The pancreas is a rare metastization site for renal cell carcinoma (RCC); this paper reports a rare presentation of metastatic RCC, of obstructive jaundice secondary to a solitary synchronous pancreatic metastasis; the authors review the existing literature on pancreatic metastization of RCC in what concerns etiopathogenesis, clinics and therapeutic options.
Clinical caseA 53-year-old man was referenced to consultation for complains of weakness, dyspepsia, back pain, choluria, weight loss and jaundice; image exams revealed a single lesion on the head of the pancreas and a tumour in the left kidney; after elective pancreaticoduodenectomy and radical nephrectomy, histology revealed a RCC, and a metastatic lesion with endoluminal growth in Wirsung duct; the follow-up was uneventful with no evidence of recurrence at 6 months.
DiscussionPancreatic metastization of RCC tend to occur many years after nephrectomy; computerize tomography imaging is the method of choice for its characterization; the R0 resection provide the longest disease-free survival possible; any patient with solitary metastatic RCC in the pancreas should be a candidate for complete surgical excision if technically feasible, for palliation and prognostic reasons.
A metastização pancreática do carcinoma de células renais (CCR) é rara; Este artigo relata um caso clinico de CCR com apresentação clínica de icterícia obstrutiva secundária a metastização pancreática solitária e síncrona, e realiza uma revisão da literatura existente sobre metastização pancreática do CCR, no que concerne à etiopatogenia, clínica e terapêutica.
Caso clínicoHomem, de 53 anos, com um quadro clínico de astenia, dispepsia, lombalgia, colúria, perda ponderal e ictericia. Imagiologicamente identificou-se uma lesão única na cabeça do pâncreas e uma lesão neoformativa no rim esquerdo. Após duodenopancreatectomia e nefrectomia radical, o estudo histopatológico revelou um CCR e uma lesão pancreática metastática, com crescimento endoluminal do canal de Wirsung. O seguimento decorreu sem intercorrências ou evidência de recorrência aos 6 meses de pós-operatório.
DiscussãoA metastização pancreática do CCR tende a ocorrer vários anos após nefrectomia; a tomografia computadorizada é o método de escolha para o estudo imagiológico; a única opção terapêutica curativa é a excisão cirúrgica; um doente com metastização pancreática isolada de CCR deve ser candidato a metastectomia completa no sentido de aumentar a sobrevida livre de doença.
Renal cell carcinoma (RCC) is the third most frequent genitourinary malignancy.1 It is estimated that 25–33% of patients with RCC have metastases at presentation.2–5 RCC has the potential to metastasize to almost any site.6 Although the pancreas is not a common site of metastasis from RCC,7 it is one of the few from which metastatic tumours can occur, accounting for 2% of pancreatic tumours removed in sizable series of operations.2
The authors report a case of a 53-years-old man with a rare clinical presentation of metastatic RCC, of obstructive jaundice secondary to a solitary synchronous pancreatic metastasis.
Clinical caseA 53-year-old man was referenced to consultation for complaints of weakness, dyspepsia, back pain, choluria and weight loss (10kg in 1 month). On clinical examination he was icteric. Abdominal examination was unremarkable. By persistence of pain and jaundice the patient was sent to the emergency room for detailed imaging, including abdominal computerized tomography (CT), and blood work.
Laboratory investigation on admission was: Haemoglobin 10.0g/dL, leucocytes 3.80×109/L, urea 18mg/dL, creatinine 0.86mg/dL, total bilirubin 12.46mg/dL, direct bilirubin 6.29mg/dL, AST 70U/L, ALT 128U/L, GGT 894U/L, alkaline phosphatase 1085U/L, pancreatic amylase 730U/L and PCR 48.2mg/L.
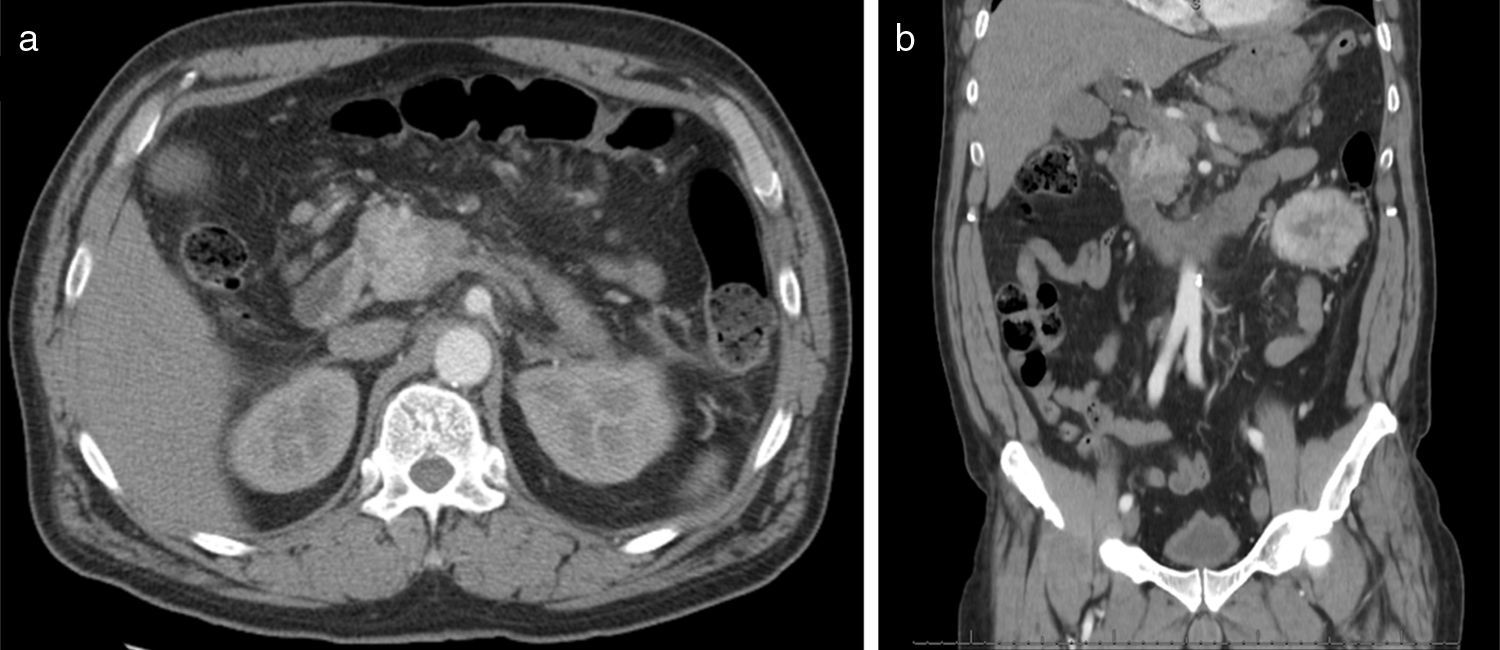
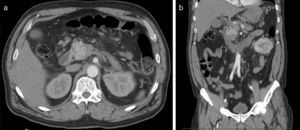
Abdominal CT scan (Fig. 1) revealed: a solid lesion on the head of the pancreas, with diameters of 30mm×30mm and lobulated limits, the lesion was most pronounced in the early arterial phases of enhancement, conditioned stenosis of the intrapancreatic portion of the bile duct, and dilatation of Wirsung's channel and main bile duct; Multiple adenopathies larger than 1cm of diameter; a large solid mass in the left kidney interesting the middle and lower thirds with 9cm×7cm×6.5cm diameters, peripheral enhancement and extensive central necrotic area, without evidence of locoregional extension or venous thrombosis.
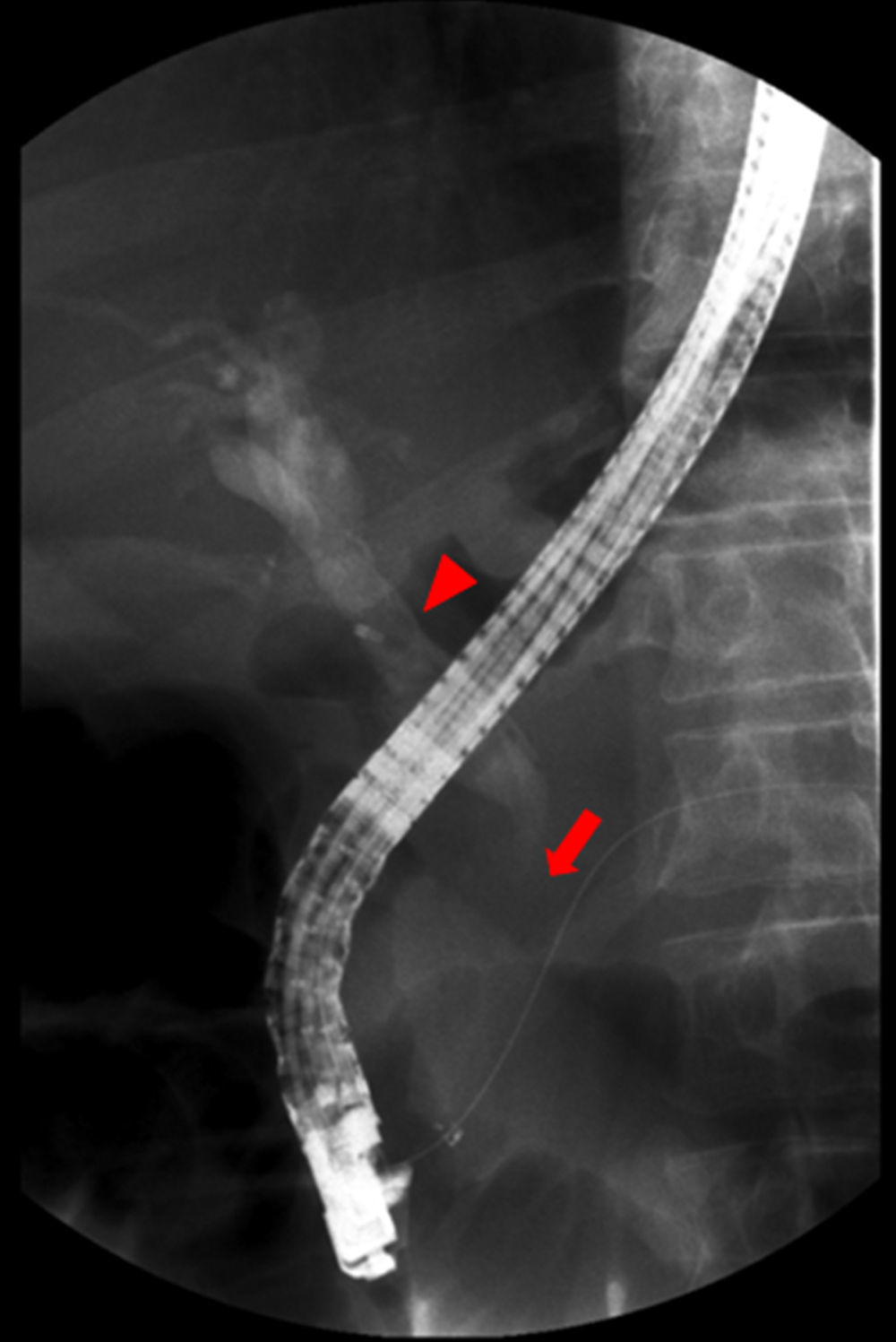
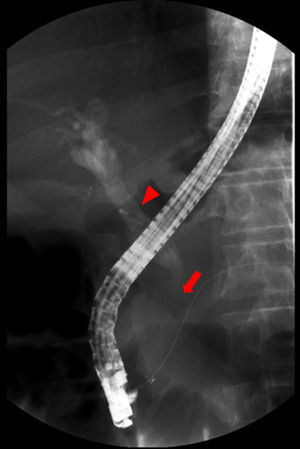
Endoscopic retrograde cholangiopancreatography (Fig. 2) revealed dilatation of main biliary duct, and a transendoscopic sphincterotomy and placement of a biliary stent (10Fr, 9cm) were performed. Abdominal magnetic resonance (MRI) and cholangio-MRI described the pancreatic lesion as discretely hypointense on T1 and discretely hyperintense on T2, with enhancement in the early phase after gadolinium injection and gradual attenuation in the later sequences.
The patient underwent elective pancreaticoduodenectomy and left radical nephrectomy (plus splenectomy, lymphadenectomy and cholecystectomy).
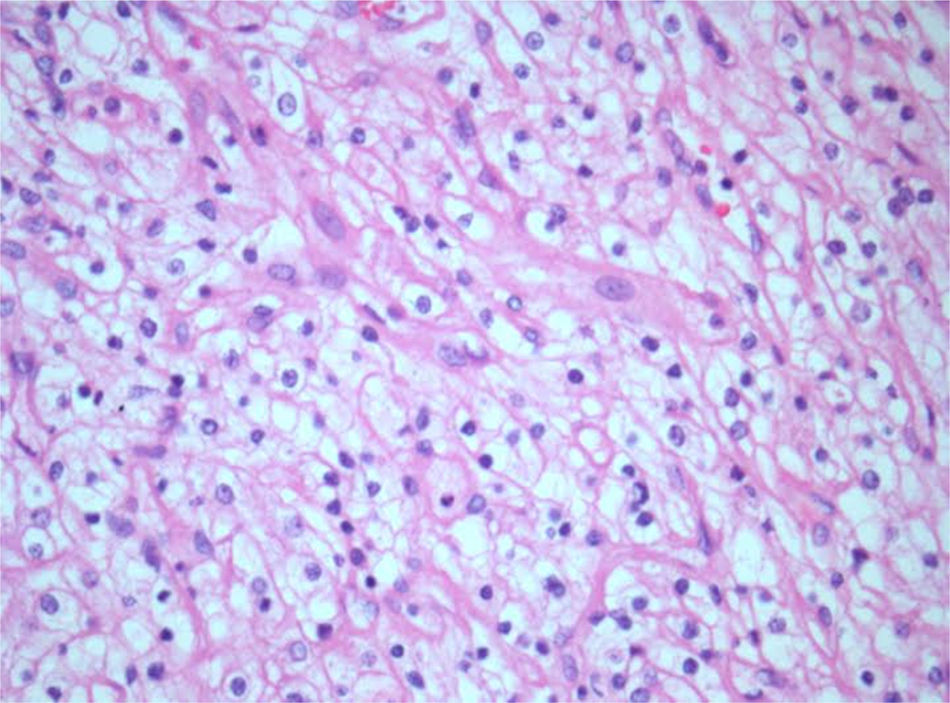
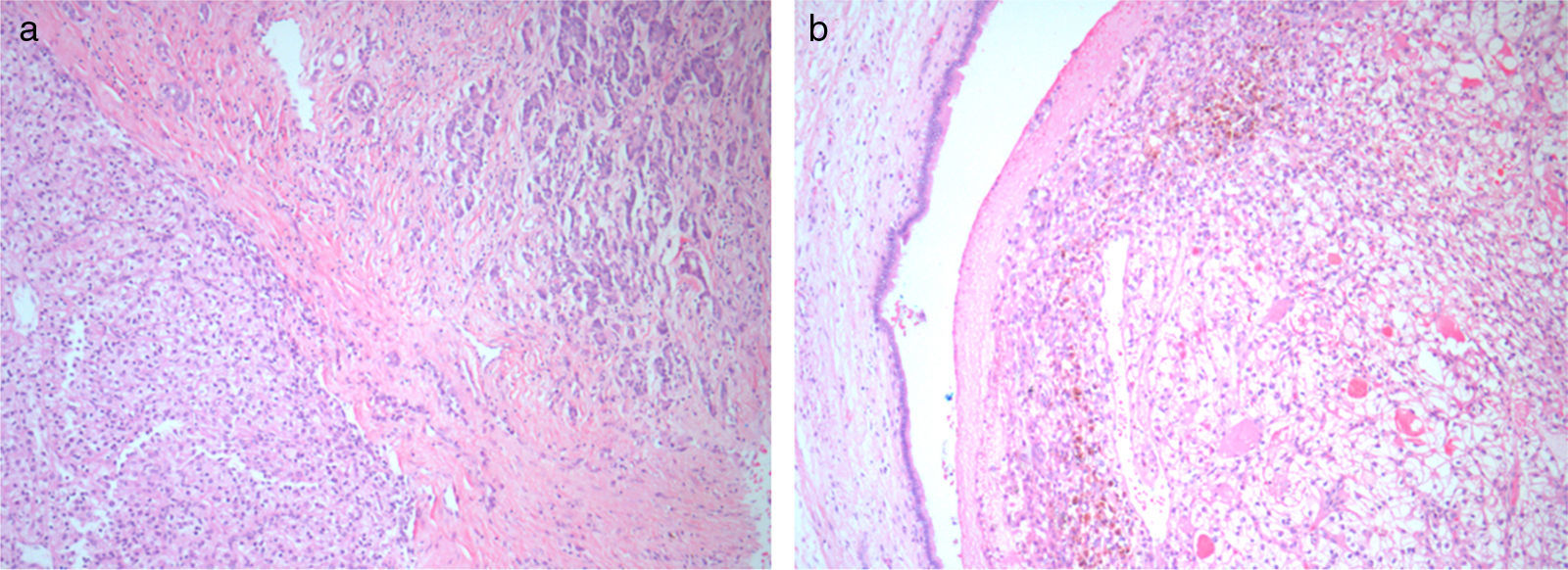
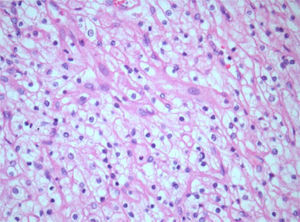
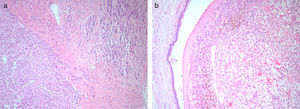
Histology and immunohistochemistry studies revealed a RCC, clear cell variant, predominantly Fuhrman grade 2, with perirenal fat infiltration and vascular invasion (Fig. 3); a lesion compatible to metastization of the described kidney neoplasia was identified in the pancreas (CK7−, CK20−, CD10+), with involvement of peripancreatic adipose tissue, ampulla of Vater, duodenum, and endoluminal growth in Wirsung duct (Fig. 4). The surgical margins were free of tumour and no metastases were found in the regional lymph nodes (pT3aN0M1).
The patient stayed 4 days in the intensive care unit due to haemodynamic instability and the total clinical stay was 10 days.
The patient had to be treated for postoperative insulin dependent diabetes. The follow-up was uneventful with no evidence of recurrence at 6 months.
DiscussionPancreatic metastasis is a rarity accounting for only 2–5% of all pancreatic malignancies.6,8 The most frequent places for RCC metastasis are: the lungs (50–60%), bones (30–40%), liver (30–40%), suprarenals and brain (5%).9 The incidence of metastasis from primary renal cell carcinoma to pancreas ranges from 0.25% to 3% of all metastatic RCC.8,10,11 However RCC is the most common primary tumour leading to solitary pancreatic metastasis,11 these are usually detected many years after nephrectomy, ranging from 6 to 8 years.10 Thompson and Heffess12 in a comprehensive review of the literature examined the clinicopathological features and prognosis for 109 patients and reported that the interval between the initial nephrectomy and the presentation of the metastasis averaged 14.6 years (range, 1 month to 32.7 years). Usually it coexists with metastases of the lungs, brain, or bones, and when it is solitary to the pancreas, it is distributed equally to all parts of it (head, body, and tail).13
The mode of spread of renal cell carcinoma to the pancreas is controversial, it may occur by haematogenous or lymphatic dissemination, the direct spread to the pancreas is not thought to occur.14,15 Haematogenous spread may occur along the draining collateral veins of a hypervascular renal tumour with or without associated renal vein thrombosis.11,16 Lymphogenous spread may occur via retrograde lymph flow through lymphatic routes from the head of the pancreas to the dorsal side of the renal artery.16,17 According to Sellner et al.,18 local lymphogenous or local venous spread through abnormal lymphatic or venous communications between the RCC and the pancreas19 cannot play an important role, because this would fail to explain the lack of relationship between the site of isolated pancreatic metastases and the side affected by the primary tumour: i.e., the left or right kidney. The localization anywhere in the pancreas irrespective of the site of the RCC argues, instead, in favour of a haematogenous systemic spread. However, a systemic spread would not explain the discrepancy between the relative frequency of multiple pancreatic metastases and the absence of metastases to other organs. The most likely explanation for this unique behaviour of isolated pancreatic metastases would seem to lie in the special biology of the tumour. The high affinity of some renal cancer cells for the parenchyma of the pancreas is supported by some reports of metachronous late metastases which again occurred solely in the residual pancreas.19
In 2006, Sellner et al.18 identified 236 cases of isolated pancreatic metastasis of RCC, either asymptomatic in 35% of the cases, or presenting with abdominal pain (20%), GI bleeding (20%), obstructive jaundice (9%), weight loss (9%), pancreatitis and diabetes (3% each), and pancreatic exocrine/endocrine dysfunction. Isolated pancreatic metastases are often found with routine surveillance imaging for primary lesions or as an incidental finding on imaging.6 Symptoms are tumour diameter-dependent, more frequent in those with more than 45mm.10 The lesions can be multifocal in approximately 30% of patients and are resectable in approximately 80% of cases.20
The radiologic features of metastatic RCC lesions closely resemble the appearance of primary RCC.11 CT imaging shows lesions that are most pronounced in the early arterial phases of enhancement, which hyperenhancing reflects the hypervascular nature of this tumours.6,11 This feature aids in detecting RCC as well as distinguishing it from primary adenocarcinoma of the pancreas, which is typically hypovascular and appears nonenhancing on CT imaging.11 In the case presented, imagological evaluation demonstrated a typical well defined lesion, with characteristic hypervascularity manifested by contrast enhancement. The most common type RCC metastasis, reported in 50–73% of cases, is that of a solitary, localized, encapsulated, well-defined mass. A second pattern of multiple pancreatic lesions has been reported in 5–10% of cases and a third pattern of diffuse metastatic infiltration causing generalized enlargement of the organ in 15–44% of cases.6 Of note, pancreatic neuroendocrine tumours are also hypervascular and contrast avid on cross sectional imaging, such that distinguishing them from RCC metastases to the pancreas can be difficult.21 On MRI, pancreatic lesions typically appear hypointense, compared with normal gland tissue on unenhanced T1 weighted images, both with and without fat saturation.3 Following intravenous contrast injection, homogeneous enhancement is typically demonstrated in smaller lesions and rim enhancement in larger ones.3 On T2 weighted images, the lesions are slightly heterogeneous and moderately hyperintense.19 Hypointense nodules are sometimes visible on T2 weighted images, especially in the diffusely enlarged type.19 Diffusion weighted imaging was recently included in the standard MRI protocol; metastatic lesions typically also show a hyperintensity signal in sequences with high b-values (700–1000).19
On endoscopic ultrasound (EUS) pancreatic metastases appear as solid intraparenchymal space-occupying lesions with an internal structure that is much more hypoechoic than the normal pancreatic tissue, or isoechoic. These lesions are homogeneous, round, well circumscribed and associated with enhancement through transmission of the ultrasonic beam.19 At power Doppler or colour Doppler evaluation and with ultrasound contrast agent, metastatic pancreatic tumours from RCC are hypervascular.19 The clinical features and findings on imaging tests often do not adequately distinguish the nature of the injury, in this sense EUS-guided biopsy can obtain material for cytology and in some cases immunocytochemistry techniques that allow us to have a more accurate diagnosis,22 especially in the case of unresectable lesions, although it is also difficult to diagnose primary or metastatic RCC in small biopsies because of the wide variety of histologic appearance,10 and also should be taken into account the risk of bleeding, which is increased due to the high vascularity of the tumour.6
Although metastasis to the pancreas is most commonly associated with disseminated systemic disease, RCC typically spreads to the pancreas as an isolated lesion, often making it amenable to surgical treatment.21 Currently, management depends on the general condition of the patient, the extent and location of the lesion, and the concurrent metastases at other sites. In the case of duodenum metastasis procedures from classic pancreaticoduodenectomy (Whipple procedure) to interventional embolization have been reported.6 Surgical resection of primary RCC and metastatic deposits remains the most effective treatment10 since chemotherapy, radiotherapy and hormonal therapy have proven ineffective.10 Surgical procedures vary according to the site and the extent of the lesion, including distal pancreatectomy, cephalic pancreaticoduodenectomy or tumour enucleation.22
The outcome of isolated pancreatic RCC metastasis is clearly more favourable with a mean 5-years survival ranging from 43% to 88%.10 In a systematic review of more than 400 patients with RCC pancreatic metastasis, Tanis et al.23 found that long disease-free interval after resection of primary tumour, a single, asymptomatic metastatic deposit with central necrosis and complete excision of the secondary lesion are associated with good prognosis. In addition, other authors found that synchronous had worse prognosis when compared to metachronous solitary metastasis.10 Although the 5-year survival rate of untreated pancreatic metastatic patients is <13%, the outcome of surgical resection of RCC pancreatic metastasis is even superior to surgical resection of primary pancreatic adenocarcinoma or to treatment of isolated pancreatic metastases from neoplasms other than RCC.11 Approximately 2–6% of patients with metastatic RCC present with isolated metastatic lesions amenable to surgical resection.11 Surgical resection of metastatic disease limited to the pancreas has a 5-year survival rate of 29–53%1,11,17 and 5-year disease-free survival rate of 5–23%.1 The largest single centre series of pancreatic resection for RCC metastasis reported a great 5-year survival benefit after surgical resection compared with conservative treatment of unresectable disease (53% vs 26%).24 Radical lymph node dissection is not necessary, as peripancreatic lymph node involvement is uncommon, which may be one of the factors that explain the favourable outcomes achieved with surgery.11
Chemotherapy and radiotherapy have generally proved to be ineffective for primary RCC or metastatic disease. Despite promising results with immunotherapy using IL-2, a complete response occurred in less than 15% and was rarely durable, emphasizing that complete resection of the primary and the metastatic lesions whenever possible is the best treatment.24 In recent years, with the advent of target therapies, the prognosis of patients with metastatic RCC has improved.6 However, in the absence of dramatic results, surgical excisions of isolated metastasis continue to play a role in the treatment of metastatic RCC.6
We opted not to perform EUS fine needle aspiration before surgery because negative results do not change the previously established surgical strategy due to low sensitivity of this technique.
In conclusion, solitary pancreatic metastasis from RCC is a rare pancreatic tumour and it is very difficult to differentiate it from pancreatic adenocarcinoma. The R0 resection is the best approach available to provide the patient with the longest disease-free survival possible, as described in the literature reviewed, and any patient with solitary metastatic RCC in the pancreas should be a candidate for complete surgical excision, if medically and technically feasible, both for palliation and prognostic reasons, as it appears to prolong disease-free survival.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.