COVID 19 & vaccines: Development and practice
More infoThe objective of this systematic review is to give a comprehensive interpretation of menstrual cycle changes after the COVID-19 vaccination. Additionally, it is imperative to assess reports of menstrual changes following vaccination to dispel concerns that COVID-19 vaccines hinder the likelihood of pregnancy in the long run. A literature review was conducted using digital databases to systematically identify the studies reporting any menstrual abnormalities after the COVID-19 vaccine. Detailed patient-level study characteristics including the type of study, sample size, administered vaccines, and menstrual abnormalities were abstracted. A total of 78 138 vaccinated females were included in this review from 14 studies. Of these, 39 759 (52.05%) had some form of a menstrual problem after vaccination. Due to the lack of published research articles, preprints were also included in this review. Menorrhagia, metrorrhagia, and polymenorrhea were the most commonly observed problems and the overall study-level rate of menstrual abnormality ranged from 0.83% to 90.9%. Age, history of pregnancy, systemic side-effects of COVID-19, smoking, and second dose of COVID-19 vaccine were predictors of menstrual problems after vaccination.
Alteraciones menstruales tras la vacunación contra la COVID-19: revisión sistemática Resumen El objetivo de esta revisión sistemática es aportar una interpretación amplia sobre los cambios de los ciclos menstruales tras la vacunación contra la COVID-19. Además, es imperativo evaluar los informes sobre los cambios menstruales tras la vacunación, para disipar preocupaciones en cuanto a que las vacunas contra la COVID-19 dificultan la probabilidad de embarazo a largo plazo. Se realizó una revisión de la literatura utilizando bases de datos digitales para identificar sistemáticamente los estudios que reportan cualquier alteración menstrual tras la vacuna contra la COVID-19. Se resumieron las características detalladas del estudio al nivel del paciente, incluyendo tipo de estudio, tamaño de la muestra, vacunas administradas, y alteraciones menstruales. Se incluyó en la revisión a un total de 78 138 mujeres vacunadas, procedentes de 14 estudios. De ellas, 39 759 (52,05%) tuvieron algún tipo de problema menstrual tras la vacunación. Debido a la falta de artículos de investigación publicados, también se incluyeron preimpresos en esta revisión. Menorragia, metrorragia, y polimenorrea fueron los problemas más comúnmente observados, oscilando la tasa global de alteraciones menstruales a nivel de estudios entre el 0,83 y el 90,9%. La edad, los antecedentes de embarazos, los efectos secundarios sistémicos de la COVID-19, el tabaquismo y la segunda dosis de la vacuna contra la COVID-19 fueron factores predictivos de problemas menstruales tras la vacunación.
Billions of vaccines have been administered internationally after the first Coronavirus disease 2019 (COVID-19) vaccines became authorized for use in the emergency pandemic situation in December 2020.1–3 Several COVID-19 vaccines are presently being administered worldwide and can be divided into 3 main groups. The first group consists of mRNA-based vaccines, namely Pfizer-BioNTech (BNT162b2) and Moderna (mRNA-1273). The second and third groups include inactivated whole virus vaccines (Sinopharm/BIBP-CorV and Sinovac Biotech) and recombinant adenoviral vector vaccines (Johnson &Johnson/Janssen/Ad26.COV2.S, Oxford-AstraZeneca/ChAdOx1 ncov-19, and Sputnik V) Their use has not been without evidence of both mild side effects such as fatigue, headache and myalgias, and more severe adverse events following administration.4,5
The menstrual cycle is an important indicator of women’s overall health, as irregular and prolonged cycles have been found to increase the risk of premature mortality.6 During the initial phases of COVID-19 mass vaccination campaigns, there were reservations that these vaccines could potentially affect the menstrual cycle in women. This idea was reinforced when many women reported that their menstrual cycle unexpectedly changed after vaccination.7 Previously, HPV vaccination was also reported to cause menstrual disturbances.8 Consequently, cross-sectional and cohort studies were conducted to identify the possible association between receiving a COVID-19 vaccine and changes in the menstrual cycle. To further evaluate this link, we devised a systematic review with a comprehensive search of databases to compile the available literature on post-vaccination changes in the menstrual cycle in women.
The objective of this systematic review is to give a comprehensive interpretation of menstrual cycle changes after the COVID-19 vaccination. Our findings can equip doctors with clear and substantial information to counsel patients regarding possible post-vaccination menstrual cycle changes and how to deal with them accordingly. Such guidance is especially crucial for women who depend on knowledge of their menstrual cycle to plan when or when not to achieve pregnancy.8 Additionally, it is imperative to assess reports of menstrual changes following vaccination to dispel concerns that COVID-19 vaccines hinder the likelihood of pregnancy in the long run.9
MethodsThe following review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.10 Data were acquired from original investigations published on the topic. All data is available from the references section of Table 1.
Study characteristics.
| Author (year) [ref] | Study design | Region | Age range (years) | Vaccine(s) administered (n) | Menstrual problems (%) [type of vaccine] | Summary/Outcome/Predictors |
|---|---|---|---|---|---|---|
| Alghamdi (2021)11 | Cross-sectional | Saudi Arabia | Women aged between 18 and 45 years | Pfizer-BioNTech (2601)AstraZeneca (1560) | Menorrhagia (0.42%) [Pfizer-BioNTech]Metrorrhagia (0.26%) [Pfizer-BioNTech] (0.44%) [AstraZeneca]Mean=0.33% |
|
| Alvergne (2021)12* | Cross-sectional | United Kingdom | Women aged greater than 18 years, having ever menstruated and currently living in the UK | Pfizer-BioNTech (2335)AstraZeneca (2600) | Menorrhagia (11.5%)Metrorrhagia (6.1%)Polymenorrhea (1.5%) [Type of vaccine=NR] |
|
| Male (2021)13* | Cross-sectional study | United kingdom | Women over 18 years of age | Pfizer-BioNTech (778)AstraZeneca (346)Moderna (136)J & J/Janssen (8)Unspecified (5) | No significant changes reported |
|
| Anjorin (2022)14 | Cross-sectional study | Africa | 18 years of age and above | Pfizer-BioNTech (88)AstraZeneca (754)Moderna (17)Sputnik V (7)Sinopharm (44)Others (59) | Menstrual disorder (0.51%) [Type of vaccine=NR] |
|
| Edelman (2022)15 | Retrospective Cohort study | United States | 18 till 45 years | Pfizer-BioNTech (1326)Moderna (835)J & J/Janssen (168)Unspecified (74)Unvaccinated (1556) | No significant changes reported |
|
| Lagana (2022)16 | Cross-sectional | Italy | Females between 15 and 45 years | Pfizer-BioNTech (133)AstraZeneca (9)Moderna (19)J & J/Janssen (3) | Menorrhagia (55.6%) [AstraZeneca] (24.8%) [Pfizer-BioNTech] (15.8%) [Moderna] (66.7%) [J & J/Janssen]Hypomenorrhea (11.1%) [AstraZeneca] (21.8%) [Pfizer-BioNTech] (36.8%) [Moderna] (0) [J & J/Janssen]Polymenorrhea (11.1%) [AstraZeneca] (6.8%) [Pfizer-BioNTech] (10.5%) [Moderna] (0) [J & J/Janssen] |
|
| Lee (2022)17* | Cross-sectional | United States | 18–45 years | Pfizer-BioNTech (21 620)Moderna (13 001)J & J/Janssen (3469)AstraZeneca (751)Novavax (61)Other (204)Unspecified (23) | Menorrhagia (42.1%)Breakthrough bleeding (post-menopausal) (3.41%) [Type of vaccine=NR] |
|
| Mersel (2022)18 | Cross-sectional | Saudi Arabia | Female participants over the age of menarche | Pfizer-BioNTech (731) | Menorrhagia (34.9%)Polymenorrhea (60.5%)Oligomenorrhea (30.4%)Dysmenorrhea (62.4%) |
|
| Muhaidat (2022)19 | Cross-sectional | MENA (Middle-East and North Africa) | Female participants over the age of menarche (mean age of 34.3 ± 8.5 years) | Pfizer-BioNTech (1089)AstraZeneca (295)Sinopharm (794) | Menorrhagia (19.46%)Polymenorrhea (18.18%)Metrorrhagia (2.2%)Dysmenorrhea (21.25%)Worsening premenstrual symptoms (3.92%) [Type of vaccine=NR] |
|
| Trogstad (2022)20* | Cohort study | Norway | Norwegian women aged 18–30 years | Pfizer-BioNTech (3295)Moderna (2020)Other (285)Unspecified (88) | Menorrhagia (13.6%)Polymenorrhea (12%)Dysmenorrhea (14.6%) [Type of vaccine=NR] |
|
| Woon (2022)21* | Cohort study | Norway | Women over 18 having regular periods or withdrawal | Pfizer-BioNTech (65)Moderna (11)AstraZeneca (3) | No significant changes reported |
|
| Zhang (2022)22* | Cross-sectional | China | 18–45 years | N=13 118 [Vaccine names=NR] | Menorrhagia (0.21%)Metrorrhagia (12.74%)Hypomenorrhea (7.95%)Amenorrhea (12.62%) |
|
| Dar-Odeh (2022)23 | Cross-sectional | Jordan and Saudi Arabia | 18–45 years | Pfizer-BioNTech (241)AstraZeneca (91)Sinopharm (158) | Menstrual disturbances (4.8%) |
|
| Cheng (2022)24 | Cross-sectional | China | 18–45 years | Inactivated vaccines-Sinopharm, CoronaVac (1264) | Menstrual changes (2.1 % after first dose and 1.0% after second dose)Menstruation delay (1.4% after 1st dose and 0.6% after second dose)Early menstruation (0.4% after first dose and 0.3% after second dose)Menorrhagia (0.2% after first dose and 0.1% after second dose) |
|
Footnotes: * preprint; NR=not reported
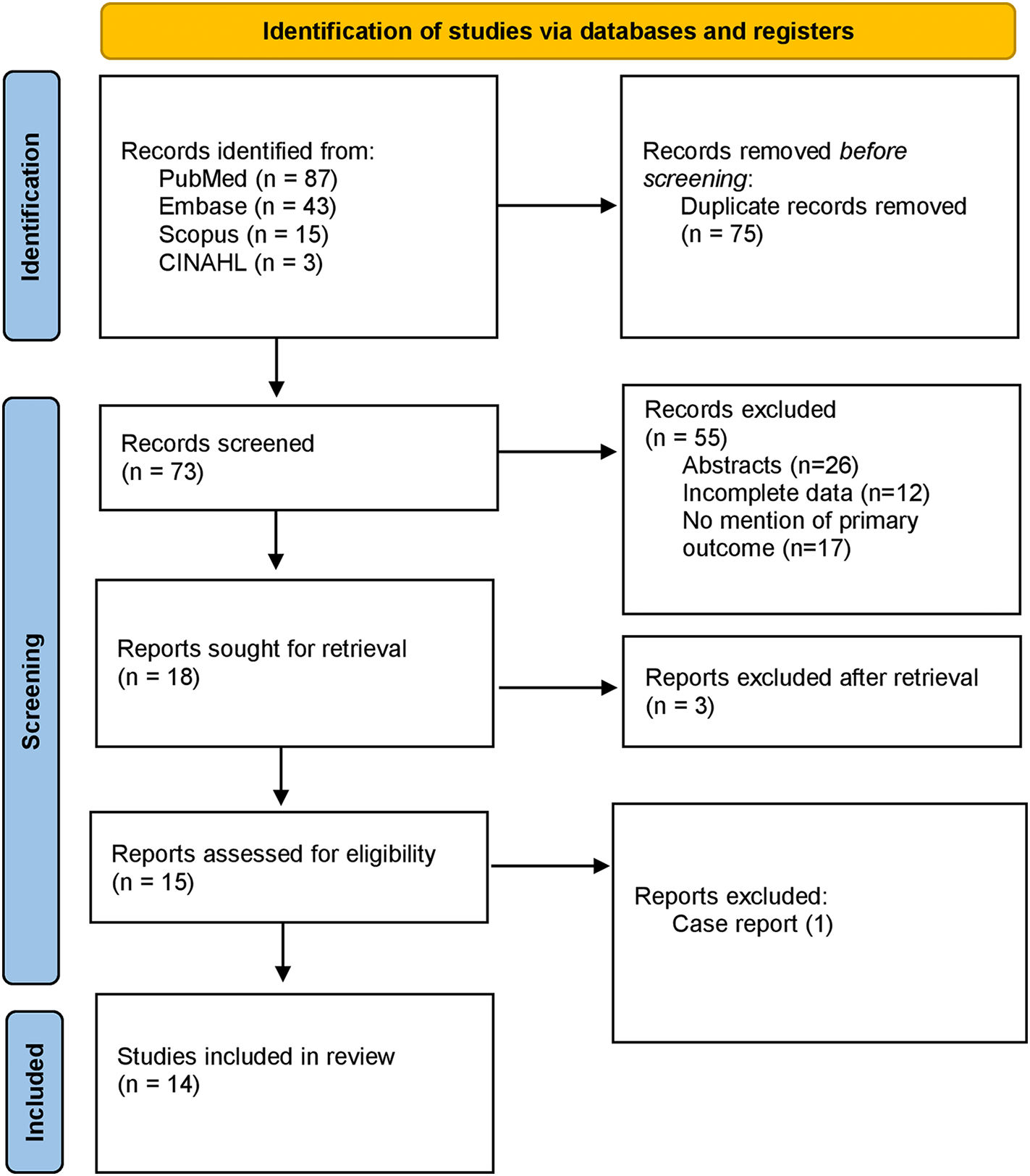
PubMed, CINAHL, Web of Science, and Scopus databases were examined with various Medical Subject Headings (MeSH) keywords to identify the studies of interest (from March 2020 to May 2022). Moreover, pre-print services including Medrxiv and biorxiv were also used to search for unpublished pre-prints. No time filters were applied on the search and backward snowballing by the screening of relevant articles and their references were also performed to extract unidentified articles missed in the primary search. The MeSH was comprised of 2 subsets: one for COVID-19 vaccines using the keywords “COVID-19 vaccine”, “COVID-19 vaccination programs”, “mRNA vaccines”, “COVID-19 mRNA vaccines”, “vector vaccines”, “COVID-19 vector vaccines”, “protein subunit vaccine”, “COVID-19 protein subunit vaccine”, and the other for menstrual problems including “women health”, “women-related problems”, “menstrual irregularities”, “menstrual problems”, “dysmenorrhea”, “menorrhagia”, “metrorrhagia”, “amenorrhea”, “oligomenorrhea”, “hypomenorrhea”, “premenstrual syndrome”. The 2 subsets were systematically combined using the Boolean operators. The results were downloaded from all given combinations into the Covidence library for review. The search strategy is shown in Fig. 1.
Three investigators (A.A., A.M., and T.Z.) reviewed the abstracts and titles for the original articles extracted in the initial search and selected the articles (also pre-prints) that reported menstrual problems associated with COVID-19 vaccines independently, including clinical trials and observational studies. Finally, abstracts, case reports, or articles published in languages other than English were not considered for analysis. Patients with prior menstrual problems unrelated to COVID-19 vaccines were also excluded from the analysis. All data was validated by the last author (U.I.); in the case of missing data authors of the original articles were contacted. The final search ended on May 15, 2022.
Data extractionThe data about the menstrual problems associated with COVID-19 vaccines were extracted independently by 3 authors (A.A., A.M., and T.Z.). Detailed study and patient-level baseline characteristics including the type of study, sample size, number of menstrual irregularities, type of vaccines, age, and summary of the studies were abstracted.
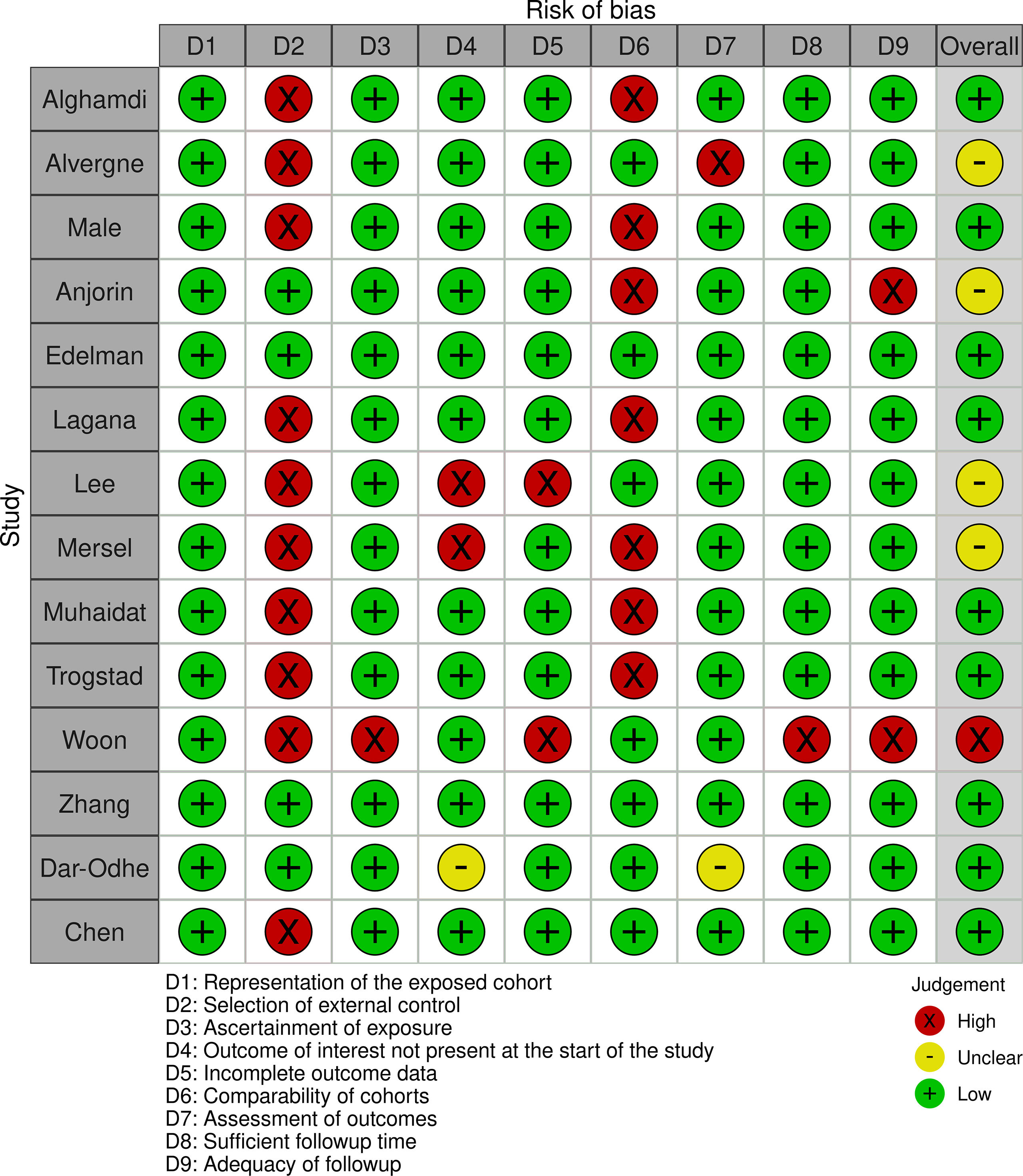
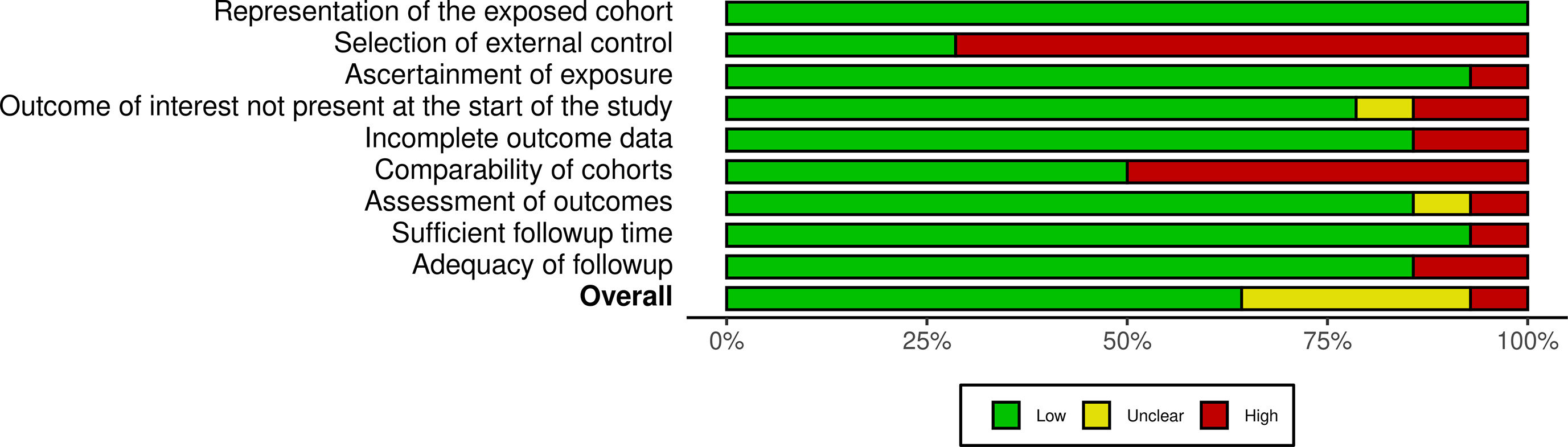
Quality assessmentThe overall quality was not the exclusion criteria for this review as a thorough assessment of vaccine-associated menstrual problems is desired. The methodological quality of observational studies was performed using the Newcastle-Ottawa scale. Supplementary Figs. S1 and S2 summarize the quality assessment for these studies. PRISMA checklist is available in Supplementary Data S3.
The overall quality was not the exclusion criteria for this review as a thorough assessment of vaccine-associated menstrual problems is desired. The methodological quality of observational studies was performed using the Newcastle-Ottawa scale. Supplementary Figs. S1 and S2 summarize the quality assessment for these studies. PRISMA checklist is available in Supplementary Data S3.
ResultsSearch resultsThe primary search elicited 148 articles. After the removal of duplicates (75), 73 articles were screened for review of the full text. Of these, 59 studies were excluded based on different reasons. A total of 14 observational studies (3 cohort and 11 cross-sectional) qualified for the analysis.11–24 Most of the studies (12 out of 14) were from developed countries (Saudi Arabia, USA, Norway, UK, and Italy) while only 2 studies were conducted in underdeveloped nations of East Africa. The search strategy is shown in Fig. 1 and the PRISMA checklist is given as Supplementary Data S3.
Study characteristics and outcomesA total of 78 138 vaccinated females were included in this review from 14 studies. Of these, 39 759 (52.05%) had some form of a menstrual problem after vaccination. All studies were published between 2021 and 2022 and 3/12 studies showed no causal relationship between menstrual abnormalities and the COVID-19 vaccine. The various types of vaccines administered to the patients include Sinopharm, Sinovac, CoVac, Modera, Astrazenica, Pfizer, CoronaVax, Novavax, and J & J/Janssen. The overall study-level rate of menstrual abnormality ranged from 0.83% to 90.9%. The criteria of menstrual abnormalities were defined in all included studies and menorrhagia was the most commonly observed adverse symptom of COVID-19 vaccines. Detailed symptoms and their percentages are tabulated in Table 1. Due to the lack of published research articles, preprints were also included in this review (6/12). Age, history of pregnancy, systemic side-effects of COVID-19, smoking, and second dose of COVID-19 vaccine were predictors of menstrual problems after vaccination. Additional information is shown in Supplementary table S4 and S5.
DiscussionThis systematic review represents the most contemporary and largest evidence on the predictors and rate of menstrual problems after COVID-19 vaccination, including 78 138 patients. Furthermore, it shows that a significant number of women (52.05%) have experienced menstrual abnormalities after the COVID-19 vaccine. However, there is heterogeneity in the occurrence of menstrual abnormalities among women following COVID-19 vaccination. Most of the cross-sectional studies reported in literature were unable to report casual relationship between menstrual irregularities and COVID-19 vaccination status. Nonetheless, a few studies reported various menstrual problems (menorrhagia, oligomenorrhea, and dysmenorrhea) post-COVID-19 vaccinations. In 3 studies, increase in female age was found to be a predictor of menstrual problems after COVID-19 vaccination. Other predictors of menstrual abnormalities after COVID-19 vaccinations include smoking, history of pregnancy, and second dose of vaccine; however, these studies were mainly questionnaire based cross-sectional studies and a causal relationship of the above-mentioned predictors with post-vaccination menstrual irregularities requires further validation.
A prospective cohort from the U.S. to date comprising 3959 women concluded that there was approximately a 1-day change in menstrual cycle in the vaccinated cohort compared to the unvaccinated cohort. However, the study’s sample population consisted largely of ethnically Caucasian women, with lower BMIs, not using hormonal contraception, who attained college level or higher education, indicating ease of utilization for menstrual tracking apps, and regular menstrual cycles on average, which meant that this sample was not representative of the majority of women residing in the United States.15 Another pre-print prospective cohort study from the UK concurred with the findings of the US cohort that vaccination was associated with a small but statistically significant delay in the next period.21 Both studies examined cohorts vaccinated with viral vector and mRNA-based vaccines but not inactivated viral vaccines used in regions like Asia and the Middle East.
The study conducted by Cheng et al on Chinese Healthcare workers reporting menstrual abnormalities after COVID-19 vaccination is notable for being one of the few large studies having participants who underwent inactivated viral vector vaccination rather than mRNA-based vaccination. The 1392 HCWs working in obstetrics/gynecology who had received at least 1 vaccine dose were included, of which 1264 (90.8%) were females and 1047 (75.2%) received 2 doses. Only 22 and 10 participants had menstrual disorders after the first and second vaccine dose, respectively. However, given that stress and psychological distress itself is one of the factors for menstrual abnormalities, these healthcare workers may have already been exposed to both factors close to or after their vaccination leading to menstrual irregularities. Further biochemical and endocrine studies based on larger sample sizes may help to clarify this confounding factor.
The US cohort study included 6 post-vaccination menstrual cycles, whereas the UK cohort study included up to 3 post-vaccination cycles. All studies, including the prospective cohorts, recognized the necessity of following more cycles over longer periods to determine long-term consequences.
Two retrospective surveys, performed in Saudi Arabia and the UK respectively, reported low incidence and a low probability that menstrual abnormalities could be linked to COVID-19 vaccination. In these studies, it was reasoned that menstrual changes could be accounted for by underlying platelet/clotting disorders or women already having delayed menstrual cycles at the time that they got their vaccine doses.11,13 The remaining 10 retrospective studies reported a proportion of women in whom menstrual irregularities could be associated with COVID-19 vaccination. In a Norwegian cross-sectional survey based on 5756 women recruited via random sampling, around 39%–41% of women experienced some form of post-vaccination menstrual change.20 Symptoms included heavier bleeding, longer as well as shorter than usual cycles, unexpected breakthrough bleeding in those using contraceptives, and dysmenorrhea. Such symptoms were experienced more frequently following the second dose.16,18,20 Previous severe acute respiratory syndrome coronavirus-2 (SARS-COV-2) infection also resulted in more frequent post-vaccination menstrual changes.12,19 These findings allude to a possible cumulative immune effect due to repeated antigen exposure in addition to environmental factors brought on by the pandemic itself, such as increased experiences of psychological distress and domestic workload.
Gynecological disorders such as endometriosis, menorrhagia, fibroids, PCOS, and adenomyosis predisposed participants to increase in post-vaccination menstrual in 1 study.17 However, other studies found such disorders to not affect the post-vaccination menstrual cycle flow and timing.12,13 These points must be considered when counseling patients with preexisting gynecological and menstrual disorders regarding the post-vaccination menstrual change. Essentially, while the effect of the COVID-19 vaccine is mostly short-lived, the long-lasting impact of chronic SARS-COV-2 infection could potentially render the menstrual cycle prone to abnormalities.13 It should be noted that results from retrospective studies, the majority of which utilized online surveys based on smartphone menstrual cycle tracking apps, are subject to multiple sources of bias, such as selection and recall bias, due to the self-reporting nature of these retrospective studies. Furthermore, most of the retrospective studies were cross-sectional in design, which cannot be utilized to establish causality between variables. Confounding variables such as socioeconomic status may also serve to hinder the establishment of a causal link as participants with access to smartphones would tend to be more educated and belong to upper socioeconomic strata compared to participants from lower socioeconomic backgrounds who may not have access to such apps.
It is known that the hypothalamic–pituitary–ovarian axis which regulates the timing and length of the menstrual cycle can be affected by various biological and environmental stressors.25–27 The hypothesis that menstrual irregularities occurred due to COVID-19 pandemic stress rather than the vaccination itself also cannot be ruled out based on available evidence. When compared to the vaccinated group, the unvaccinated control group experienced no alterations in the menstrual cycle during the pandemic.15,28 However, the intense immune response after SARS-COV-2 infection, and similarly to the administration of COVID-19 vaccines, could be a potential stressor that changes the hypothalamic–pituitary–ovarian axis.29–31 The particular antigen which causes this immune response is debatable. Both COVID-19-related spike protein and adjuvants used in vaccination have been considered possible culprits. COVID-19-related spike protein is found on the surface of the SARS-COV-2 virus. All vaccines result in the formation of neutralizing antibodies and activation of immune cells via the release of pro-inflammatory markers like cytokines and interferons against this protein to prevent or mitigate future infection from SARS-COV-2.32 Cyclic breakdown and restoration of the uterine endometrium are mediated by innate immune cells residing in the endometrium.33 Activation of these immune cells could be responsible for heavier, irregular, and untimely bleeding. Hormone levels involved in the menstrual cycle could also be affected by vaccine-induced-immunological changes including but not limited to thyroid abnormalities, which could also disrupt menstrual cycles.33,34 Another plausible hypothesis for post-vaccination menstrual changes could be immune-mediated vaccine-induced thrombocytopenia, a phenomenon that has previously been observed resulting from vaccination with several other vaccines such as measles, hepatitis, and diphtheria vaccines.35,36
This meant that given the dosing schedule between 2 injections (21 and 28 days for Pfizer-BioNTech and Moderna, respectively), the first dose would be administered in the early follicular phase.15 The post-vaccine immune response stressor is hypothesized to affect the development of the dominant follicle during the follicular phase which would, in turn, affect the cycle length.37,38 Retrospective studies also found that using estradiol-containing contraceptives lowered the odds of women experiencing post-vaccination menstrual disturbances12,17,21 which could be the result of the anti-inflammatory and immunomodulatory roles played by Estrogen and Estradiol, which have a protective effect against severe COVID-19-related clinical outcomes.39,40 Although 1 study mentioned that those using hormonal contraception experienced heavier flow following vaccination, it reasoned that this counterintuitive finding was likely due to reporting or self-selection bias.13 An acute severe systemic illness such as severe COVID-19 could permanently disturb the endocrinological homeostatic regulation of the menstrual cycle41,42 compared to vaccination by COVID-19 vaccines. Of the studies included, only 4 examined the effects of inactivated viral particle vaccine Sinopharm. Post-vaccination variations in cycle length were mainly seen in individuals who received 2 doses of the mRNA COVID-19 vaccines in 1 menstrual cycle. Thus, inactivated viral particle vaccines can serve as a safer alternative for those patients suffering from previous menstrual abnormalities. Consequently, all major studies so far conclude and reiterate that the effect of COVID-19 vaccinations on the menstrual cycle is mild, temporary, and of no long-term clinical consequences.
The primary limitation of this systematic review was a lack of data from randomized clinical trials investigating the effect of COVID-19 vaccines on the menstrual cycle. Most surveys included in our analysis were performed in the US, UK, and Norway, which are countries belonging to the ‘Global North’ in terms of health access and utilization by women, due to which, their findings cannot be extrapolated to the rest of the world, particularly countries with low healthcare access and resources such as Pakistan and India, where smartphone apps used for collecting and tracking menstrual data by users may not be as prevalent as they are in the countries where the studies were conducted.43 With a lack of peer-reviewed evidence from South Asia, which includes population centers such as Pakistan and India, it cannot be stated with certainty whether COVID-19 vaccination would have similar low reported menstrual abnormalities. Given the current body of evidence, such trials are imperative in establishing or refuting a causal relationship. Regardless, this first-of-its-kind review provides important information to clinicians regarding possible menstrual cycle changes following vaccination and addresses relevant reservations against vaccination.
ConclusionThis review provides evidence that females subjected to COVID-19 vaccines may experience menstrual abnormalities, including but not limited to menorrhagia, metrorrhagia, and polymenorrhea. Such abnormalities can impact daily life activities and ultimately impair a female's quality of life. Nevertheless, most people who reported a change in their menstrual cycle were observed to be intermittent and self-limiting. Due to the changed prioritization governed by the COVID-19 pandemic, women are less likely to seek medical assistance about menstrual abnormalities after vaccination. Therefore, further prospective cohort studies are needed to identify the temporal link between menstrual cycle changes.
Expert opinionDue to the rapidity of emerging information and the evolving nature of COVID-19 disease itself, conclusions regarding the impact of COVID-19 vaccination on female menstrual cycles can only be drawn once more high-powered studies, such as randomized control trials or longitudinal prospective population-based studies, enrolling genetically and socioeconomically diverse female populations are conducted. With the variance in epigenetic findings due to genetic polymorphisms, those populations at risk may be identified and offered counseling to stem the rise of misconceptions that may lead to vaccine hesitancy in populations prone to the spread of vaccine misinformation, such as countries in the developing world already struggling with the rapidly increasing burden of communicable diseases. Studies included in this review provide clear direction to the question of the long-term impact of both infections by COVID-19 and vaccination using the relatively newer technique of mRNA-based vaccines as opposed to conventional inactivated viral particle vaccines. While the studies themselves may have the selection and recall bias owing to the retrospective and self-reporting nature of their study designs, prospective studies also provide similar insight into this phenomenon. As the long-term adverse effects of chronic COVID-19 infection become clear, the multisystem repercussions of COVID-192 in female patients, in particular, can no longer be overlooked. As available evidence has shown, chronic COVID-19 infection may have more adverse effects on the menstrual cycle than COVID-19 vaccines, which have so far presented no long-term adverse events linked to fertility or reproduction. The complex interplay between inflammatory markers and hormonal disruptions due to COVID-19 infections, and the added factor of mRNA-based vaccination, may potentially be more damaging to the fertility and long-term health of women already suffering from previously diagnosed menstrual irregularities. Careful consideration must be given to patients suffering from previous menstrual and fertility abnormalities. Personalized counseling based on updated evidence for their concerns should be formulated, ensuring that all such patient concerns are adequately addressed through empathic health communications. Community-based engagement, particularly those led by women, will lead to better acceptance of COVID-19 vaccination, especially in vaccine-resistant areas such as Pakistan and Afghanistan, where endemic polio has already jeopardized national vaccination efforts. Health communication in these regions must focus on the low adverse impact of vaccination compared to the severe long-term effects of infection with COVID-19.
The following are the supplementary data related to this article.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vacun.2022.07.001.
Conflict of Interest and Authorship Conformation FormPlease check the following as appropriate:
- •
All authors have participated in (a) conception and design, or analysis and interpretation of the data; (b) drafting the article or revising it critically for important intellectual content; and (c) approval of the final version.
- •
This manuscript has not been submitted to, nor is under review at, another journal or other publishing venue.
- •
The authors have no affiliation with any organization with a direct or indirect financial interest in the subject matter discussed in the manuscript
- •
The following authors have affiliations with organizations with direct or indirect financial interest in the subject matter discussed in the manuscript:
Concept: JM, UI
Methodology: JM
Statistical analysis: SMJZ
First draft: MN, SA, MAR, AS, AS, AAK, TK, HK
Final draft: AM, SMJZ, JM, UI
Approval and supervision: UI
FundingThe authors declare no funding for this manuscript.
Data availabilityAll the data is available within the manuscript.