Colombia designed and adopted a vaccination plan against COVID-19 that will immunise 35 million people. The study aim was to detect the level of willingness to accept vaccination against COVID-19.
MethodsA telephone survey of 11,721 people aged 80 and over, affiliated with a health insurer, was carried out. The respondents were the affiliates or their relatives or caregivers.
ResultsThe average age was 85.0 years (SD: 4.5), with no differences between sexes; 3344 (28.5%) referred to a previous diagnostic test for COVID-19 and 73 were positive, giving an incidence of 622.8 per 100,000 people (95% CI: 491–778). Regarding attitude to vaccination against SARS-CoV-2, 1/4 respondents refrained from giving an opinion or were neutral. When the respondent was a relative, the acceptance of the vaccine was 60.4% (95% CI: 59.5–61.3) with differences by gender: men 62.2% (95% CI: 60.8–63.6) and women 59.2 (95% CI: 58.0–60.3), P<.05. When the respondent was the potential recipient of the vaccine, the acceptance of the vaccine was 61.7% (95% CI: 59.4–64.0) and this also differed by gender: 70.2% in men (95% CI: 66, 9–73.5) and 55.1% in women (95% CI: 52.0–58.3), P<.05.
ConclusionThe relatively low acceptance of vaccination against COVID-19 in Colombia poses significant challenges to achieve herd immunity that would allow control of the pandemic.
Objetivo Colombia diseñó y adoptó un plan de vacunación contra la COVID-19. El objetivo de esta investigación fue conocer la disposición para aceptar la vacunación contra esa enfermedad de personas de 80 y más años.
Métodos Se realizó una encuesta telefónica a 11.721 personas de 80 y más años, afiliadas a una aseguradora de salud, en la que se indagó sobre la intención a recibir la vacuna. Los respondientes fueron los afiliados o sus familiares o cuidadores.
Resultados El promedio de edad fue 85,0 años (SD: 4,5), sin diferencias entre sexos; 3.344 participantes (28,5%) refirieron el antecedente de haberse realizado una prueba diagnóstica previa de COVID-19 y 73 resultaron positivos. La incidencia acumulada de infección por SARS-CoV-2 desde marzo de 2020 fue de 622,8 por 100.000 personas (IC95%: 491-778 por 100.000). Respecto a la actitud hacia la vacunación contra la COVID-19, uno de cada 4 respondientes se abstuvo de opinar o se manifestó neutro. Cuando el respondiente era un familiar, la aceptación de la vacuna era del 60,4% (IC95%: 59,5-61,3) con diferencias por sexo: entre los hombres era del 62,2% (IC95%: 60,8-63,6) y en mujeres del 59,2 (IC95%: 58,0-60,3), con p<0,05. Cuando el respondiente fue el potencial receptor de la vacuna, la aceptación de la vacuna fue del 61,7% (IC95%: 59,4-64,0) y también difería por sexos: 70,2% en hombres (IC95%: 66,9-73,5) y 55,1% en mujeres (IC95%: 52,0-58,3), con p<0,05.
Conclusión La relativamente baja aceptación de la vacunación contra la COVID-19 en Colombia plantea retos importantes para lograr el control de la pandemia.
After somewhat more than a year of pandemic which started in Wuhan, China,1 by 3 February 2021, COVID-19 had caused more than 104.3 million confirmed cases and more than 2.2 million deaths.2 By the same date, Colombia had recorded more than 2.1 million cases and about 55,000 deaths. Multiple efforts by society and governments in different countries to contain or mitigate the transmission of the disease had led to widely varying results and high costs for economies and healthcare systems. After one year of pandemic, progress in knowledge of the genetic structure of the virus had made it possible to develop more than 100 vaccine candidates that promised to make an important contribution to its control. According to the World Health Organization, on 1 February, there were 15 vaccines which were highly advanced in the process of development, trials and approval by the medical authorities in different countries.3
Colombia designed and officially adopted a vaccination plan against COVID-19, with the aim of immunising at least 35 million individuals.4 Given the restrictions in the availability of vaccines, the vaccination plan focussed on and prioritised the highest-risk populations, emphasising the prevention of mortality. Phase 1 therefore involved the vaccination of medical workers who were at the front line of care, together with individuals aged 80 or more years, among whom a high level of lethality had been recorded.5
The announcement of the development of vaccines against COVID-19led to a considerable flow of erroneous information (“fake news”) around the world regarding their safety and efficacy, accompanied by increasingly delirious “conspiracy theories” about the “true” reasons for the development of these vaccines. This flood of tendentious information in the social networks may affect acceptance of vaccination and hinder the goal of achieving a sufficiently high level of herd immunity to control the pandemic. Multiple surveys have shown that factors such as the perceived risk of falling ill and concern about the safety of vaccines may influence the decision to vaccinate against COVID-19, reducing its acceptance.6
In December 2020, the Colombian National Administrative Department for Statistics (DANE) carried out a survey in 23 cities of the country regarding the interest felt by individuals in vaccinating against COVID-19.7 The result was that 35.9% of men and 42.7% of women expressed their lack of interest in receiving the vaccination.
However, there is still little information about the degree of interest in vaccination among those people at highest risk. Although the previous survey was segmented according to age, it does not specify results for those aged over 80 years, who are the first population to be vaccinated.
This paper presents the results of a survey applied exclusively to individuals aged 80 years and over who are insured by a Colombian health insurance company, with the aim of establishing the degree to which they are prepared to accept vaccination against COVID-19.
MethodsType of studyA cross-sectional study was performed in individuals aged 80 years and more who were insured by a Colombian health insurance company.
Study populationThe insurance company, Mutual SER EPS, covers about 2.2 million members, amounting to 20.4% of the population of 6of the 8departments in the Caribe region of Colombia. 2.5% of the population covered by Mutual SER EPS is above the age of 80 years (54,000 individuals), a percentage which is similar to the total population in Colombia above 80 years old (2.0%) (DANE 2020).
Sample populationOnly those older than 80 years who were members of EPS were included, as they correspond to the first population that will have access to vaccination in Colombia.
Sample sizeA sample size of 8154 is required for a population of 54,000 people to estimate a 50% frequency of vaccine acceptance with an absolute error of 1% and a confidence level of 95%.
Sample selection procedureThe sample was selected by convenience within the process of locating potential vaccine recipients undertaken by the EPS. To implement the vaccination plan against COVID-19, the insurance company tried to telephonically locate all of its members over the age of 80 years. Those who answered were requested to answer a survey about the acceptance of vaccination.
Information sourcesThe survey was answered by the member or their carer. It asked about the demographic characteristics of the respondents, their history of health service use over the previous year, whether they planned to be vaccinated or not and if they had any opinion (favourable or unfavourable) about this question.
Result definitionThe main result of the survey was the verbal expression of whether or not the member would receive the vaccine. According to the member’s answer, 2 analytical categories were obtained: those who wanted to be vaccinated, and those who did not.
Statistical analysisThe answers were included in a database and processed using a MS Excel® spreadsheet and described using the analytical tools of the said application and those of EPIDAT 3.1. A description of demographic characteristics was prepared and the frequency of acceptance of vaccination, using averages, medians, or proportions, according to the level of variable distribution. 95% confidence intervals were estimated for the distribution of certain variables. The proportions of acceptance of vaccination were compared according to sex, and the differences were analysed using statistical significance tests, χ2, with a 95% level of significance.
Ethical considerationsThis paper is based on data collected in an operative survey implemented by a health services provider in compliance with its functions and for public health decision-making. It therefore did not require approval by an ethics committee.
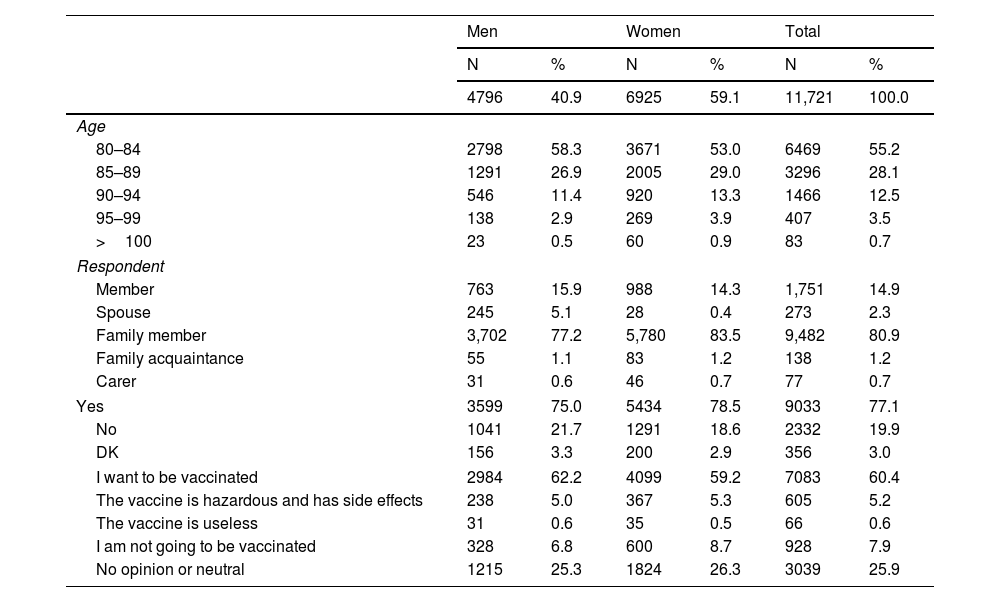
ResultsFrom 23 January to 1 February, 11,721 members aged 80 years and over were contacted by telephone, amounting to 21.6% of the total population in this age group. Their average age was 85.0 years (SD: 4.5), with no differences between the sexes; 83.3% of the interviewees were less than 90 years old, and 6925 (59.1%) were women (Table 1).
Characteristics of the population surveyed and proportion of acceptance of the vaccine against COVID-19 in members of the Mutual SER EPS Health Insurance Company, 2021
| Men | Women | Total | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| 4796 | 40.9 | 6925 | 59.1 | 11,721 | 100.0 | |
| Age | ||||||
| 80–84 | 2798 | 58.3 | 3671 | 53.0 | 6469 | 55.2 |
| 85–89 | 1291 | 26.9 | 2005 | 29.0 | 3296 | 28.1 |
| 90–94 | 546 | 11.4 | 920 | 13.3 | 1466 | 12.5 |
| 95–99 | 138 | 2.9 | 269 | 3.9 | 407 | 3.5 |
| >100 | 23 | 0.5 | 60 | 0.9 | 83 | 0.7 |
| Respondent | ||||||
| Member | 763 | 15.9 | 988 | 14.3 | 1,751 | 14.9 |
| Spouse | 245 | 5.1 | 28 | 0.4 | 273 | 2.3 |
| Family member | 3,702 | 77.2 | 5,780 | 83.5 | 9,482 | 80.9 |
| Family acquaintance | 55 | 1.1 | 83 | 1.2 | 138 | 1.2 |
| Carer | 31 | 0.6 | 46 | 0.7 | 77 | 0.7 |
| Yes | 3599 | 75.0 | 5434 | 78.5 | 9033 | 77.1 |
| No | 1041 | 21.7 | 1291 | 18.6 | 2332 | 19.9 |
| DK | 156 | 3.3 | 200 | 2.9 | 356 | 3.0 |
| I want to be vaccinated | 2984 | 62.2 | 4099 | 59.2 | 7083 | 60.4 |
| The vaccine is hazardous and has side effects | 238 | 5.0 | 367 | 5.3 | 605 | 5.2 |
| The vaccine is useless | 31 | 0.6 | 35 | 0.5 | 66 | 0.6 |
| I am not going to be vaccinated | 328 | 6.8 | 600 | 8.7 | 928 | 7.9 |
| No opinion or neutral | 1215 | 25.3 | 1824 | 26.3 | 3039 | 25.9 |
Source: calculations based on survey data.
3344 (28.5%) of the respondents stated that they had taken a COVID-19 diagnostic test; of these, 73 were found to be positive, for an accumulated incidence of confirmed COVID-19 cases of 622.8 per 100,000 people (CI 95%: 491–778 per 100,000).
Respecting attitudes to vaccination against COVID-19, 1 of every 4respondents either abstained or expressed neutrality. The percentage of acceptance varied depending on whether the respondent were a member (a potential recipient of the vaccine) or a family member or carer. The acceptance rate for family members was 60.4% (CI 95%: 59.5–61.3), although this varied depending on the sex of the potential recipient. When the potential recipient was a man, the acceptance rate expressed by family members was 62.2% (CI 95%: 60.8–63.6) while for female recipients it was 59.2% (CI 95%: 58.0–60.3), where P<.05.
When the respondent was the potential recipient of the vaccine, the general acceptance rate for the same was 61.7% (CI 95%: 59.4–64.0), and here too it differed according to sex: 70.2% for men (CI 95%: 66.9–73.5) and 55.1% for women (CI 95%: 52.0–58.3), where P<.05.
More women (14.4%; CI 95%: 13.6–15.3) than men (12.4%; CI 95%: 11.5–13.4) agreed with the following statements: the vaccine is hazardous, it has side effects; the vaccine is useless and I will not take it (P<.05).
DiscussionAlthough there is sufficient evidence that vaccination is the most cost-effective public health measure to save lives and prevent disabilities, and that it is also the fairest in health terms,8,9 the development of vaccines against COVID-19has generated multiple controversies and has accentuated the impact of anti-vaccination movements, especially against coronavirus.6
The results of a survey on the possible acceptance of a vaccine against COVID-19 in 13,426 randomly selected individuals in 19 countries with high rates of COVID-19 varied widely (from 54.85% to 88.6%). Acceptance rates in Asian countries (China, South Korea and Singapore) were higher than 80%, showing strong trust in central government; the lowest levels of acceptance were found in France, Poland, and Russia (at under 59%).10 In the United States, the acceptance rate stood at 67% in a survey at the start of May 2020 among individuals above the age of 55 years old.11
On the other hand, although anti-vaccination movements have never been as important in Colombia, our study shows worrying results about the acceptance of vaccination against COVID-19. They are very similar to those recorded by the Pulso Social Survey by the DANE in December 2020.7 However, the fact that in the population aged 80 years and above (the first priority group for access to vaccination), about 26% had no clear opinion regarding vaccination indicates that they require intervention to raise awareness and express the support of the insurance company. For example, one challenge would be to transport individuals to health centres for vaccination and back, as this would make vaccination a more secure process for members. Such support for patients would add to their security.
The limitations of this study include not having examined the specific reasons for rejecting vaccination, and not having classified acceptance of the same according to the specific characteristics of the several vaccines that are available. Nevertheless, its strengths include the use of a large population sample, exclusively targeting individuals aged 80 or more years, and, although the selectin was not random, it did consist of an important proportion of the individuals who would be vaccinated first in Colombia.
To conclude, the relatively low level of acceptance of vaccination against COVID-19 in Colombia (the Pulso Social Survey) and among members aged 80 years and over in the Mutual SER EPS Insurance Company sets major challenges if collective immunity is to be achieved that will permit the control of the pandemic. On the other hand, the existence of a high proportion of individuals who either have no opinion or are neutral offers an opportunity to raise awareness and support this population during the vaccination process.
AuthorshipAll of the authors contributed equally to: 1) the conception and design of the study, or data acquisition, or data analysis and interpretation; 2) the draft of the paper or the critical review of the intellectual content, and 3) the definitive approval of the version presented here.
FinancingThe telephonic survey was financed by the Mutual SER EPS Health Insurance Company. None of the authors received any fee or grant to participate in this study.
Please cite this article as: Alvis-Guzman N, et al. Disposición a recibir la vacuna contra COVID-19 en población de 80 y más años en Colombia 2021. Vacunas. 2021. https://doi.org/10.1016/j.vacun.2021.07.005