Vaccine safety is a major barrier to the uptake of the COVID-19 vaccine by pregnant women. To bring confidence among pregnant women towards vaccine intake, there is a need to synthesize evidence on safety profile of vaccination.
ObjectiveTo assess adverse events (AEs) following COVID-19 vaccination among pregnant women.
Materials and methodsA vaccine safety surveillance was conducted at 2 rural primary health centers (PHC) located in Anantapur District, India. A total of 420 pregnant women were monitored for AEs following COVID-19 vaccination for a period of 30 min and followed for 1 month for late reactions through telephonic interviews. All AEs were subjected to causality and severity assessment. Descriptive statistics were used to represent adverse events.
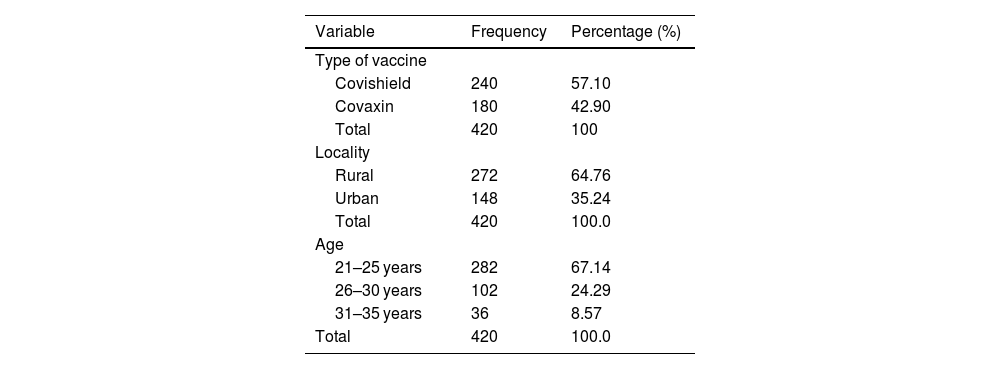
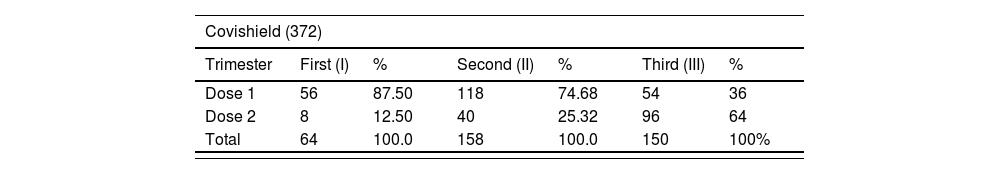
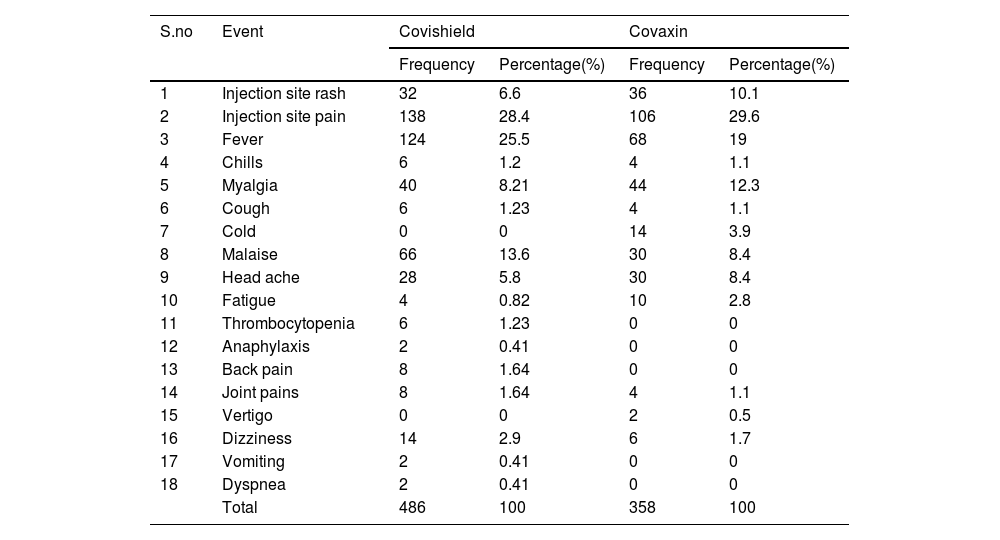
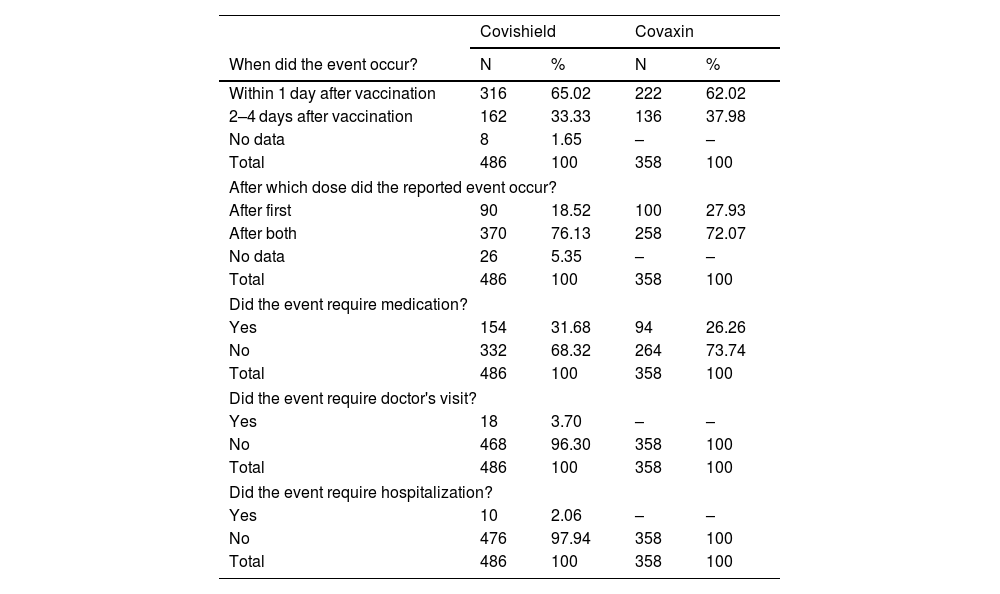
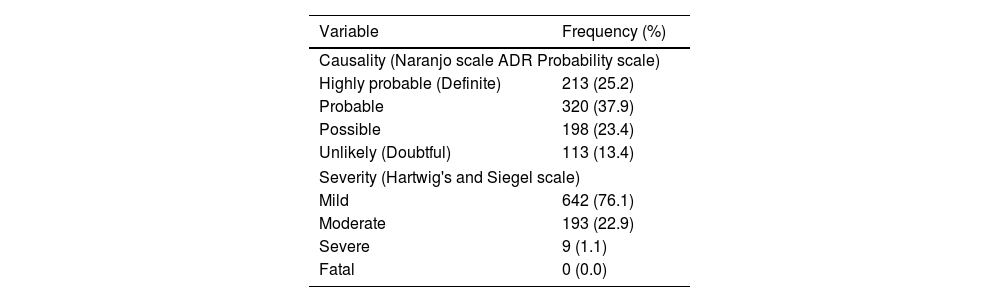
ResultsThe COVID-19 vaccine acceptance rate among pregnant women was 64.4%. A total of 420 pregnant women received 670 vaccine doses (Covishield = 372, Covaxin = 298) against COVID-19. Majority of vaccine intake was observed during the second trimester. The incidence rate of AEs following the COVID-19 vaccine among pregnant women was 93.8%, and the majority include injection site pain (28.4%, 29.6%), fever (25.5%, 19.0%), myalgia (8.21%, 12.3%), and malaise (13.6%, 8.4%). Most AEs notified are probable and mild in nature.
ConclusionThe COVID-19 vaccine acceptance rate among pregnant women was 64.4%. A 30 days incidence rate of AEs following COVID-19 vaccination among pregnant women was 93.8%, with the most common mild events like injection site pain, and fever. A further follow-up cohort study by taking an adequate sample size was recommended to capture fetal–maternal outcomes.
La seguridad de la vacuna es una barrera importante para la adopción de la vacuna COVID-19 por parte de las mujeres embarazadas. Para llevar confianza entre las mujeres embarazadas hacia la ingesta de vacunas, es necesario sintetizar la evidencia sobre el perfil de seguridad de la vacunación.
ObjetivoEvaluar los eventos adversos (EA) después de la vacunación contra la COVID-19 en mujeres embarazadas.
Materiales y métodosSe llevó a cabo una vigilancia de la seguridad de las vacunas en dos centros rurales de atención primaria de salud (PHC) ubicados en el distrito de Anantapur, India. Un total de 420 mujeres embarazadas fueron monitoreadas para detectar EA después de la vacunación COVID-19 durante un período de 30 minutos y seguidas durante un mes para detectar reacciones tardías a través de entrevistas telefónicas. Todos los EA se sometieron a una evaluación de causalidad y gravedad. Se utilizaron estadísticas descriptivas para representar los eventos adversos.
ResultadosLa tasa de aceptación de la vacuna COVID-19 entre las mujeres embarazadas fue del 64,4%. Un total de 420 mujeres embarazadas recibieron 670 dosis de vacunas (Covishield = 372, Covaxin = 298) contra COVID-19. La mayoría de la ingesta de vacunas se observó durante el segundo trimestre. La tasa de incidencia de EA después de la vacuna COVID-19 entre las mujeres embarazadas fue del 93,8%, y la mayoría incluye dolor en el lugar de la inyección (28,4%, 29,6%), fiebre (25,5%, 19,0%), mialgia (8,21%, 12,3%) y malestar general (13,6%, 8,4%). La mayoría de los EA notificados son de naturaleza probable y leve.
ConclusiónLa tasa de aceptación de la vacuna COVID-19 entre las mujeres embarazadas fue del 64,4%. Una tasa de incidencia de EA a 30 días después de la vacunación contra COVID-19 entre las mujeres embarazadas fue del 93,8%, con los eventos leves más comunes como dolor en el lugar de la inyección y fiebre. Se recomendó un estudio de cohorte de seguimiento adicional mediante la toma de un tamaño de muestra adecuado para capturar los resultados maternos fetales.