Cervical cancer is the second most common form of cancer in women. In order to prevent this disease, several vaccines like Cervarix [2vHPV], Cecolin, Cervarix quadrivalent and Gardasil [9vHPV]), have been included in the vaccination program of some countries. This study as a part of Health Technology Assessment (HTA), aimed to assess the feasibility of inclusion of Human Papilloma Virus (HPV) vaccine in the national vaccination program of Iran.
Subject and methodsThis is a qualitative case study composed of semi structured interviews with a purposive sample of experts working extensively in the field of women's health. Data saturation reached at the 12th interview. The data was analyzed using the framework method.
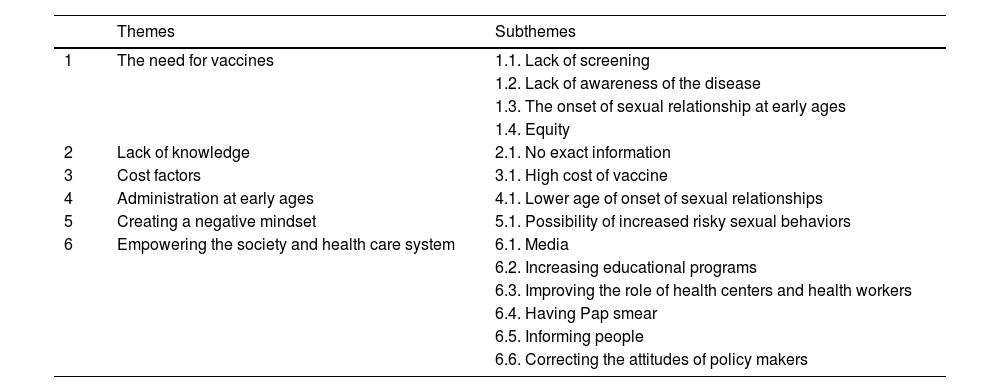
ResultsA total of 14 subthemes under 6 themes of the need for vaccines, lack of knowledge, cost factors, administration at early ages, creating a negative mindset, and empowering the society were identified.
ConclusionIncluding HPV vaccine in the national vaccination program not only seems justifiable, but also its absence in the vaccination program violates some aspects of medical ethics principles. Therefore, the government must take steps to increase public awareness about human papillomavirus and include the aforementioned vaccine in the national vaccination program.
El cáncer de cuello uterino es la segunda forma más común de cáncer en las mujeres. Para prevenir esta enfermedad, se han incluido dos vacunas (Cervarix [2vHPV], Cecolin, Cervarix quadrivalent, Gardasil [9vHPV]) y Gardasil [9vHPV]), en el programa de vacunación de algunos países. Este estudio, como parte de la Evaluación de Tecnología Sanitaria (HTA), tuvo como objetivo evaluar la viabilidad de la inclusión de la vacuna contra el Virus del Papiloma Humano (VPH) en el programa nacional de vacunación de Irán.
Sujeto y métodosEste fue un estudio de caso cualitativo que reclutó entrevistas semiestructuradas con una muestra intencional de expertos que trabajan extensamente en el campo de la salud de la mujer. Saturación de datos alcanzada en la 12ª entrevista. Los datos se analizaron utilizando el método framework.
ResultadosSe identificaron un total de 14 subtemas bajo 6 temas de necesidad de vacunas, falta de conocimiento, factores de costo, administración a edades tempranas, creación de una mentalidad negativa y empoderamiento de la sociedad.
ConclusiónLa inclusión de la vacuna contra el VPH en el programa nacional de vacunación no solo parece justificable, sino que su ausencia en el programa de vacunación viola algunos aspectos de los principios de la ética médica. Por lo tanto, el gobierno debe tomar medidas para aumentar la conciencia pública sobre el virus del papiloma humano e incluir la vacuna antes mencionada en el programa nacional de vacunación.