19 vaccine was 2.07 ± 3.18days: 1.67 ± 3.1 for Ad26.COV2S; 2.51 ± 3.43 for mRNA-1273 and 1.64 ± 2.8 for BNT162b2. The onset interval for mRNA-1273 vaccine recipients was 0.84 to 0.87days longer than that of the other vaccine recipients. The results revealed that 128821 (50.13%) subjects recovered and 128173 (49.87%) did not recover from adverse events caused by COVID-19 vaccinesTwenty-one diagnoses were observed according to the System Organ Class (SOC), generally associated with general disorders and conditions at the injection site, cardiovascular, neurological, pulmonary, musculoskeletal, and connective tissue, gastrointestinal, skin (connective tissue) disorders, including type I, II and III delayed hypersensitivity reactions (HSRs), were more associated with mRNA and adenovirus vector vaccines. They are proposed as disabling diseases secondary to COVID-19 vaccines |
| Kim et al. 202243 | Prospective, descriptive cohort study. Investigating severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses after various COVID-19 vaccinations in healthcare workers | Blood samples were collected from 497 vaccinated healthcare workers. Inoculated vaccines were ChAdOx1 (AstraZeneca/Oxford), BNT162b2 (Pfizer/BioNTech), JNJ-78436735 (Janssen), and mRNA-1273 (Moderna). Each sample was tested for antibodies against SARS-CoV-2 using the Elecsys Anti-SARS-CoV-2 S assay (Roche Diagnostics), the SARS-CoV-2 IgG II Quant assay (Abbott), and R-FIND SARS-CoV-2 Neutralising Antibody Kit (SG medical Inc.). They use different target antibodies in the 3 assays: 1) antibodies (including IgG) against SARS-CoV-2S protein RBD, 2) IgG antibodies (including neutralising antibodies) against the RBD of the S1 subunit of the SARS-CoV-2S protein, and 3) neutralising antibodies. As there are differences in the target antigen, measurement method and positive cut-off criteria for each assay, their results showed differences in the antibody positivity rate of the same subject. Therefore, they analysed the differences according to the vaccine type, days since vaccination, and the relationship between adverse effects and antibody quantities. They used a questionnaire to investigate adverse events related to vaccination, which they classified into different grades: Grade 1 (no discomfort at all), Grade 2 (discomfort, but no problems in daily life), Grade 3 (symptoms requiring self-medication), Grade 4 (symptoms requiring outpatient treatment), and Grade 5 (symptoms requiring hospitalisation). Regardless of the adverse event, subjects were graded according to the need for treatment.They found 99 subjects (5%) with a positive rate of 96% -100% in the 3 antibody assays regardless of vaccine type. The antibody-positive rate of the completed vaccination groups reached 96%–100% and antibody quantities increased significantly 2weeks after vaccination. Antibody values measured at approximately 3months after BNT162b2 inoculation significantly correlated with adverse events.The study comprised individuals aged 20–50years, 26.4% and 27%, respectively. A total of 80.9% (402 of 497 subjects) were female. A total of 79.9% (397 of 497 cases) completed vaccination. Those who completed vaccination with ChAdOx1 and BNT162b2 accounted for 61% (303 of 497 cases) and 17.7% (88 of 497 cases). The time since the last vaccine administration and measurement of antibody-positive rates ranged from 0days to 117days. Those who had passed ≥15days and experienced Grade 3 or higher symptoms with the first or second dose were classified into the adverse event group.In the ChAdOx1 group, they observed a significant association between adverse events and age, but not with antibody titre. With BNT162b2, antibody titres were higher in the adverse events group (measured approximately 3months after vaccination) |
| Finsterer et al. 202144 | Systematic review. Evaluating the causal relationship between SARS-CoV-2 vaccinations and Guillain-Barre syndrome as a side effect of SARS-CoV-2 (ScoVaG) vaccination, its pathophysiology, clinical presentation, treatment, and outcome | This review summarises and discusses ScoVaG based on recent research reports. It identifies 9 articles reporting 18 patients with ScoVaG and one more report on another patient under review. Patients ranged in age from 20 to 86years, 9 patients were male, and 10 were female. In all 19 cases ScoVaG developed after the first dose of vaccine: 14 patients with AstraZeneca, four patients with Pfizer-BioNTech, and one patient with Johnson & Johnson.The latency between vaccination and onset of Guillain-Barre syndrome ranged from 3h to 39days. Treatment included: intravenous immunoglobulins (IVIGs) (n=13 patients), steroids (n=3 patients), or no therapy (n=3 patients). Two patients required plasmapheresis because IVIGs were ineffective, 6 patients required mechanical ventilation, one case received pregabalin for dysaesthesias. Only one case recovered completely, and partial recovery was achieved in 9 cases (still disabled). The outcome was not reported in 9 patients.They conclude that Guillain-Barre syndrome may develop time-linked to the first dose of a SARS-CoV-2 vaccine. They show a causal relationship between COVID-19 vaccination and ScoVaG. All patients had cranial nerve involvement, in some cases Guillain-Barre syndrome or subtypes could not be defined because of atypical presentation, also known as pseudo-Guillain-Barre syndrome, they received the same treatment and diagnosis regardless of their clinical condition.They suggest the study of the following mechanisms: 1) cross-reaction between foreign antigens and self-antigens, 2) over-activation of antigen presenting cells and subsequent autoimmune response, and 3) activation of polyclonal B cells or bystanders leading to cytokine synthesis and activation of autoreactive T cells.They propose the mechanisms of molecular mimicry, protein contaminants, and adenovirus vector proteins. They also indicate that there is an increased likelihood of neurological side effects after administration of the first dose due to the production of higher immunoglobulin peaks after the first exposure.) |
| Umezawa et al. 202345 | Case report. Presenting aQP4-IgG-positive neuromyelitis optica spectrum disorder (NMOSD) following administration of the COVID-19 mRNA vaccine BNT162b2 | They report a case of a 52-year-old woman who developed an aquaporin (AQP)-4-IgG-positive NMOSD 14days after the first dose of BNT162b21. She experienced neck pain, weakness in the left arm and leg, numbness of the left hand and impaired sensation of temperature in her right leg. MRI showed T2WI hyperintense lesions in the postrema area and cervical spinal cord ranging from C1 to C6 and Gadolinium-enhanced lesions from C3 to C5, especially in the left lateral column, coincidentally on the side where she received the vaccine (left arm).Twenty-eight days later she showed improvement after high dose glucocorticosteroid therapy: 2cycles of therapy, each of 1000mg intravenous methylprednisolone for 3days (first cycle started 21days after vaccination, second cycle at 28days), continued with 40mg oral prednisone for 16days and a tapering dose for 2 more weeks. SARS-CoV-2 infection was ruled out by negative polymerase chain reaction (PCR) test and absence of antibodies against SARS-CoV-2N protein. Family history was negative for any neurological disorders and autoimmune diseases.They suggest that for the development of NMOSD to be AQP4-IgG-positive, several steps occur after blood–brain barrier (BBB) breakdown, including complement activations and astrocyte lysis after BBB breakdown. They claim that post-vaccination immune responses (interleukin-6 (IL-6) signalling pathways and humoral factors) cause alterations and increased permeability in the BBB. Through damaged BBB, plasma cells producing AQP4Abs could be recruited to the central nervous system (CNS) and AQP4Abs bind to the cervical medulla and area postrema.They propose AQP4Abs as a biomarker, widely recognised as a specific biomarker of NMOSD, with a direct role in astrocyte damage in NMOSD. Pathophysiology lies in astrocyte lysis and not demyelination, thought to be another subtype of NMOSD, namely MOG-IgG-positive NOSMD.Putative causality is established in terms of temporal association with vaccine, vaccine type, and AQP4-IgG status. |
| Hetland et al. 202246 | Clinical trial in a human model. To investigate whether key inflammatory markers related to NETosis, and endothelial damage are increased after ChAdOx1 nCoV-19 vaccination and whether their levels are associated with the severity of side effects from the vaccination | The mechanism of thrombosis in VITT associated with high levels of neutrophil extracellular traps (NETs) has been studied previously. The main finding of this study was that severe side effects (VITT) are associated with high levels of NETs. Also, measurements of calprotectin contained in NETs together with DNA histone and granular enzymes, as well as syndecan-1, discriminated between patients with VITT and those with prolonged symptoms and signs, but without VITT.They indicate key markers for NETosis, such as H3-NETs and calprotectin, as well as syndecan-1 for endotheliopathy, can be used as prognostic factors to predict the severity of complications associated with ChAdOx1 vaccination. Five patients with VITT, 10 with prolonged symptoms and cutaneous haemorrhages but without VITT, and 15 with brief and mild symptoms after vaccination were examined.The sample was divided into 3 groups according to different degrees of vaccine complications, including a control group consisting of 20 healthy, non-COVID-19- vaccinated blood donors, sampled in 2015. Group 1 (consisting of patients with VITT, symptom onset 7–10days after vaccination); group 2 (patients who had contacted health services with prolonged symptoms (mild to severe headaches) and signs (cutaneous haemorrhages) with a median onset of 1.5days after vaccination and a duration of 2.8weeks; group 3 (consisting of patients with no symptoms or brief, mild symptoms (mild headaches) onset 0–2days after vaccination). Samples from vaccinees were collected between 7 and 64days after receiving the ChAdOx1 vaccine.Levels of H3-NETs and calprotectin in vaccinated individuals were markedly increased in VITT patients compared to vaccinees with milder vaccination-associated symptoms (r=0.818, p<.0001) with the severity of vaccination side effects. Syndecan-1 levels in vaccinees were also positively correlated to side effects after ChAdOx1 nCoV-19 vaccination (r=0.590, p<.001).The authors recommend using NET inflammatory markers and calprotectin as confirmatory tests in diagnosing VITT. |
| Mingot-Castellano et al. 202247 | Narrative review. The study objective was to describe the main autoimmune haematological disorders caused by SARS-CoV-2 vaccination | They describe commonly used therapies, potentially involved vaccine-related mechanisms (BNT162b2, mRNA-1273, ChAdOx,1, and Ad26.CoV2.S), and the autoimmune pathophysiology caused. They recommend guidelines to promptly recognise and manage these adverse effects. The structures affected by these autoimmune haematological disorders include blood vessels, platelets, and red blood cells. They associate them with SARS-CoV-2 mRNA and adenoviral vector vaccines. They conclude that SARS-CoV-2 vaccines, like other vaccines, are able to induce antibodies against self-antigens of vaccinated subjects that trigger mechanisms leading to autoimmune haematological diseases. They demonstrate molecular mimicry between viral and self antigens.They report cases of secondary immune thrombocytopaenia (ITP), immune thrombotic thrombocytopaenic purpura (iTTP), autoimmune haemolytic anaemia (AIHA), Evans syndrome, aplastic anaemia, antiphospholipid syndrome (APS), catastrophic APS (CAPS), and vaccine-induced thrombotic thrombocytopaenia (VITT). Antibodies directed against the cationic platelet chemokine platelet factor 4 (PF4) have been detected in VITT, which is able to activate platelets. VITT patients present with thrombocytopaenia and may develop thrombosis in unusual locations, such as the cerebral bed. The management of these adverse events is similar when the vaccine is not the trigger, except for the recommendation to avoid rituximab to ensure adequate immunisation against SARS-CoV-2 (by immunostimulatory underproduction). Prompt and accurate diagnosis is essential to initiate appropriate treatment and avoid thromboembolic complications or life-threatening bleeding such as VITT.These findings suggest that disrupting the monocyte-endothelial-platelet axis could restore the immune dysregulation observed in all adverse events secondary to administration of COVID-19 vaccines and could be a potential therapeutic alternative in the near future. |
| Watanabe et al. 202248 | Case series. Histopathological analysis of skin reactions after coronavirus disease vaccination with BNT162b2 | They report a case series of adverse events after COVID-19 vaccination and reveal the mechanism underlying the increment in number of infiltrated plasmacytoid dendritic cellsCase 1: A 47-year-old woman who developed a wheal at the site of inoculation 2days after receiving the initial dose of BNT162b2. Treated with topical steroids and oral antihistamines.Case 2: A 51-year-old woman experienced generalised petechial erythema with fever (38.5°C), genital bleeding, thrombocytopaenia, liver dysfunction and disseminated intravascular coagulation 2days after her the first dose of BNT162b2. She was diagnosed with macrophage activation syndrome (MAS), in which vaccination triggered excessive cytokine production. She received anti-inflammatory therapy (high-dose intravascular immunoglobulin therapy and intravenous methylprednisolone).Immunohistological analysis of the rash in both cases showed infiltration of plasmacytoid dendritic cells (p-DCs), were immunohistochemically positive for CD123+ BDCA2+ (markers of plasmacytoid dendritic cells).They propose that p-DCs infiltrate the skin as a cutaneous response to the COVID-19 vaccine, where they produce IFN-I, which causes skin disorders. Pathological findings showed intraepidermal spongiosis, liquefaction degeneration, and dermal infiltration by lymphocyte-dominant inflammatory cells.The pathogenesis of cutaneous adverse events, following COVID-19 vaccination, is caused by an immune response similar to that elicited by SARS-CoV-2 infection. The authors confirm the mechanism of virus entry into the body, Toll-like receptors (TLR3, TLR7, and TLR8) on the surface of p-DC recognise the viral mRNA and produce IFN-I. They indicate that excessive production of IFN-I causes the side effects of the vaccine. They also point out that myxovirus-resistance protein 1-exposed skin-derived IFN-I is a cause of skin disorder after COVID-19 vaccination.In both cases the numbers of p-DCs were elevated based on numbers of p-DC in HIV-associated psoriasis and psoriasis vulgaris.They suggest the pathogenic contribution of the BNT162b2 mRNA vaccine, it is possible that p-DCs infiltrate perivascular and perieccrine sweat glands of the mid-dermis. Therefore, they speculate that the cutaneous adverse events are the consequence of an immune response that mimics the response seen following SARS-CoV-2 infection. |
| Abbasi et al. 202249 | Case report. Presenting a case of vaccine-induced thrombotic thrombocytopaenia (VITT) of splanchnic veins after first dose of AstraZeneca | They describe the case of a 36-year-old woman who experienced epigastric pain 2weeks after vaccination with the first dose of AstraZeneca. COVID-19 polymerase chain reaction (PCR) test was negative.Suggestive laboratory results (low fibrinogen, high D-dimer, and low platelets), the presence of thrombosis in an unusual area, along with the fact that the patient was healthy with no history of thrombosis or thrombotic triggers, other than COVID-19 vaccine administration, confirmed the suspicion of VITT. In addition, a right ovarian corpus luteum cyst and a mildly thickened endometrium were detected. She was given Apixaban as anticoagulation and intravenous immunoglobulin (IVIG) (1g/kg for 2days), her platelets improved. For long-term treatment, apixaban was given for 6months and recanalisation of the previously thrombosed veins was confirmed by CT scan 5months after discharge.They compare adenoviral vector-based vaccines (AstraZeneca's ChAdOx1 and Janssen's Ad26.COV2.S), both associated with the adverse effect of VITT, which causes thrombi formation in unusual sites, mainly in cerebral and splanchnic veins, predominantly in females. It causes headache, abdominal pain, neurological symptoms, and fever. It is hypothesised that certain vaccine components, including the adenovirus vector, trigger the formation of antibodies against platelet factor 4 (PF4). They propose the mechanism of antigen–antibody complexes that form and trigger a cascade of reactions that eventually lead to consumption coagulopathy. They highlight the similarity of the pathogenesis to heparin-induced thrombocytopaenia. VITT is an autoimmune phenomenon. Laboratory results in VITT reflect its proposed pathophysiology: low platelets, low fibrinogen, and high D-dimer, in addition to elevated anti-PF4 titres are classic findings. This indicates that the absence of heparin exposure means that VITT mimics autoimmune heparin-induced thrombocytopaenia (aHIT). Treatment includes non-heparin anticoagulants, intravenous immunoglobulin, and plasmapheresis; surgical intervention (mechanical thrombectomy) may be necessary. Mortality has been associated with cerebral haemorrhage.The prothrombotic state is subsequently achieved when PF4-antiPF4 complexes crosslink Fc gamma RIIA receptors on platelets, monocytes, and neutrophils to trigger intracellular pathways that bring about the hypercoagulable state.In summary, they hypothesise that the adenoviral vector precipitates antibody formation, reinforces the theory that SARS-CoV-2 vaccines composed of viral vectors send more attenuated inflammatory signals or via other mechanisms contribute to innate immune activation, not just by mRNA vaccines and “endogenous adjuvant potential”. |
| Chow et al. 202250 | Case report. Presenting a case of biopsy-confirmed lymphohistiocytic myocarditis after mRNA vaccination (mRNA-1273/Moderna) | They describe the case of a previously healthy 45-year-old woman diagnosed with a lymphohistiocytic myocarditis with scattered eosinophils and an ill-defined granuloma, suggestive of a hypersensitivity reaction (HSR) following the first dose of mRNA-1273 vaccine. She presented with multiple intermittent episodes of palpitations lasting seconds to minutes, ventricular tachycardia, exercise intolerance, generalised fatigue with exertional dyspnoea and a syncopal episode resulting from (deemed to be) a seizure, one week after vaccination.Histological and immunohistochemical, and cardiac imaging findings were compatible with myocarditis. The endomyocardial biopsy specimen showed patchy endocardial and intramyocardial lymphohistiocytic infiltrates with scattered eosinophils and focal myocyte injury. CD3 and CD68 immunostains confirmed the lymphocytic and histiocytic nature of the infiltrate, respectively. They observed a focal histiocytic collection suggestive of an ill-defined granuloma.They confirmed the temporal relationship of the onset of the patient's symptoms in relation to the first dose of mRNA-1273 vaccine and the worsening of symptoms after the second dose, in view of the findings of myocarditis induced by the HSR mechanism, if anaphylactoid (HSR) or anaphylactic allergic reaction is suspected, the patient should not be re-exposed to the allergen, the risk would outweigh the benefit. She was treated with prednisone and showed clinical improvement.They confirm the mechanism of autoimmune dysregulation resulting in lymphocytic and eosinophilic infiltration of the myocardium due to molecular mimicry of the SARS-CoV-2 spike protein, nonspecific systemic inflammatory response, and by vaccine mRNA activation of cytokine storm. |
| Zlotnik et al. 202251 | Case report. Reporting a case of aunti-LGI1 autoimmune encephalitis following COVID-19 mRNA BNT162b2 vaccination | They describe the case of a 48-year-old man presenting with rapidly progressive cognitive impairment and mild hyponatraemia diagnosed with anti LGI1 AE, following the second dose of Pfizer's COVID-19 mRNA vaccine. Two and a half weeks following vaccination he experienced severe impairments in short-term memory, temporal orientation, abstraction, and language skills. Montreal Cognitive Assessment (MoCA) score of 18/30 (normal range: 26/30). Adrenal finding consistent with adenoma, with no other signs of solid tumour. He had not developed COVID-19 infection prior to vaccination, serology was positive in response to vaccination. Cerebrospinal fluid analysis showed normal protein and glucose without pleocytosis. CSF cultures were negative. Autoimmune cell-based encephalitis panel (Euroimmune) was positive for anti-LGI1Ab in both serum and CSF.He was initially treated with high-dose methylprednisolone (1g/24h for 5days), with a good response. His MoCA score after treatment improved to 22/30. He continued treatment with oral prednisolone (1mg/kg) and his MoCA score 2weeks later improved to 25/30, with marked improvement in temporal orientation, short-term memory, and language, although executive skills remained impaired. Serum sodium levels normalised 3weeks after symptom onset. He continued to improve gradually 4months later, MoCA score 27/30, executive skills remained impaired.Autoimmune limbic encephalitis with LGI1 antibodies (Anti-LGI1 AE) is characterised by rapidly progressive cognitive impairment or dementia, psychiatric disorders, faciobrachial dystonic seizures (FBDS), and refractory hyponatraemia. It responds to immunotherapy treatment including steroids, intravenous immunoglobulin, and other immunosuppressive agents.The mechanisms discussed above are proposed: hyperimmune or inflammatory reaction after exposure to spike protein; activation of APCs; mRNA chain; autoimmunity through molecular mimicry; other pathways such as: release of anti-idiotype antibodies against certain antigen-specific antibody regions; activation of pre-existing dysregulated immune pathways in predisposed subjects; amplification of antibody-dependent immunity or other forms of immune enhancement with re-exposure to virus following vaccination, direct cell invasion through spike protein interaction, transcription, and induction of many target genes, leading to synthesis and release of pyrogenic cytokines (IL-1, IL-6, TNF-α, and prostaglandin-E2) in blood, mimicking the response to natural infection. Neuroinvasion and neuroimmune cross-talk is confirmed as inducing of neurological symptoms produced by BNT162b2 vaccination against SARS-CoV-2. A causal mechanism is suggested by temporal association between vaccination and disease development in the absence of other possible intercurrent triggering events.The mechanisms discussed could be summarised as: pathogenic/viral hypothesis, paraneoplastic hypothesis, and autoimmune hypothesis. The cytokine storm that induces iMCD is caused by uncontrolled infection (pathogen hypothesis), autoantibodies, or autoreactive T cells associated with predisposing germline mutations (autoimmune hypothesis), germline mutations in genes regulating inflammation (autoinflammatory hypothesis) and/or somatic mutations in monoclonal lymph node cells that lead to ectopic cytokine secretion (paraneoplastic hypothesis). |
| Ameratunga et al. 202252 | Case report. Reporting a case of fatal fulminant necrotising eosinophilic myocarditis following the initial dose of the Pfizer-BioNTech mRNA COVID-19 (BNT162b2, Comirnaty): an idiosyncratic hypersensitivity reaction | They describe a case of fatal fulminant necrotising myocarditis in a 57-year-old woman after the first dose of BNT162b2 vaccine, discounting other causes. She died after 3days, with no history of autoimmunity or allergic disease. Toxicology screen was negative, complement and cytokine studies were not undertaken because ante mortem blood was not available. Viral PCR tests for SARS-CoV-2 were negative.Histological examination at autopsy showed multifocal aggregates of lymphoid cells, histiocytes and abundant eosinophils, with focal myocyte necrosis in the free walls of both ventricles, inter-ventricular septum and around the conduction system (sino-atrial and atrio-ventricular nodes). No parasitic organisms or giant cells were identified. The left pleural space mass showed a thymoma (WHO subtype AB).The temporal association is compatible with hypersensitivity reaction (HSR), other causes have been excluded and the histomorphology is compatible with the diagnosis. Cardiac injury is likely to be mediated by the highly toxic content of granules (anaphylatoxins), including eosinophil cationic protein, eosinophil major basic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin. In mice, eosinophil-derived IL-4 is required to cause cardiac damage. The immunopathology of this disorder is confirmed by mechanisms mediated by cytotoxic T cells producing IL-5, release of anti-idiotype antibodies against certain regions of antigen-specific antibodies, and direct cell invasion through spike protein interaction. The spike effect of the COVID-19 vaccine is compared with the virus to produce adverse events following SARS-CoV-2 vaccines. SARS-CoV-2 uses the angiotensin-converting enzyme receptor 2 (ACE2). Cardiac myocytes express ACE2 receptors that bind to the spike glycoprotein, translated from the mRNA vaccine. A specific ACE2-induced hypersensitivity reaction would not explain the pathogenesis of this disorder, indicating that it can be triggered by other drugs and vaccines. Sensitisation prior to the development of the disease would not be necessary, it can occur after the first exposure to a drug or vaccine. Histological features showed an inflammatory infiltrate composed of T cells and macrophages, eosinophils, B cells and plasma cells, however, this was not identified as fulminant necrotising eosinophilic myocarditis.) |
| Duijster et al. 202353 | Retrospective descriptive cohort study. Duration: 4months.1 To describe the nature and potential risk factors associated with menstrual abnormalities based on spontaneously reporting data and data from a prospective cohort event monitoring (CEM) study | Summary of reports of menstrual abnormalities registered by the Netherlands Pharmacovigilance Centre Lareb in the spontaneous reporting system between February 2021 and April 2022. They also performed a logistic regression analysis on menstrual abnormalities reported in the CEM study to assess the association between individual characteristics, prior SARS-CoV-2 infection, and hormonal contraceptive use and the occurrence of menstrual abnormalities after COVID-19 vaccination.The study showed a high incidence of menstrual disorders among women aged 54years and older, an observation supported by analysis of spontaneous reports. They analysed over 24000 spontaneous reports of menstrual abnormalities (amenorrhoea/oligomenorrhoea and heavy menstrual bleeding), ≥500 episodes (n=16929 women) in the CEM study. The CEM study had an incidence of 41.4 per 1000 women ≤54years. A significant association was observed for the age group 25–34years (OR=2.18, 95% CI 1.45–3.41) and the Pfizer vaccine (OR=3.04, 95% CI 2.36–3.93).They suggest a link between COVID-19 vaccination and menstrual abnormalities. They propose the mechanism of increased vaccine reactogenicity in women. Due to the stronger immediate response to the antigen, modulated through the innate immune system (sex-differentiated immunity). Both abnormal bleeding and irregular menstrual cycle could be associated with the spike effect, reinforcing the hypothesis of “endogenous adjuvant potential” although antibody responses in this vaccine are not reported. |
Please cite this article as: Morlanes Pallás R. Innate and adaptative immune mechanisms of COVID-19 vaccines. Serious adverse events associated with SARS-CoV-2 vaccination: A systematic review. Vacunas. 2024. https://doi.org/10.1016/j.vacun.2024.01.001.